Factors Associated with Risk of Diabetic Complications in Novel Cluster-Based Diabetes Subgroups: A Japanese Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Blood Measurements
2.3. Definition of Diabetes Subgroups and Diabetic Complications
2.4. Cluster Analysis
2.5. Statistical Analysis
3. Results
3.1. Cluster Distribution and Characteristics at Baseline
3.2. Survival Analysis for the Development of Diabetic Complications
4. Discussion
4.1. Distribution and Clinical Features of Subgroups
4.2. Association of Five Diabetic Subgroups with Diabetic Complications
4.2.1. Diabetic Kidney Disease (DKD)
4.2.2. Diabetic Retinopathy
4.2.3. Coronary Artery Disease
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, 14–31. [Google Scholar] [CrossRef]
- Udler, M.S. Type 2 Diabetes: Multiple Genes, Multiple Diseases. Curr. Diab. Rep. 2019, 19, 55. [Google Scholar] [CrossRef]
- Pearson, E.R. Type 2 diabetes: A multifaceted disease. Diabetologia 2019, 62, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, W.-Y.; Glicksberg, B.S.; Gottesman, O.; Tamler, R.; Chen, R.; Bottinger, E.P.; Dudley, J.T. Identification of type 2 diabetes subgroups through topological analysis of patient similarity. Sci. Transl. Med. 2015, 7, 311ra174. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.; Gavin, J.R., 3rd; Aguilar, R.B. The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema. Diabetes Care 2016, 39, 179–186. [Google Scholar] [CrossRef]
- Bancks, M.P.; Casanova, R.; Gregg, E.W.; Bertoni, A.G. Epidemiology of diabetes phenotypes and prevalent cardiovascular risk factors and diabetes complications in the National Health and Nutrition Examination Survey 2003-2014. Diabetes Res. Clin. Pract. 2019, 158, 107915. [Google Scholar] [CrossRef]
- Thorens, B.; Rodriguez, A.; Cruciani-Guglielmacci, C.; Wigger, L.; Ibberson, M.; Magnan, C. Use of preclinical models to identify markers of type 2 diabetes susceptibility and novel regulators of insulin secretion—A step towards precision medicine. Mol. Metab. 2019, 27, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, E.; Storm, P.; Karajamaki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, X.; Zhu, Z.; Ji, L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol. 2019, 7, 9–11. [Google Scholar] [CrossRef]
- Zaharia, O.P.; Strassburger, K.; Strom, A.; Bönhof, G.J.; Karusheva, Y.; Antoniou, S.; Bódis, K.; Markgraf, D.F.; Burkart, V.; Müssig, K.; et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: A 5-year follow-up study. Lancet Diabetes Endocrinol. 2019, 7, 684–694. [Google Scholar] [CrossRef]
