Serum Klotho in Living Kidney Donors and Kidney Transplant Recipients: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Literature Review
2.2. Selection Criteria
2.3. Data Abstraction
2.4. Statistical Analysis
3. Results
3.1. Serum Klotho after Kidney Transplantation
3.2. Serum Klotho after Living Kidney Donation
3.3. Evaluation for Publication Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, M.C.; Shi, M.; Zhang, J.; Pastor, J.; Nakatani, T.; Lanske, B.; Razzaque, M.S.; Rosenblatt, K.P.; Baum, M.G.; Kuro-o, M.; et al. Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010, 24, 3438–3450. [Google Scholar] [CrossRef] [Green Version]
- Kuro-o, M. Klotho and aging. Biochim. Biophys. Acta 2009, 1790, 1049–1058. [Google Scholar] [CrossRef]
- Bian, A.; Neyra, J.A.; Zhan, M.; Hu, M.C. Klotho, stem cells, and aging. Clin. Interv. Aging 2015, 10, 1233–1243. [Google Scholar] [PubMed] [Green Version]
- Hu, M.C.; Shi, M.; Cho, H.J.; Adams-Huet, B.; Paek, J.; Hill, K.; Shelton, J.; Amaral, A.P.; Faul, C.; Taniguchi, M.; et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J. Am. Soc. Nephrol. 2015, 26, 1290–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henao Agudelo, J.S.; Baia, L.C.; Ormanji, M.S.; Santos, A.R.P.; Machado, J.R.; Saraiva Câmara, N.O.; Navis, G.J.; de Borst, M.H.; Heilberg, I.P. Fish Oil Supplementation Reduces Inflammation but Does Not Restore Renal Function and Klotho Expression in an Adenine-Induced CKD Model. Nutrients 2018, 10, 1283. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Liu, T.; Zhao, M.; Fu, H.; Wang, J.; Xu, Q. Protective Effects of Moderate Ca Supplementation against Cd-Induced Bone Damage under Different Population-Relevant Doses in Young Female Rats. Nutrients 2019, 11, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Castañeda, J.R.; Rodelo-Haad, C.; Pendon-Ruiz de Mier, M.V.; Martin-Malo, A.; Santamaria, R.; Rodriguez, M. Klotho/FGF23 and Wnt Signaling as Important Players in the Comorbidities Associated with Chronic Kidney Disease. Toxins (Basel) 2020, 12, 185. [Google Scholar]
- Buendía, P.; Carracedo, J.; Soriano, S.; Madueño, J.A.; Ortiz, A.; Martín-Malo, A.; Aljama, P.; Ramírez, R. Klotho Prevents NFκB Translocation and Protects Endothelial Cell From Senescence Induced by Uremia. J. Gerontol A Biol. Sci. Med. Sci. 2015, 70, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Buendía, P.; Merino, A.; Madueño, J.A.; Peralbo, E.; Ortiz, A.; Martín-Malo, A.; Aljama, P.; Rodríguez, M.; Ramírez, R. Klotho modulates the stress response in human senescent endothelial cells. Mech. Ageing Dev. 2012, 133, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Martín-Núñez, E.; Ferri, C.; Hernández-Carballo, C.; Tagua, V.G.; Delgado-Molinos, A.; López-Castillo, Á.; Rodríguez-Ramos, S.; Cerro-López, P.; López-Tarruella, V.C.; et al. FGF23 and Klotho Levels are Independently Associated with Diabetic Foot Syndrome in Type 2 Diabetes Mellitus. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komaba, H.; Fukagawa, M. FGF23-parathyroid interaction: Implications in chronic kidney disease. Kidney Int. 2010, 77, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodelo-Haad, C.; Santamaria, R.; Muñoz-Castañeda, J.R.; Pendón-Ruiz de Mier, M.V.; Martin-Malo, A.; Rodriguez, M. FGF23, Biomarker or Target? Toxins (Basel) 2019, 11, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Tsai, J.P.; Wang, L.H.; Lee, M.C.; Hsu, B.G. Positive correlation of serum fibroblast growth factor 23 with peripheral arterial stiffness in kidney transplantation patients. Clin. Chim. Acta 2020, 505, 9–14. [Google Scholar] [CrossRef]
