Recommendations for Dental Care during COVID-19 Pandemic
Abstract
1. Introduction
2. Experimental Section
2.1. Composition of the Recommendation Development Group
2.2. The End-User of the Recommendations
2.3. Data Searches
2.4. Synthesis of Literature
- Virus transmission and pathogenicity.
- Testing.
- Assessment of individual COVID-19 risk.
- Classification of emergency level for dental treatments.
- Measures by health care professionals to limit virus spread, including masks, face shields, coats, gloves, and double shoes.
- Measures to limit nosocomial infection at dental clinics, including disinfection, isolation rooms, ventilation, waiting rooms, dining, and locker rooms.
- Measures applied by patients including overshoes, masks, and mouth rinses.
- Information collected from legal and ethical frameworks relevant to COVID-19 pandemic in dentistry.
3. Results
3.1. Virus Transmission and Pathogenicity
3.2. Testing
3.3. Individual Risk Assessment
3.4. Classification of Emergency Level for Dental Treatments
3.5. Measures by Health Care Professionals to Limit Virus Spread, Including Masks, Face Shields, Coats, Gloves, Double Shoes
3.5.1. Use of Masks
3.5.2. Use of Face Shields/Goggles
3.5.3. Additional Measures (Gowns, Overshoes, Gloves)
3.6. Measures to Limit Nosocomial Infection at Dental Clinics, including Disinfection, Isolation Rooms, Ventilation, Waiting Rooms, Dining, and Locker Rooms
3.6.1. Disinfectants against Human Coronavirus
3.6.2. Ventilation
3.6.3. Isolation Rooms
3.6.4. Air Disinfection
3.7. Measures Applied by Patients including Hand Hygiene, Overshoes, Masks, and Mouth Rinse
3.7.1. Hand Hygiene
3.7.2. Masks
3.7.3. Other Measures
3.7.4. Mouth Rinse
3.8. Aspects Collected from Legal and Ethical Frameworks Relevant to COVID-19 Pandemic in Dentistry
4. Recommendations
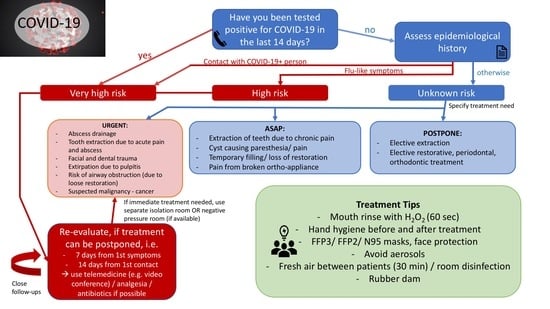
4.1. Individual Risk Assessment
- –
- Very high risk: The patient scores at least 1 YES in epidemiological history AND/OR patient has been tested positive for COVID-19
- –
- High risk: The patient scores at least 0 YES in epidemiological history and at least 1 in clinical manifestation
- –
- Unknown risk: The patient scores 0 YES in epidemiological history and 0 YES in clinical manifestation.
Rationale of the Authors
4.2. Patient Triage Recommendation
4.2.1. General Recommendations
4.2.2. Triage Recommendations
- Do you have pain? Yes/No
- If yes, where is the pain and from how long? Yes/No
- Is the pain associated with swelling and limited opening of the mouth? Yes/No
- Have you taken any medication, like paracetamol/ibuprofen/Aspirin? If yes, did you find any relief? Yes/No
- Do you have any underlying medical conditions? If yes, which one.