- Safai, N.; Ali, A.; Rossing, P.; Ridderstråle, M. Stratification of type 2 diabetes based on routine clinical markers. Diabetes Res. Clin. Pract. 2018, 141, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.B.; Pedersen, M.; Tanaka, H.; Ohsugi, M.; Overgaard, R.V.; Lynge, J.; Almind, K.; Vasconcelos, N.-M.; Poulsen, P.; Keller, C.; et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 2014, 37, 796–804. [Google Scholar] [CrossRef]
- Ahuja, V.; Kadowaki, T.; Evans, R.W.; Kadota, A.; Okamura, T.; El Khoudary, S.R.; Fujiyoshi, A.; Barinas-Mitchell, E.J.M.; Hisamatsu, T.; Vishnu, A.; et al. Comparison of HOMA-IR, HOMA-β% and disposition index between US white men and Japanese men in Japan: The ERA JUMP study. Diabetologia 2015, 58, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Ai, M.; Schaefer, E.J.; Otokozawa, S.; Asztalos, B.F.; Nakajima, K.; Zhou, Y.; Liu, C.-T.; Jacques, P.F.; Cupples, L.A.; et al. Ethnic Differences in Glucose Homeostasis Markers between the Kyushu-Okinawa Population Study and the Framingham Offspring Study. Sci. Rep. 2016, 6, 36725. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakajima, A.; Tanaka, K.; Saito, H.; Iwasaki, T.; Oda, A.; Kanno, M.; Shimabukuro, M.; Asahi, K.; Watanabe, T.; Kazama, J.J. Blood pressure control in chronic kidney disease according to underlying renal disease: The Fukushima CKD cohort. Clin. Exp. Nephrol. 2019. [Google Scholar] [CrossRef]
- Levy, J.C.; Matthews, D.R.; Hermans, M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998, 21, 2191–2192. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Yasuda, H.; Sanada, M.; Kitada, K.; Terashima, T.; Kim, H.; Sakaue, Y.; Fujitani, M.; Kawai, H.; Maeda, K.; Kashiwagi, A. Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res. Clin. Pract. 2007, 77 (Suppl. 1), S178–S183. [Google Scholar] [CrossRef]
- Harada-Shiba, M.; Arai, H.; Ishigaki, Y.; Ishibashi, S.; Okamura, T.; Ogura, M.; Dobashi, K.; Nohara, A.; Bujo, H.; Miyauchi, K.; et al. Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 2017. J. Atheroscler. Thromb. 2018, 25, 751–770. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Dennis, J.M.; Shields, B.M.; Henley, W.E.; Jones, A.G.; Hattersley, A.T. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: An analysis using clinical trial data. Lancet Diabetes Endocrinol. 2019, 7, 442–451. [Google Scholar] [CrossRef]
- Kodama, K.; Tojjar, D.; Yamada, S.; Toda, K.; Patel, C.J.; Butte, A.J. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care 2013, 36, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Yabe, D.; Seino, Y.; Fukushima, M.; Seino, S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr. Diab. Rep. 2015, 15, 602. [Google Scholar] [CrossRef]