- Razzaque, M.S. The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009, 5, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef]
- Zou, D.; Wu, W.; He, Y.; Ma, S.; Gao, J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018, 19, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erben, R.G.; Andrukhova, O. FGF23-Klotho signaling axis in the kidney. Bone 2017, 100, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olauson, H.; Lindberg, K.; Amin, R.; Jia, T.; Wernerson, A.; Andersson, G.; Larsson, T.E. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J. Am. Soc. Nephrol. 2012, 23, 1641–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Secreted klotho and chronic kidney disease. Adv. Exp. Med. Biol. 2012, 728, 126–157. [Google Scholar] [PubMed] [Green Version]
- Takeshita, A.; Kawakami, K.; Furushima, K.; Miyajima, M.; Sakaguchi, K. Central role of the proximal tubular αKlotho/FGF receptor complex in FGF23-regulated phosphate and vitamin D metabolism. Sci. Rep. 2018, 8, 6917. [Google Scholar] [CrossRef] [PubMed]
- Mencke, R.; Olauson, H.; Hillebrands, J.L. Effects of Klotho on fibrosis and cancer: A renal focus on mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2017, 121, 85–100. [Google Scholar] [PubMed]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [PubMed]
- Maekawa, Y.; Ishikawa, K.; Yasuda, O.; Oguro, R.; Hanasaki, H.; Kida, I.; Takemura, Y.; Ohishi, M.; Katsuya, T.; Rakugi, H. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine 2009, 35, 341–346. [Google Scholar]
- Nabeshima, Y. Toward a better understanding of Klotho. Sci. Aging Knowl. Environ. 2006, 2006, pe11. [Google Scholar]
- Jin, M.; Lv, P.; Chen, G.; Wang, P.; Zuo, Z.; Ren, L.; Bi, J.; Yang, C.W.; Mei, X.; Han, D. Klotho ameliorates cyclosporine A-induced nephropathy via PDLIM2/NF-kB p65 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 486, 451–457. [Google Scholar] [PubMed]
- Wang, Y.; Kuro-o, M.; Sun, Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 2012, 11, 410–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panesso, M.C.; Shi, M.; Cho, H.J.; Paek, J.; Ye, J.; Moe, O.W.; Hu, M.C. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int. 2014, 85, 855–870. [Google Scholar]
- Hu, M.C.; Shi, M.; Zhang, J.; Quinones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar]
- Kuro-o, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 2008, 389, 233–241. [Google Scholar] [CrossRef]
- Liu, F.; Wu, S.; Ren, H.; Gu, J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat. Cell Biol. 2011, 13, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.; Zou, Y.; Togao, O.; Pastor, J.V.; John, G.B.; Wang, L.; Shiizaki, K.; Gotschall, R.; Schiavi, S.; Yorioka, N.; et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011, 286, 8655–8665. [Google Scholar] [PubMed] [Green Version]
- Zhou, L.; Li, Y.; Zhou, D.; Tan, R.J.; Liu, Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J. Am. Soc. Nephrol. 2013, 24, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, H.; Yoshida, T.; Shiohira, S.; Kohei, J.; Mitobe, M.; Kurosu, H.; Kuro-o, M.; Nitta, K.; Tsuchiya, K. Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am. J. Physiol. Renal Physiol. 2012, 302, F1252–F1264. [Google Scholar] [CrossRef]