Rationale of the Authors
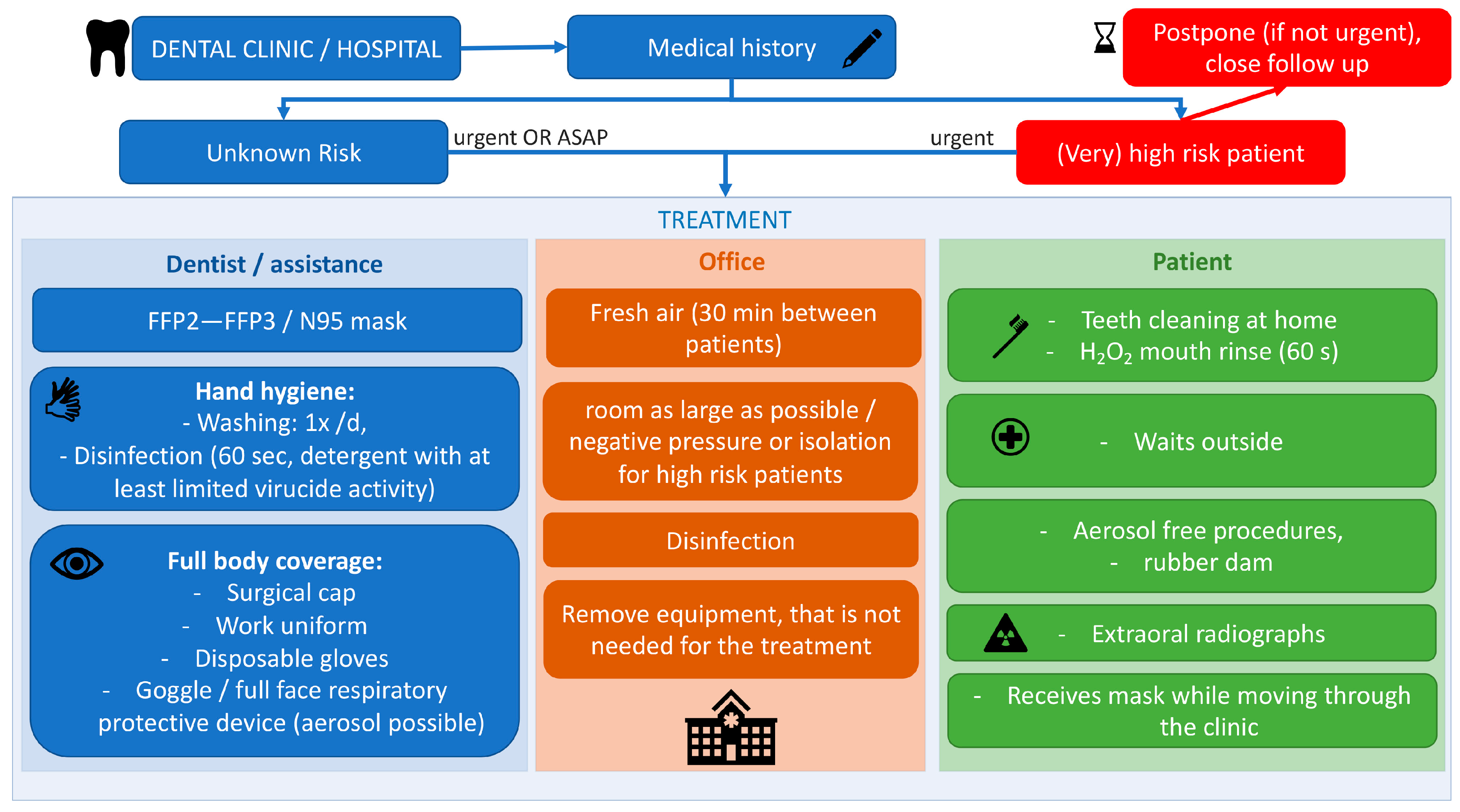
4.3. Measures to Prevent Infection in Dental Settings
4.3.1. Personal Protective Equipment for Dental Professionals
4.3.2. Recommendations Regarding the Dental Clinic
4.3.3. Recommendations to Be Followed by Patients
Rationale of the Authors
4.4. Future Perspectives for Elective Dental Care
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science 2020. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhu, J.; Zhang, Z.; Han, Y.; Huang, L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Woelfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Mueller, M.A.; Niemeyer, D.; Vollmar, P.; Rothe, C.; Hoelscher, M.; et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. MedRxiv 2020. [Google Scholar] [CrossRef]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef]
- Hoehl, S.; Rabenau, H.; Berger, A.; Kortenbusch, M.; Cinatl, J.; Bojkova, D.; Behrens, P.; Boddinghaus, B.; Gotsch, U.; Naujoks, F.; et al. Evidence of SARS-CoV-2 Infection in Returning Travelers from Wuhan, China. N. Engl. J. Med. 2020, 382, 1278–1280. [Google Scholar] [CrossRef]
- Qian, G.; Yang, N.; Ma, A.H.Y.; Wang, L.; Li, G.; Chen, X.; Chen, X. A COVID-19 Transmission within a family cluster by presymptomatic infectors in China. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) from Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Fallahi, H.R.; Keyhan, S.O.; Zandian, D.; Kim, S.G.; Cheshmi, B. Being a front-line dentist during the Covid-19 pandemic: A literature review. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 12. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Hua, F.; Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J. Dent. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Schunemann, H.J.; Zhang, Y.; Oxman, A.D. Distinguishing opinion from evidence in guidelines. BMJ 2019, 366, l4606. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Liu, X.F.; Jia, Z.F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet 2020, 395, e39. [Google Scholar] [CrossRef]
- Xia, J.; Tong, J.; Liu, M.; Shen, Y.; Guo, D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Liu., Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020. [Google Scholar] [CrossRef]
- Stadnytskyi, V.; Bax, C.E.; Bax, A.; Anfinrud, P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA 2020. [Google Scholar] [CrossRef]