- Radcliffe, N.J.; Seah, J.M.; Clarke, M.; MacIsaac, R.J.; Jerums, G.; Ekinci, E.I. Clinical predictive factors in diabetic kidney disease progression. J. Diabetes Investig. 2017, 8, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Horita, S.; Nakamura, M.; Suzuki, M.; Satoh, N.; Suzuki, A.; Seki, G. Selective Insulin Resistance in the Kidney. BioMed. Res. Int. 2016, 2016, 5825170. [Google Scholar] [CrossRef]
- Karalliedde, J.; Gnudi, L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrol. Dial. Transpl. 2016, 31, 206–213. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Stender, S.; Pietrelli, A.; Mancina, R.M.; Cespiati, A.; Petta, S.; Pelusi, S.; Pingitore, P.; Badiali, S.; Maggioni, M.; et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J. Intern. Med. 2018, 283, 356–370. [Google Scholar] [CrossRef]
- Liu, Y.L.; Reeves, H.L.; Burt, A.D.; Tiniakos, D.; McPherson, S.; Leathart, J.B.; Allison, M.E.; Alexander, G.J.; Piguet, A.C.; Anty, R.; et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 2014, 5, 4309. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef]
- Tariq, R.; Axley, P.; Singal, A.K. Extra-Hepatic Manifestations of Nonalcoholic Fatty Liver Disease: A Review. J. Clin. Exp. Hepatol. 2020, 10, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Cohney, S.; De Michieli, F.; Pinach, S.; Saba, F.; Gambino, R. Fatty Liver and Chronic Kidney Disease: Novel Mechanistic Insights and Therapeutic Opportunities. Diabetes Care 2016, 39, 1830–1845. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef] [PubMed]
- LeCaire, T.J.; Palta, M.; Klein, R.; Klein, B.E.K.; Cruickshanks, K.J. Assessing Progress in Retinopathy Outcomes in Type 1 Diabetes. Diabetes Care 2013, 36, 631. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.I.; Morrison, A.D.; Pavan, P.R.; Cuthbertson, D.D. Prevalence and Significance of Retinopathy in Subjects with Type 1 Diabetes of Less Than 5 Years’ Duration Screened for the Diabetes Control and Complications Trial. Diabetes Care 2001, 24, 522. [Google Scholar] [CrossRef]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Shimabukuro, M. Cardiac adiposity and global cardiometabolic risk: New concept and clinical implication. Circ. J. 2009, 73, 27–34. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Higa, N.; Masuzaki, H.; Sata, M.; Ueda, S. Impact of individual metabolic risk components or its clustering on endothelial and smooth muscle cell function in men. Cardiovasc. Diabetol. 2016, 15, 77. [Google Scholar] [CrossRef]