- Guan, X.; Nie, L.; He, T.; Yang, K.; Xiao, T.; Wang, S.; Huang, Y.; Zhang, J.; Wang, J.; Sharma, K.; et al. Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. J. Pathol. 2014, 234, 560–572. [Google Scholar] [PubMed]
- Huang, J.S.; Chuang, C.T.; Liu, M.H.; Lin, S.H.; Guh, J.Y.; Chuang, L.Y. Klotho attenuates high glucose-induced fibronectin and cell hypertrophy via the ERK1/2-p38 kinase signaling pathway in renal interstitial fibroblasts. Mol. Cell Endocrinol. 2014, 390, 45–53. [Google Scholar]
- Shi, M.; Flores, B.; Gillings, N.; Bian, A.; Cho, H.J.; Yan, S.; Liu, Y.; Levine, B.; Moe, O.W.; Hu, M.C. AlphaKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. J. Am. Soc. Nephrol. 2016, 27, 2331–2345. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, Z. Current understanding of klotho. Ageing Res. Rev. 2009, 8, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Olauson, H.; Mencke, R.; Hillebrands, J.L.; Larsson, T.E. Tissue expression and source of circulating αKlotho. Bone 2017, 100, 19–35. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. The emerging role of Klotho in clinical nephrology. Nephrol. Dial. Transplant. 2012, 27, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.S.; Khatami, M.R. Fibroblast Growth Factor 23 in Postrenal Transplant: An Often Forgotten Hormone. Exp. Clin. Transplant. 2016, 14, 606–616. [Google Scholar] [PubMed]
- Neyra, J.A.; Li, X.; Mescia, F.; Ortiz-Soriano, V.; Adams-Huet, B.; Pastor, J.; Hu, M.C.; Toto, R.D.; Moe, O.W. Urine Klotho Is Lower in Critically Ill Patients With Versus Without Acute Kidney Injury and Associates With Major Adverse Kidney Events. Crit. Care Explor. 2019, 1, e0016. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Niño, M.D.; Fernandez-Fernandez, B.; Ortiz, A. Klotho, the elusive kidney-derived anti-ageing factor. Clin. Kidney J. 2019, 13, 125–127. [Google Scholar]
- Christov, M.; Neyra, J.A.; Gupta, S.; Leaf, D.E. Fibroblast Growth Factor 23 and Klotho in AKI. Semin. Nephrol. 2019, 39, 57–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyra, J.A.; Hu, M.C. αKlotho and Chronic Kidney Disease. Vitam. Horm. 2016, 101, 257–310. [Google Scholar] [PubMed] [Green Version]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Klotho and chronic kidney disease. Contrib. Nephrol. 2013, 180, 47–63. [Google Scholar] [PubMed] [Green Version]
- Barker, S.L.; Pastor, J.; Carranza, D.; Quiñones, H.; Griffith, C.; Goetz, R.; Mohammadi, M.; Ye, J.; Zhang, J.; Hu, M.C.; et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol. Dial. Transplant. 2015, 30, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Seiler, S.; Rogacev, K.S.; Roth, H.J.; Shafein, P.; Emrich, I.; Neuhaus, S.; Floege, J.; Fliser, D.; Heine, G.H. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2–4. Clin. J. Am. Soc. Nephrol. 2014, 9, 1049–1058. [Google Scholar] [CrossRef]
- Wang, Q.; Su, W.; Shen, Z.; Wang, R. Correlation between Soluble α-Klotho and Renal Function in Patients with Chronic Kidney Disease: A Review and Meta-Analysis. Biomed. Res. Int. 2018, 2018, 9481475. [Google Scholar] [CrossRef] [Green Version]
- Sari, F.; Inci, A.; Dolu, S.; Ellidag, H.Y.; Cetinkaya, R.; Ersoy, F.F. High serum soluble α-Klotho levels in patients with autosomal dominant polycystic kidney disease. J. Investig. Med. 2017, 65, 358–362. [Google Scholar] [CrossRef]