- Jianyun, L.; Jieni, G.; Kuibiao, L.; Conghui, X.; Wenzhe, S.; Zhisheng, L.; Deqian, Z.; Chao, Y.; Bin, X.; Zhicong, Y. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. J. 2020, 26. [Google Scholar] [CrossRef]
- Buonanno, G.; Stabile, L.; Morawska, L. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020, 141, 105794. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, J.; Peng, J.; Li, X.; Deng, X.; Geng, Z.; Shen, Z.; Guo, F.; Zhang, Q.; Jin, Y.; et al. Detection of 2019-nCoV in Saliva and Characterization of Oral Symptoms in COVID-19 Patients. SSRN 2020, in press. [Google Scholar]
- To, K.K.-W.; Tsang, O.T.-Y.; Yip, C.C.-Y.; Chan, K.-H.; Wu, T.-C.; Chan, J.M.-C.; Leung, W.-S.; Chik, T.S.-H.; Choi, C.Y.-C.; Kandamby, D.H.; et al. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.C.; Mühlemann, B.; Veith, T.; Zuchowski, M.; Hofmann, J.; Stein, A.; Edelmann, A.; Corman, V.M.; Drosten, C. An analysis of SARS-CoV-2 viral load by patient age. Ger. Res. Netw. Zoonotic Infect. Dis. Website 2020, in press. [Google Scholar]
- Ganyani, T.; Kremer, C.; Chen, D.; Torneri, A.; Faes, C.; Wallinga, J.; Hens, N. Estimating the generation interval for COVID-19 based on symptom onset data. MedRxiv 2020. [Google Scholar] [CrossRef]
- Xing, Y.; Mo, P.; Xiao, Y.; Zhao, O.; Zhang, Y.; Wang, F. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef]
- Nishiura, H.; Kobayashi, T.; Miyama, T.; Suzuki, A.; Jung, S.; Hayashi, K.; Kinoshita, R.; Yang, Y.; Yuan, B.; Akhmetzhanov, A.R.; et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). medRxiv 2020. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 2020, 25, 2000180. [Google Scholar] [CrossRef]
- Wax, R.S.; Christian, M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can. J. Anaesth. 2020, 1–9. [Google Scholar] [CrossRef]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef]
- Tang, Y.W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512–e00520. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, V.; Chawla, A.; Logani, A. Rapid detection of SARS-CoV-2 in saliva: Can an endodontist take the lead in point-of-care COVID-19 testing? Int. Endod. J. 2020. [Google Scholar] [CrossRef]
- Lv, H.; Wu, N.C.; Tak-Yin Tsang, O.; Yuan, M.; Perera, R.A.P.M.; Leung, W.S.; So, R.T.Y.; Chun Chan, J.M.; Yip, G.K.; Hong Chik, T.S.; et al. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jacofsky, D.; Jacofsky, E.M.; Jacofsky, M. Understanding Antibody Testing for COVID-19. J. Arthroplast. 2020, 27, S0883–S5403. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, A.J.; Kekäläinen, E.; Kallio-Kokko, H.; Mannonen, L.; Kortela, E.; Vapalahti, O.; Kurkela, S.; Lappalainen, M. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Eurosurveillance 2020, 25, 2000603. [Google Scholar] [CrossRef]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Geng, B.; Muenker, M.C.; Moore, A.J.; Vogels, C.B.F.; et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. medRxiv 2020. [Google Scholar] [CrossRef]
- Williams, E.; Bond, K.; Zhang, B.; Putland, M.; Williamson, D.A. Saliva as a non-invasive specimen for detection of SARS-CoV-2. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020, S0163–S4453. [Google Scholar] [CrossRef]
- Alizargar, J.; Etemadi, S.M.; Aghamohammadi, M.; Hatefi, S. Saliva samples as an alternative for novel coronavirus (COVID-19) diagnosis. J. Formos. Med. Assoc. 2020, S0929–S6646. [Google Scholar] [CrossRef]
- Sapkota, D.; Thapa, S.B.; Hasséus, B.; Jensen, J.L. Saliva testing for COVID-19? Br. Dent. J. 2020, 228, 658–659. [Google Scholar] [CrossRef]
- Bi, Q.; Wu, Y.; Mei, S.; Ye, C.; Zou, X.; Zhang, Z.; Liu, X.; Wei, L.; Truelove, S.A.; Zhang, T.; et al. Epidemiology and Transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Meng, S.; Wu, Y.J.; Mao, Y.P.; Ye, R.X.; Wang, Q.Z.; Sun, C.; Sylvia, S.; Rozelle, S.; Raat, H.; et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect. Dis. Poverty 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Polish Dental Association (PTS). COVID-19 a Praca Lekarza Dentysty: Wytyczne PTS Uaktualnione. Available online: https://pts.net.pl/covid-19-a-praca-lekarza-dentysty-wytyczne-pts-uaktualnione/ (accessed on 21 March 2020).
- Ather, A.; Patel, B.; Ruparel, N.B.; Diogenes, A.; Hargreaves, K.M. Coronavirus Disease 19 (COVID-19): Implications for Clinical Dental Care. J. Endod. 2020, 46, 584–595. [Google Scholar] [CrossRef] [PubMed]
- European Center for Disease Prevention and Control 2020-risk. Available online: https://www.ecdc.europa.eu/en/current-risk-assessment-novel-coronavirus-situation (accessed on 20 May 2020).
- Vereinigung der Kantonszahnärztinnen und Kantonszahnärzte der Schweiz. Positionspapier. Available online: https://www.sso.ch/fileadmin/upload_sso/5_Newsletter/2020/200323_Positionspapier2_D.pdf?fbclid=IwAR1fLdsyDdAGzWz70NOZb6Q_0hzxEgUyBTVi9ohgL3mKLm8H7FajCsm1BiY (accessed on 26 March 2020).
- American Dental Association (ADA). Return to Work Interim Guidance Toolkit. Available online: https://success.ada.org/~/media/CPS/Files/Open%20Files/ADA_Return_to_Work_Toolkit.pdf (accessed on 15 May 2020).