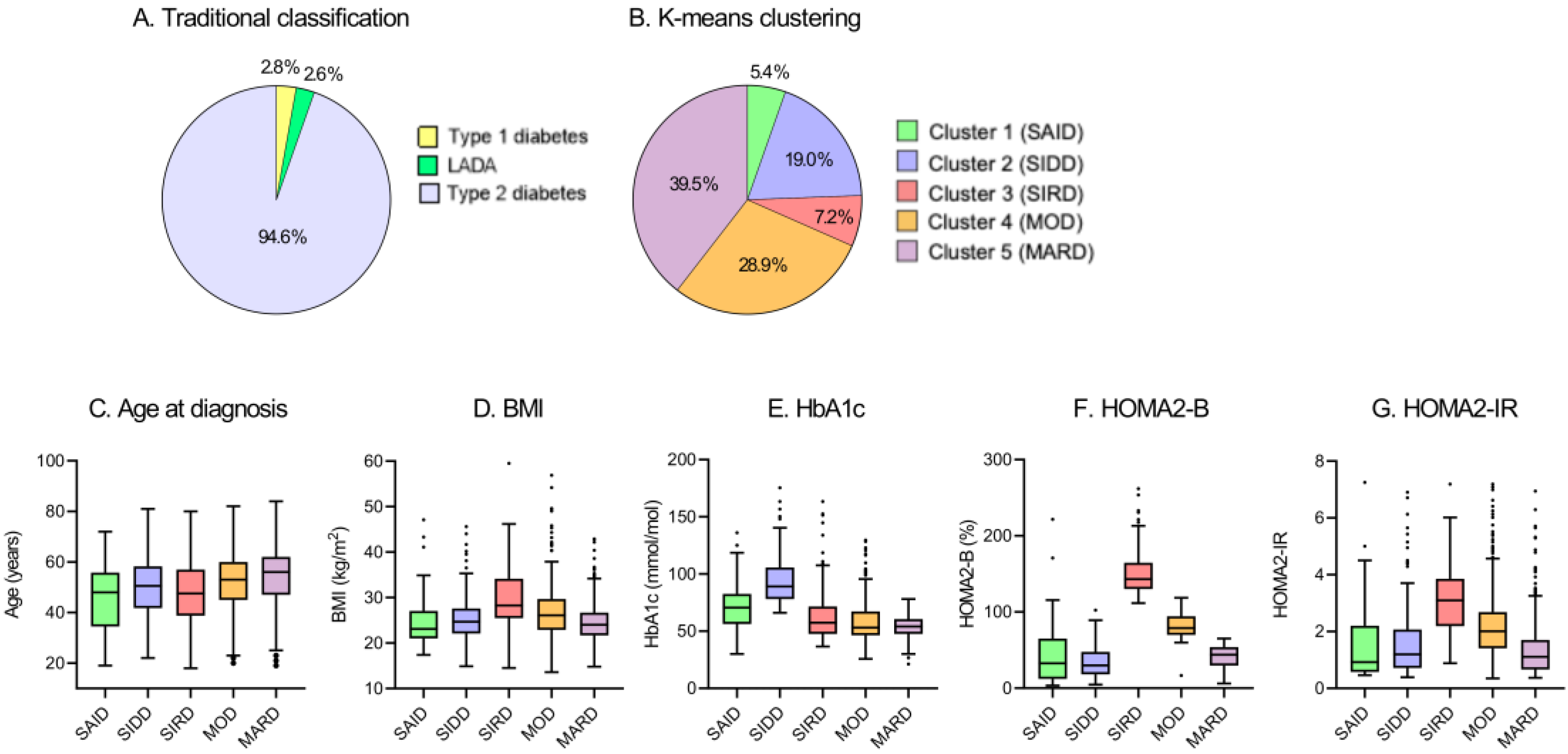

| Cluster 1: SAID | Cluster 2: SIDD | Cluster 3: SIRD | Cluster 4: MOD | Cluster 5: MARD | p-Value | |
|---|---|---|---|---|---|---|
| n (%) | 68 (5.4) | 238 (19.0) | 90 (7.2) | 363 (28.9) | 496 (39.5) | |
| Male, % | 48.5 | 58.0 | 50.0 | 49.0 | 60.3 | 0.008 |
| Age, Years | 55 (41–62) | 57 (49–65) | 54 (41–64) | 57 (49–65) | 61 (53–68) | <0.001 |
| Age at Diagnosis, Years | 48 (35–56) | 51 (42–58) | 48 (39–57) | 53 (45–60) | 56 (47–62) | <0.001 |
| Diabetes Duration, Years | 5 (0–9) | 3 (0–10) | 2 (0–7) | 1 (0–7) | 3 (0–10) | 0.005 |
| BMI, kg/m2 | 23.1 (21.0–27.0) | 24.7 (22.1–27.6) | 28.3 (25.5–34.1) | 26.1 (22.9–29.7) | 24.0 (21.7–26.7) | <0.001 |
| Systolic Blood Pressure, mmHg | 133 (118–152) | 132 (120–146) | 137 (122–153) | 133 (120–148) | 133 (122–146) | 0.630 |
| Diastolic Blood Pressure, mmHg | 80 (71–89) | 76 (69–85) | 79 (71–88) | 78 (70–86) | 77 (69–84) | 0.066 |
| Smoking, % | 20.6 | 23.5 | 21.1 | 21.5 | 15.1 | 0.046 |
| Family History of Diabetes, % | 42.6 | 46.2 | 36.7 | 37.2 | 36.1 | 0.090 |
| Plasma Glucose, mg/dL | 193 (139–263) | 237 (180–294) | 135 (109–214) | 136 (112–196) | 142 (120–181) | <0.001 |
| HbA1c, % | 8.6 (7.3–9.7) | 10.3 (9.3–11.8) | 7.4 (6.5–8.7) | 7.0 (6.4–8.3) | 7.1 (6.5–7.7) | <0.001 |
| HbA1c, mmol/mol | 70.5 (56.3–82.5) | 89.1 (78.1–105.7) | 57.4 (47.5–71.6) | 53.0 (46.4–67.2) | 54.1 (47.5–60.6) | <0.001 |
| HbA1c at the Follow-Up, % | 7.7 (7.0–8.5) | 7.4 (6.8–8.3) | 6.8 (6.2–7.2) | 6.7 (6.3–7.2) | 6.9 (6.6–7.3) | <0.001 |
| HbA1c at the Follow-Up, mmol/mol | 60.4 (53.0–69.8) | 57.6 (50.8–66.8) | 50.3 (44.2–54.6) | 49.7 (45.3–55.2) | 51.9 (48.1–56.3) | <0.001 |
| HOMA2-B | 32.7 (12.2–65.2) | 19.8 (18.0–47.6) | 143.2 (130.1–164.6) | 78.9 (69.8–94.4) | 44.0 (29.6–53.8) | <0.001 |
| HOMA2-IR | 0.92 (0.58–2.20) | 1.20 (0.72–2.07) | 3.09 (2.19–3.86) | 2.01 (1.41–2.68) | 1.11 (0.66–1.70) | <0.001 |
| eGFR, mL/min/1.73 m2 | 84 (67–103) | 87 (69–103) | 73 (52–88) | 79 (64–90) | 77 (64–91) | <0.001 |
| Triglycerides, mg/dL | 99 (62–161) | 123 (84–172) | 148 (97–207) | 133 (97–183) | 106 (79–160) | <0.001 |
| LDL Cholesterol, mg/dL | 112 (93–130) | 118 (94–145) | 123 (94–146) | 117 (92–143) | 117 (94–140) | 0.422 |
| Hypertension, % | 63.2 | 70.6 | 84.4 | 76.9 | 77.2 | 0.008 |
| Dyslipidemia, % | 73.5 | 83.2 | 95.6 | 88.7 | 82.7 | <0.001 |
| CKD, % | 14.7 | 13.4 | 34.4 | 19.6 | 20.6 | 0.001 |
| Proteinuria, % | 16.2 | 20.6 | 27.0 | 22.5 | 16.8 | 0.093 |
| NAFLD, % | 33.8 | 53.8 | 66.7 | 63.4 | 44.2 | <0.001 |