- Memmos, E.; Sarafidis, P.; Pateinakis, P.; Tsiantoulas, A.; Faitatzidou, D.; Giamalis, P.; Vasilikos, V.; Papagianni, A. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. 2019, 20, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongprayoon, C.; Hansrivijit, P.; Leeaphorn, N.; Acharya, P.; Torres-Ortiz, A.; Kaewput, W.; Kovvuru, K.; Kanduri, S.R.; Bathini, T.; Cheungpasitporn, W. Recent Advances and Clinical Outcomes of Kidney Transplantation. J. Clin. Med. 2020, 9, 1193. [Google Scholar] [CrossRef] [Green Version]
- Thongprayoon, C.; Kaewput, W.; Kovvuru, K.; Hansrivijit, P.; Kanduri, S.R.; Bathini, T.; Chewcharat, A.; Leeaphorn, N.; Gonzalez-Suarez, M.L.; Cheungpasitporn, W. Promises of Big Data and Artificial Intelligence in Nephrology and Transplantation. J. Clin. Med. 2020, 9, 1107. [Google Scholar] [CrossRef] [Green Version]
- Cheungpasitporn, W.; Thongprayoon, C.; Vaitla, P.K.; Chewcharat, A.; Hansrivijit, P.; Koller, F.L.; Mao, M.A.; Bathini, T.; Salim, S.A.; Katari, S.; et al. Degree of Glomerulosclerosis in Procurement Kidney Biopsies from Marginal Donor Kidneys and Their Implications in Predicting Graft Outcomes. J. Clin. Med. 2020, 9, 1469. [Google Scholar] [CrossRef]
- Leeaphorn, N.; Thongprayoon, C.; Chon, W.J.; Cummings, L.S.; Mao, M.A.; Cheungpasitporn, W. Outcomes of kidney retransplantation after graft loss as a result of BK virus nephropathy in the era of newer immunosuppressant agents. Am. J. Transplant. 2020, 20, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.L.; Delmonico, F.L. Living-donor kidney transplantation: a review of the current practices for the live donor. J. Am. Soc. Nephrol. 2005, 16, 2098–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizusaki, K.; Hasuike, Y.; Kimura, T.; Nagasawa, Y.; Kuragano, T.; Yamada, Y.; Nojima, M.; Yamamoto, S.; Nakanishi, T.; Ishihara, M. Inhibition of the Mammalian Target of Rapamycin May Augment the Increase in Soluble Klotho Levels in Renal Transplantation Recipients. Blood Purif. 2019, 47 (Suppl. 2), 12–18. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Yang, A.; Wu, J.; Zhou, J.; Meng, S.; Zhu, C.; Wang, J.; Shen, S.; Ma, J.; Liu, D. The Value of Older Donors’ Klotho Level in Predicting Recipients’ Short-Term Renal Function. Med. Sci. Monit. 2018, 24, 7936–7943. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, S.J.; Ahn, S.; Min, S.-I.; Min, S.-K.; Ha, J. The Potential Role of Klotho as a Prognostic Biomarker in Deceased Donor Kidney Transplantation. Transplantation 2018, 102, S539. [Google Scholar] [CrossRef]
- Kubota, M.; Hamasaki, Y.; Masuda, T.; Hashimoto, J.; Takahashi, Y.; Saito, A.; Yuasa, R.; Muramatsu, M.; Sakai, K.; Shishido, S. Fgf 23-Aklotho axis and phosphate metabolism in the early post-kidney transplantation period. In Pediatric Transplantation; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Tan, S.J.; Crosthwaite, A.; Langsford, D.; Obeysekere, V.; Ierino, F.L.; Roberts, M.A.; Hughes, P.D.; Hewitson, T.D.; Dwyer, K.M.; Toussaint, N.D. Mineral adaptations following kidney transplantation. Transpl. Int. 2017, 30, 463–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloglu, İ.; Turkmen, K.; Selçuk, N.Y.; Tonbul, H.Z.; Erdur, F.M. Fgf-23 and Klotho Levels in Renal Transplant Patients and Comparison with Hemodialysis Patients. In Nephrology Dialysis Transplantation; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Tartaglione, L.; Pasquali, M.; Rotondi, S.; Muci, M.L.; Leonangeli, C.; Farcomeni, A.; Fassino, V.; Mazzaferro, S. Interactions of sclerostin with FGF23, soluble klotho and vitamin D in renal transplantation. PLoS ONE 2017, 12, e0178637. [Google Scholar] [CrossRef] [PubMed]