- Italian Recommendations. Indicazioni Operative Per L’attività Odontoiatrica Durante la Fase 2 Della Pandemia COVID-19. Available online: https://fnomceo-my.sharepoint.com/personal/m_molinari_fnomceo_it/_layouts/15/onedrive.aspx?id=%2Fpersonal%2Fm%5Fmolinari%5Ffnomceo%5Fit%2FDocuments%2Fdocumento%20finale%2Epdf&parent=%2Fpersonal%2Fm%5Fmolinari%5Ffnomceo%5Fit%2FDocuments&originalPath=aHR0cHM6Ly9mbm9tY2VvLW15LnNoYXJlcG9pbnQuY29tLzpiOi9nL3BlcnNvbmFsL21fbW9saW5hcmlfZm5vbWNlb19pdC9FZGVMcF9YdXNHaE1naGNVWTRTeG5wWUJMZEhFcWtxTktORXNMaTJsVjUtZHB3P3J0aW1lPVNSaU8xdnI0MTBn (accessed on 15 May 2020).
- European Federation of Periodontology (EFP). EFP Suggestions for the Management of a Dental Clinic during the Covid-19 Pandemic. Available online: https://www.efp.org/publications/EFP-Infographic-COVID19.pdf (accessed on 15 May 2020).
- ADA Interim Guidance for Management of Emergency and Urgent Dental Care. Available online: https://www.ada.org/~/media/CPS/Files/COVID/ADA_Int_Guidance_Mgmt_Emerg-Urg_Dental_COVID19?utm_source=adaorg&utm_medium=VanityURL&utm_content=interimguidance-flowcharts&utm_campaign=covid-19 (accessed on 15 May 2020).
- Italian Society of Periodontology and Implantology (SIdP). COVID-2019 Norme per L’attività Odontoiatrica. Available online: https://www.sidp.it/media-download/taxtbu3.pdf?v=11032020174011 (accessed on 27 March 2020).
- Scottish Dental Clinical Effectiveness Programme. Emergency Dental Care. Available online: http://www.sdcep.org.uk/wp-content/uploads/2013/03/EDC+Guidance.pdf (accessed on 23 March 2020).
- Wang, X.; Pan, Z.; Cheng, Z. Association between 2019-nCoV transmission and N95 respirator use. J. Hosp. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Hu, T.; Liu, L.; Chen, R.; Guo, Q.; Yang, L.; Cheng, Y.; Huang, J.; Du, L. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J. Evid. Based Med. 2020. [Google Scholar] [CrossRef]
- Noti, J.D.; Lindsley, W.G.; Blachere, F.M.; Cao, G.; Kashon, M.L.; Thewlis, R.E.; McMillen, C.M.; King, W.P.; Szalajda, J.V.; Beezhold, D.H. Detection of Infectious Influenza Virus in Cough Aerosols Generated in a Simulated Patient Examination Room. Clin. Infect. Dis. 2012, 54, 1569–1577. [Google Scholar] [CrossRef]
- Lee, S.A.; Hwang, D.C.; Li, H.Y.; Tsai, C.F.; Chen, C.W.; Chen, J.K. Particle Size-Selective Assessment of Protection of European Standard FFP Respirators and Surgical Masks against Particles-Tested with Human Subjects. J Healthc Eng 2016, 2016, 8572493. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Advice on the Use of Masks in the Community, during Home Care and in Healthcare Settings in the Context of the Novel Coronavirus (COVID-19) Outbreak. 6 April 2020. Available online: https://www.who.int/publications-detail/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak (accessed on 20 May 2020).
- Center for Disease Control and Prevention (CDC). Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. Available online: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html (accessed on 15 May 2020).
- Magennis, P.; Coulthard, P. FFP3 Masks with Valves Should Be Avoided to Reduce Risk to Patients during Close Interactions when a Clinician Is Unknowingly COVID Positive. Available online: https://www.baoms.org.uk/_userfiles/pages/files/professionals/covid_19/baoms_baos_ppe__avoid_using_ffp3_masks_with_valves_for_close_patient_interactions_may_2020_updated.pdf (accessed on 15 May 2020).