| Polyneuropathy, % | 35.3 | 31.1 | 23.3 | 21.8 | 16.9 | <0.001 |
| Retinopathy, % | 29.4 | 28.2 | 14.4 | 15.7 | 12.1 | <0.001 |
| Coronary artery disease, % | 14.7 | 8.8 | 6.7 | 15.4 | 10.7 | 0.038 |
| Stroke, % | 4.4 | 5.5 | 6.7 | 4.4 | 6.0 | 0.824 |
| Peripheral artery disease, % | 4.4 | 2.9 | 0.0 | 3.6 | 3.4 | 0.461 |
| Metformin, % | 11.8 | 31.1 | 23.3 | 20.7 | 25.2 | 0.005 |
| Insulin therapy, % | 58.8 | 29.4 | 23.3 | 10.2 | 21.6 | <0.001 |
| Diabetic Kidney Disease | Model 1 | Model 2 | ||||
| Variables | Events (%) | Censored | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Cluster 1: SAID | 23 (47.9) | 25 | 1.23 (0.79–1.91) | 0.361 | 1.08 (0.69–1.70) | 0.742 |
| Cluster 2: SIDD | 70 (44.3) | 88 | 1.04 (0.79–1.39) | 0.773 | 0.86 (0.60–1.23) | 0.404 |
| Cluster 3: SIRD | 28 (68.3) | 13 | 2.38 (1.58–3.57) | <0.001 | 2.19 (1.44–3.34) | <0.001 |
| Cluster 4: MOD | 117 (51.1) | 112 | 1.40 (1.10–1.79) | 0.006 | 1.28 (0.99–1.64) | 0.055 |
| Cluster 5: MARD | 156 (46.2) | 182 | 1.00 (ref) | 1.00 (ref) | ||
| Age | 1.04 (1.03–1.05) | <0.001 | 1.03 (1.02–1.04) | <0.001 | ||
| Sex (Male) | 1.05 (0.85–1.28) | 0.667 | 1.02 (0.83–1.26) | 0.841 | ||
| Diabetes Duration | 1.01 (0.99–1.02) | 0.282 | 1.01 (0.99–1.02) | 0.469 | ||
| BMI | 0.99 (0.98–1.02) | 0.911 | ||||
| HbA1c (mmol/mol) | 1.01 (0.99–1.01) | 0.059 | ||||
| eGFR | 0.98 (0.97–0.99) | <0.001 | ||||
| Smoking | 1.13 (0.86–1.48) | 0.381 | ||||
| Hypertension | 1.06 (0.83–1.14) | 0.658 | ||||
| Retinopathy | 1.42 (1.09–1.87) | 0.011 | ||||
| Diabetic Retinopathy | Model 3 | Model 4 | ||||
| Variables | Events (%) | Censored | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Cluster 1: SAID | 22 (45.8) | 26 | 2.41 (1.50–3.86) | <0.001 | 1.81 (1.10–3.00) | 0.020 |
| Cluster 2: SIDD | 64 (37.4) | 107 | 1.81 (1.32–2.48) | <0.001 | 1.04 (0.69–1.55) | 0.866 |
| Cluster 3: SIRD | 23 (29.9) | 54 | 1.33 (0.84–2.10) | 0.222 | 1.08 (0.66–1.75) | 0.770 |
| Cluster 4: MOD | 64 (20.9) | 242 | 0.98 (0.71–1.34) | 0.877 | 0.89 (0.64–1.24) | 0.490 |
| Cluster 5: MARD | 103 (23.6) | 333 | 1.00 (ref) | 1.00 (ref) | ||
| Age | 0.99 (0.98–1.00) | 0.214 | 0.99 (0.98–1.00) | 0.112 | ||
| Sex (Male) | 1.16 (0.91–1.48) | 0.233 | 1.11 (0.86–1.43) | 0.433 | ||
| Diabetes Duration | 1.04 (1.03–1.06) | <0.001 | 1.04 (1.03–1.06) | <0.001 | ||
| BMI | 0.99 (0.97–1.02) | 0.477 | ||||
| HbA1c (mmol/mol) | 1.02 (1.01–1.02) | <0.001 | ||||
| Smoking | 1.19 (0.87–1.62) | 0.277 | ||||
| Hypertension | 1.01 (0.76–1.34) | 0.948 | ||||
| Dyslipidemia | 1.02 (0.73–1.42) | 0.911 | ||||
| CKD | 1.36 (1.00–1.86) | 0.050 | ||||