- Zaare Nahandi, M.; Ardalan, M.R.; Banagozar Mohamadi, A.; Ghorbani Haghjo, A.; Jabbarpor Bonyadi, M.; Mohamadian, T. Relationship of Serum Klotho Level With ACE Gene Polymorphism in Stable Kidney Allograft Recipients. Iran. J. Kidney Dis. 2017, 11, 151–156. [Google Scholar] [PubMed]
- Bleskestad, I.H.; Thorsen, I.S.; Jonsson, G.; Skadberg, Ø.; Bergrem, H.; Gøransson, L.G. Soluble Klotho and intact fibroblast growth factor 23 in long-term kidney transplant patients. Eur. J. Endocrinol. 2015, 172, 343–350. [Google Scholar] [CrossRef]
- Malyszko, J.; Koc-Zorawska, E.; Matuszkiewicz-Rowinska, J. FGF23 and Klotho in relation to markers of endothelial dysfunction in kidney transplant recipients. Transplant. Proc. 2014, 46, 2647–2650. [Google Scholar] [CrossRef]
- Akimoto, T.; Kimura, T.; Watanabe, Y.; Ishikawa, N.; Iwazu, Y.; Saito, O.; Muto, S.; Yagisawa, T.; Kusano, E. The impact of nephrectomy and renal transplantation on serum levels of soluble Klotho protein. Transplant. Proc. 2013, 45, 134–136. [Google Scholar] [CrossRef]
- Kimura, T.; Akimoto, T.; Watanabe, Y.; Kurosawa, A.; Nanmoku, K.; Muto, S.; Kusano, E.; Yagisawa, T.; Nagata, D. Impact of Renal Transplantation and Nephrectomy on Urinary Soluble Klotho Protein. Transplant. Proc. 2015, 47, 1697–1699. [Google Scholar] [CrossRef]
- Ponte, B.; Trombetti, A.; Hadaya, K.; Ernandez, T.; Fumeaux, D.; Iselin, C.; Martin, P.Y.; de Seigneux, S. Acute and long term mineral metabolism adaptation in living kidney donors: A prospective study. Bone 2014, 62, 36–42. [Google Scholar] [CrossRef]
- Tan, S.J.; Hewitson, T.D.; Hughes, P.D.; Holt, S.G.; Toussaint, N.D. Changes in Markers of Mineral Metabolism After Living Kidney Donation. Transplant. Direct 2017, 3, e150. [Google Scholar] [CrossRef]
- Thorsen, I.S.; Bleskestad, I.H.; Jonsson, G.; Skadberg, Ø.; Gøransson, L.G. Neutrophil Gelatinase-Associated Lipocalin, Fibroblast Growth Factor 23, and Soluble Klotho in Long-Term Kidney Donors. Nephron Extra 2016, 6, 31–39. [Google Scholar] [CrossRef]
- Hong, Y.A.; Choi, D.E.; Lim, S.W.; Yang, C.W.; Chang, Y.K. Decreased parathyroid Klotho expression is associated with persistent hyperparathyroidism after kidney transplantation. Transplant. Proc. 2013, 45, 2957–2962. [Google Scholar] [CrossRef]
- Ozdem, S.; Yılmaz, V.T.; Ozdem, S.S.; Donmez, L.; Cetinkaya, R.; Suleymanlar, G.; Ersoy, F.F. Is Klotho F352V Polymorphism the Missing Piece of the Bone Loss Puzzle in Renal Transplant Recipients? Pharmacology 2015, 95, 271–278. [Google Scholar] [CrossRef]
- Krajisnik, T.; Olauson, H.; Mirza, M.A.; Hellman, P.; Akerström, G.; Westin, G.; Larsson, T.E.; Björklund, P. Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int. 2010, 78, 1024–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Follmann, D.; Elliott, P.; Suh, I.; Cutler, J. Variance imputation for overviews of clinical trials with continuous response. J. Clin. Epidemiol. 1992, 45, 769–773. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easterbrook, P.J.; Berlin, J.A.; Gopalan, R.; Matthews, D.R. Publication bias in clinical research. Lancet 1991, 337, 867–872. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- John, G.B.; Cheng, C.Y.; Kuro-o, M. Role of Klotho in aging, phosphate metabolism, and CKD. Am. J. Kidney Dis. 2011, 58, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaitzidis, R.G.; Duni, A.; Siamopoulos, K.C. Klotho, the Holy Grail of the kidney: from salt sensitivity to chronic kidney disease. Int. Urol. Nephrol. 2016, 48, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Van Londen, M.; Aarts, B.M.; Deetman, P.E.; van der Weijden, J.; Eisenga, M.F.; Navis, G.; Bakker, S.J.L.; de Borst, M.H. Post-Transplant Hypophosphatemia and the Risk of Death-Censored Graft Failure and Mortality after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2017, 12, 1301–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, K.; Mitsuiki, K.; Kuroki, Y.; Nishiki, T.; Motoyama, K.; Nakano, T.; Kitazono, T. Relative hypophosphatemia early after transplantation is a predictor of good kidney graft function. Clin. Exp. Nephrol. 2019, 23, 1161–1168. [Google Scholar] [CrossRef]
- Hu, M.C.; Moe, O.W. Klotho as a potential biomarker and therapy for acute kidney injury. Nat. Rev. Nephrol. 2012, 8, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, S.; Noris, M. Klotho in acute kidney injury: Biomarker, therapy, or a bit of both? Kidney Int. 2010, 78, 1208–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Shi, M.; Maique, J.; Shaffer, J.; Yan, S.; Moe, O.W.; Hu, M.C. Beclin 1/Bcl-2 complex-dependent autophagy activity modulates renal susceptibility to ischemia-reperfusion injury and mediates renoprotection by Klotho. American journal of physiology. Am. J. Physiol. Renal Physiol. 2020, 318, F772–F792. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Intini, A.; Stasi, A.; Divella, C.; Gigante, M.; Pontrelli, P.; Franzin, R.; Accetturo, M.; Zito, A.; Fiorentino, M.; et al. Complement Modulation of Anti-Aging Factor Klotho in Ischemia/Reperfusion Injury and Delayed Graft Function. Am. J. Transplant. 2016, 16, 325–333. [Google Scholar] [CrossRef]
- De Sandes-Freitas, T.V.; Felipe, C.R.; Aguiar, W.F.; Cristelli, M.P.; Tedesco-Silva, H.; Medina-Pestana, J.O. Prolonged Delayed Graft Function Is Associated with Inferior Patient and Kidney Allograft Survivals. PLoS ONE 2015, 10, e0144188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, S.; Dienemann, T.; Jacobi, J.; Eckardt, K.U.; Weidemann, A. Delayed graft function is associated with an increased rate of renal allograft rejection: A retrospective single center analysis. PLoS ONE 2018, 13, e0199445. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, G.H. Electrolyte and Acid-base disturbances induced by clacineurin inhibitors. Electrolyte Blood Press 2007, 5, 126–130. [Google Scholar] [PubMed] [Green Version]
- Demeule, M.; Béliveau, R. Cyclosporin inhibits phosphate transport and stimulates alkaline phosphatase activity in renal BBMV. Am. J. Physiol. 1991, 260, F518–F524. [Google Scholar] [PubMed]
- Falkiewicz, K.; Nahaczewska, W.; Boratynska, M.; Owczarek, H.; Klinger, M.; Kaminska, D.; Wozniak, M.; Szepietowski, T.; Patrzalek, D. Tacrolimus decreases tubular phosphate wasting in renal allograft recipients. Transplant. Proc. 2003, 35, 2213–2215. [Google Scholar]
- Messa, P.; Cafforio, C.; Alfieri, C. Calcium and phosphate changes after renal transplantation. J. Nephrol. 2010, 23 (Suppl. 16), S175–S181. [Google Scholar] [PubMed]
- Sakhaee, K. Post-renal transplantation hypophosphatemia. Pediatr. Nephrol. 2010, 25, 213–220. [Google Scholar]