- Lindsley, W.G.; Noti, J.D.; Blachere, F.M.; Szalajda, J.V.; Beezhold, D.H. Efficacy of face shields against cough aerosol droplets from a cough simulator. J. Occup. Environ. Hyg. 2014, 11, 509–518. [Google Scholar] [CrossRef]
- Roberge, R.J. Face shields for infection control: A review. J. Occup. Environ. Hyg. 2016, 13, 235–242. [Google Scholar] [CrossRef]
- Verbeek, J.H.; Rajamaki, B.; Ijaz, S.; Sauni, R.; Toomey, E.; Blackwood, B.; Tikka, C.; Ruotsalainen, J.H.; Kilinc Balci, F.S. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Institut, R.K. Haändehygiene in Einrichtungen des Gesundheitswesens. Bundesgesundheitsblatt 2016, 59, 1189–1220. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Escombe, A.R.; Oeser, C.C.; Gilman, R.H.; Navincopa, M.; Ticona, E.; Pan, W.; Martínez, C.; Chacaltana, J.; Rodríguez, R.; Moore, D.A.J.; et al. Natural Ventilation for the Prevention of Airborne Contagion. PloS Med. 2007, 4, e68. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zheng, X. Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. J. Thorac. Dis. 2018, 10, S2295–S2304. [Google Scholar] [CrossRef] [PubMed]
- WHO guideline. Natural Ventilation for Infection Control in Health-Care Settings. Available online: https://apps.who.int/iris/bitstream/handle/10665/44167/9789241547857_eng.pdf?sequence=1 (accessed on 20 May 2020).
- Memarzadeh, F.; Olmsted, R.N.; Bartley, J.M. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: Effective adjunct, but not stand-alone technology. Am. J. Infect. Control. 2010, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.M.; Ko, G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ. Sci. Technol. 2007, 41, 5460–5465. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.M.; Zhao, X.S.; Wen, R.F.; Huang, J.J.; Pi, G.H.; Zhang, S.X.; Han, J.; Bi, S.L.; Ruan, L.; Dong, X.P. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003, 16, 246–255. [Google Scholar]
- Darnell, M.E.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods 2004, 121, 85–91. [Google Scholar] [CrossRef]
- Tuladhar, E.; Hazeleger, W.C.; Koopmans, M.; Zwietering, M.H.; Duizer, E.; Beumer, R.R. Reducing viral contamination from finger pads: Handwashing is more effective than alcohol-based hand disinfectants. J. Hosp. Infect. 2015, 90, 226–234. [Google Scholar] [CrossRef]
- Feng, S.; Shen, C.; Xia, N.; Song, W.; Fan, M.; Cowling, B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.-L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020. [Google Scholar] [CrossRef]
- Chan, K.H.; Yuen, K.-Y. COVID-19 epidemic: Disentangling the re-emerging controversy about medical facemasks from an epidemiological perspective. Int. J. Epidemiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Using Face masks in the Community; European Centre for Disease Prevention and Control: Solna Municipality, Sweden, 2020. [Google Scholar]
- Prussin, A.J., 2nd; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 78. [Google Scholar] [CrossRef]
- Eggers, M.; Koburger-Janssen, T.; Eickmann, M.; Zorn, J. In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash against Respiratory and Oral Tract Pathogens. Infect. Dis. Ther. 2018, 7, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral. Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Meiller, T.F.; Silva, A.; Ferreira, S.M.; Jabra-Rizk, M.A.; Kelley, J.I.; DePaola, L.G. Efficacy of Listerine Antiseptic in reducing viral contamination of saliva. J. Clin. Periodontol. 2005, 32, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.; Schiff, G.; Echler, G.; Prince, A.; Feller, M.; Briner, W. In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse. J. Dent. Res. 1990, 69, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Carrouel, F.; Conte, M.P.; Fisher, J.; Gonçalves, L.S.; Dussart., C.; Llodra, J.C.; Bourgeois., D. COVID-19: A Recommendation to Examine the Effect of Mouthrinses with β-Cyclodextrin Combined with Citrox in Preventing Infection and Progression. J. Clin. Med. 2020, 9, 1126. [Google Scholar] [CrossRef]