| Coronary Artery Disease | Model 5 | Model 6 | ||||
| Variables | Events (%) | Censored | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Cluster 1: SAID | 5 (8.6) | 53 | 0.79 (0.32–1.96) | 0.607 | 0.80 (0.43–3.04) | 0.643 |
| Cluster 2: SIDD | 43 (19.8) | 174 | 1.49 (1.02–2.17) | 0.041 | 1.30 (0.78–2.18) | 0.313 |
| Cluster 3: SIRD | 19 (22.6) | 65 | 1.94 (1.17–3.22) | 0.011 | 1.39 (0.81–2.38) | 0.233 |
| Cluster 4: MOD | 46 (15.0) | 261 | 1.15 (0.79–1.66) | 0.464 | 1.04 (0.71–1.52) | 0.841 |
| Cluster 5: MARD | 76 (17.2) | 367 | 1.00 (ref) | 1.00 (ref) | ||
| Age | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 | ||
| Sex (Male) | 1.60 (1.18–2.17) | 0.002 | 1.31 (0.90–1.90) | 0.162 | ||
| Diabetes Duration | 1.02 (0.99–1.03) | 0.070 | 1.02 (1.00–1.04) | 0.047 | ||
| BMI | 1.01 (0.98–1.04) | 0.725 | ||||
| HbA1c (mmol/mol) | 1.01 (0.99–1.01) | 0.296 | ||||
| Smoking | 1.49 (1.05–2.13) | 0.027 | ||||
| Hypertension | 2.68 (1.66–4.35) | <0.001 | ||||
| Dyslipidemia | 2.03 (1.21–3.42) | 0.008 | ||||
| CKD | 1.38 (0.98–1.94) | 0.064 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanabe, H.; Saito, H.; Kudo, A.; Machii, N.; Hirai, H.; Maimaituxun, G.; Tanaka, K.; Masuzaki, H.; Watanabe, T.; Asahi, K.; et al. Factors Associated with Risk of Diabetic Complications in Novel Cluster-Based Diabetes Subgroups: A Japanese Retrospective Cohort Study. J. Clin. Med. 2020, 9, 2083. https://doi.org/10.3390/jcm9072083
Tanabe H, Saito H, Kudo A, Machii N, Hirai H, Maimaituxun G, Tanaka K, Masuzaki H, Watanabe T, Asahi K, et al. Factors Associated with Risk of Diabetic Complications in Novel Cluster-Based Diabetes Subgroups: A Japanese Retrospective Cohort Study. Journal of Clinical Medicine. 2020; 9(7):2083. https://doi.org/10.3390/jcm9072083
Chicago/Turabian StyleTanabe, Hayato, Haruka Saito, Akihiro Kudo, Noritaka Machii, Hiroyuki Hirai, Gulinu Maimaituxun, Kenichi Tanaka, Hiroaki Masuzaki, Tsuyoshi Watanabe, Koichi Asahi, and et al. 2020. "Factors Associated with Risk of Diabetic Complications in Novel Cluster-Based Diabetes Subgroups: A Japanese Retrospective Cohort Study" Journal of Clinical Medicine 9, no. 7: 2083. https://doi.org/10.3390/jcm9072083
APA StyleTanabe, H., Saito, H., Kudo, A., Machii, N., Hirai, H., Maimaituxun, G., Tanaka, K., Masuzaki, H., Watanabe, T., Asahi, K., Kazama, J., & Shimabukuro, M. (2020). Factors Associated with Risk of Diabetic Complications in Novel Cluster-Based Diabetes Subgroups: A Japanese Retrospective Cohort Study. Journal of Clinical Medicine, 9(7), 2083. https://doi.org/10.3390/jcm9072083