- Cheungpasitporn, W.; Thongprayoon, C.; Hansrivijit, P.; Medaura, J.; Chewcharat, A.; Bathini, T.; Mao, M.; Erickson, S. Impact of admission calcium-phosphate product on 1-year mortality among hospitalized patients. Adv. Biomed. Res. 2020, 9, 14. [Google Scholar]
- Thongprayoon, C.; Cheungpasitporn, W.; Mao, M.A.; Erickson, S.B. Calcium-phosphate product and its impact on mortality in hospitalized patients. Nephrology (Carlton) 2020, 25, 22–28. [Google Scholar] [PubMed]
- Pichler, G.; Haller, M.C.; Kainz, A.; Wolf, M.; Redon, J.; Oberbauer, R. Prognostic value of bone- and vascular-derived molecular biomarkers in hemodialysis and renal transplant patients: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2017, 32, 1566–1578. [Google Scholar]
- Lentine, K.L.; Patel, A. Risks and outcomes of living donation. Adv. Chronic Kidney Dis. 2012, 19, 220–228. [Google Scholar]
- Ommen, E.S.; Winston, J.A.; Murphy, B. Medical risks in living kidney donors: absence of proof is not proof of absence. Clin. J. Am. Soc. Nephrol. 2006, 1, 885–895. [Google Scholar]
- Asgari, E.; Hilton, R.M. One size does not fit all: Understanding individual living kidney donor risk. Pediatr. Nephrol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehrman-Ekholm, I.; Elinder, C.G.; Stenbeck, M.; Tydén, G.; Groth, C.G. Kidney donors live longer. Transplantation 1997, 64, 976–978. [Google Scholar] [CrossRef]
- Okamoto, M.; Akioka, K.; Nobori, S.; Ushigome, H.; Kozaki, K.; Kaihara, S.; Yoshimura, N. Short- and long-term donor outcomes after kidney donation: analysis of 601 cases over a 35-year period at Japanese single center. Transplantation 2009, 87, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.N.; Foley, R.; Tan, L.; Rogers, T.; Bailey, R.F.; Guo, H.; Gross, C.R.; Matas, A.J. Long-term consequences of kidney donation. N. Engl. J. Med. 2009, 360, 459–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segev, D.L.; Muzaale, A.D.; Caffo, B.S.; Mehta, S.H.; Singer, A.L.; Taranto, S.E.; McBride, M.A.; Montgomery, R.A. Perioperative mortality and long-term survival following live kidney donation. JAMA 2010, 303, 959–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keeffe, L.M.; Ramond, A.; Oliver-Williams, C.; Willeit, P.; Paige, E.; Trotter, P.; Evans, J.; Wadström, J.; Nicholson, M.; Collett, D.; et al. Mid- and Long-Term Health Risks in Living Kidney Donors: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2018, 168, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.X.; Meirambayeva, A.; Huang, A.; Kim, J.; Prasad, G.V.; Knoll, G.; Boudville, N.; Lok, C.; McFarlane, P.; Karpinski, M.; et al. Cardiovascular disease in kidney donors: Matched cohort study. BMJ 2012, 344, e1203. [Google Scholar] [CrossRef] [Green Version]
- Musgrove, J.; Wolf, M. Regulation and Effects of FGF23 in Chronic Kidney Disease. Annu. Rev. Physiol. 2020, 82, 365–390. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M. Forging forward with 10 burning questions on FGF23 in kidney disease. J. Am. Soc. Nephrol. 2010, 21, 1427–1435. [Google Scholar] [CrossRef] [Green Version]
- Marthi, A.; Donovan, K.; Haynes, R.; Wheeler, D.C.; Baigent, C.; Rooney, C.M.; Landray, M.J.; Moe, S.M.; Yang, J.; Holland, L.; et al. Fibroblast Growth Factor-23 and Risks of Cardiovascular and Noncardiovascular Diseases: A Meta-Analysis. J. Am. Soc. Nephrol. 2018, 29, 2015–2027. [Google Scholar] [CrossRef] [Green Version]