- Popkin, D.L.; Zilka, S.; Dimaano, M.; Fujioka, H.; Rackley, C.; Salata, R.; Griffith, A.; Mukherjee, P.K.; Ghannoum, M.A.; Esper, F. Cetylpyridinium Chloride (CPC) Exhibits Potent, Rapid Activity Against Influenza Viruses in vitro and in vivo. Pathog. Immun. 2017, 2, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Esper, F.; Buchheit, K.; Arters, K.; Adkins, I.; Ghannoum, M.A.; Salata, R.A. Randomized, double-blind, placebo-controlled clinical trial to assess the safety and effectiveness of a novel dual-action oral topical formulation against upper respiratory infections. BMC Infect. Dis. 2017, 17, 74. [Google Scholar] [CrossRef]
- Jones, S.T.; Cagno, V.; Janeček, M.; Ortiz, D.; Gasilova, N.; Piret, J.; Gasbarri, M.; Constant, D.A.; Han, Y.; Vuković, L.; et al. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020, 6, eaax9318. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Jiang, H.; Zhou, J.; Lam, P.; Zhang, L.; et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in theupper respiratory tracts of rhesus macaques. J. Virol. 2011, 85, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Cui, B.; Duan, X.; Zhang, P.; Zhou, X.; Yuan, Q. Saliva: Potential diagnostic value and transmission of 2019-nCoV. Int. J. Oral Sci. 2020, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- CED. Code of Ethics for Dentists in the European Union. Available online: https://europeanmovement.eu/wp-content/uploads/2017/05/CED-Code-of-Ethics.pdf (accessed on 23 March 2020).
- Alperovitch, A.; Dreifuss-Netter, F.D.R.; Dickele, A.M.; Gaudray, P.; Coz, P.L.; Rouvillois, P.; Roux, M.; Waquet, P. Ethical Issues Raised by a Possible Influenza Pandemic. Available online: https://www.ccne-ethique.fr/sites/default/files/publications/avis_106_anglais.pdf (accessed on 3 April 2020).
- Centers for Disease Control and Prevention (CDC). How Coronavirus Spreads. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prepare/transmission.html (accessed on 28 March 2020).
- Sokol, D. Virulent epidemics and scope of healthcare workers’ duty of care. Emerg. Infect. Dis. 2006, 12, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Sokol, D. Ethics and epidemics. Am. J. Bioeth. 2008, 8, 28–29. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurzawska-Comis, K.; Becker, K.; Brunello, G.; Gurzawska, A.; Schwarz, F. Recommendations for Dental Care during COVID-19 Pandemic. J. Clin. Med. 2020, 9, 1833. https://doi.org/10.3390/jcm9061833
Gurzawska-Comis K, Becker K, Brunello G, Gurzawska A, Schwarz F. Recommendations for Dental Care during COVID-19 Pandemic. Journal of Clinical Medicine. 2020; 9(6):1833. https://doi.org/10.3390/jcm9061833
Chicago/Turabian StyleGurzawska-Comis, Katarzyna, Kathrin Becker, Giulia Brunello, Agata Gurzawska, and Frank Schwarz. 2020. "Recommendations for Dental Care during COVID-19 Pandemic" Journal of Clinical Medicine 9, no. 6: 1833. https://doi.org/10.3390/jcm9061833
APA StyleGurzawska-Comis, K., Becker, K., Brunello, G., Gurzawska, A., & Schwarz, F. (2020). Recommendations for Dental Care during COVID-19 Pandemic. Journal of Clinical Medicine, 9(6), 1833. https://doi.org/10.3390/jcm9061833