- Grabner, A.; Amaral, A.P.; Schramm, K.; Singh, S.; Sloan, A.; Yanucil, C.; Li, J.; Shehadeh, L.A.; Hare, J.M.; David, V.; et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab. 2015, 22, 1020–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, W.E.; Ferro, C.J.; Edwards, N.C.; Chue, C.D.; Lin, E.L.; Taylor, R.J.; Cockwell, P.; Steeds, R.P.; Townend, J.N. Cardiovascular Effects of Unilateral Nephrectomy in Living Kidney Donors. Hypertension 2016, 67, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M.G.; Massy, Z.A.; Brandenburg, V.M.; Mazzaferro, S.; Cozzolino, M.; Ureña-Torres, P.; Bover, J.; Goldsmith, D. Bone: A new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol. 2014, 2, 427–436. [Google Scholar] [CrossRef]
- Hum, J.M.; O’Bryan, L.M.; Tatiparthi, A.K.; Cass, T.A.; Clinkenbeard, E.L.; Cramer, M.S.; Bhaskaran, M.; Johnson, R.L.; Wilson, J.M.; Smith, R.C.; et al. Chronic Hyperphosphatemia and Vascular Calcification Are Reduced by Stable Delivery of Soluble Klotho. J. Am. Soc. Nephrol. 2017, 28, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yoon, J.; An, S.W.; Kuro-o, M.; Huang, C.L. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J. Am. Soc. Nephrol. 2015, 26, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cha, S.K.; An, S.W.; Kuro, O.M.; Birnbaumer, L.; Huang, C.L. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat. Commun. 2012, 3, 1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, F.; Lofaro, D.; Gigliotti, P.; Perri, A.; Vizza, D.; Toteda, G.; Lupinacci, S.; Armentano, F.; Papalia, T.; Bonofiglio, R. Soluble Klotho levels in adult renal transplant recipients are modulated by recombinant human erythropoietin. J. Nephrol. 2014, 27, 577–585. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Henríquez-Palop, F.; Martín-Núñez, E.; Pérez-Delgado, N.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. Effect of Paricalcitol on FGF-23 and Klotho in Kidney Transplant Recipients. Transplantation 2016, 100, 2432–2438. [Google Scholar] [CrossRef]
- Prather, A.A.; Epel, E.S.; Arenander, J.; Broestl, L.; Garay, B.I.; Wang, D.; Dubal, D.B. Longevity factor klotho and chronic psychological stress. Transl. Psychiatry 2015, 5, e585. [Google Scholar] [CrossRef] [Green Version]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-la, O.A.; Gutiérrez, Á.; Castillo, M.J. Alcohol consumption and S-Klotho plasma levels in sedentary healthy middle-aged adults: A cross sectional study. Drug Alcohol Depend. 2019, 194, 107–111. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la, O.A.; Jurado-Fasoli, L.; Ruiz, J.R.; Castillo, M.J.; Gutiérrez, Á. Role of Exercise on S-Klotho Protein Regulation: A Systematic Review. Curr. Aging Sci. 2018, 11, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-la, O.A.; Martinez-Tellez, B.; Ruiz, J.R.; Gutiérrez, Á.; Castillo, M.J. Adherence to the Mediterranean diet, dietary factors, and S-Klotho plasma levels in sedentary middle-aged adults. Exp. Gerontol. 2019, 119, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Liu, D. Klotho: A Promising Biomarker Closely Related to Kidney Transplant. Exp. Clin. Transplant. 2018, 16, 253–258. [Google Scholar] [PubMed]
- Neyra, J.A.; Moe, O.W.; Pastor, J.; Gianella, F.; Sidhu, S.S.; Sarnak, M.J.; Ix, J.H.; Drew, D.A. Performance of soluble Klotho assays in clinical samples of kidney disease. Clin. Kidney J. 2020, 13, 235–244. [Google Scholar] [CrossRef] [PubMed]

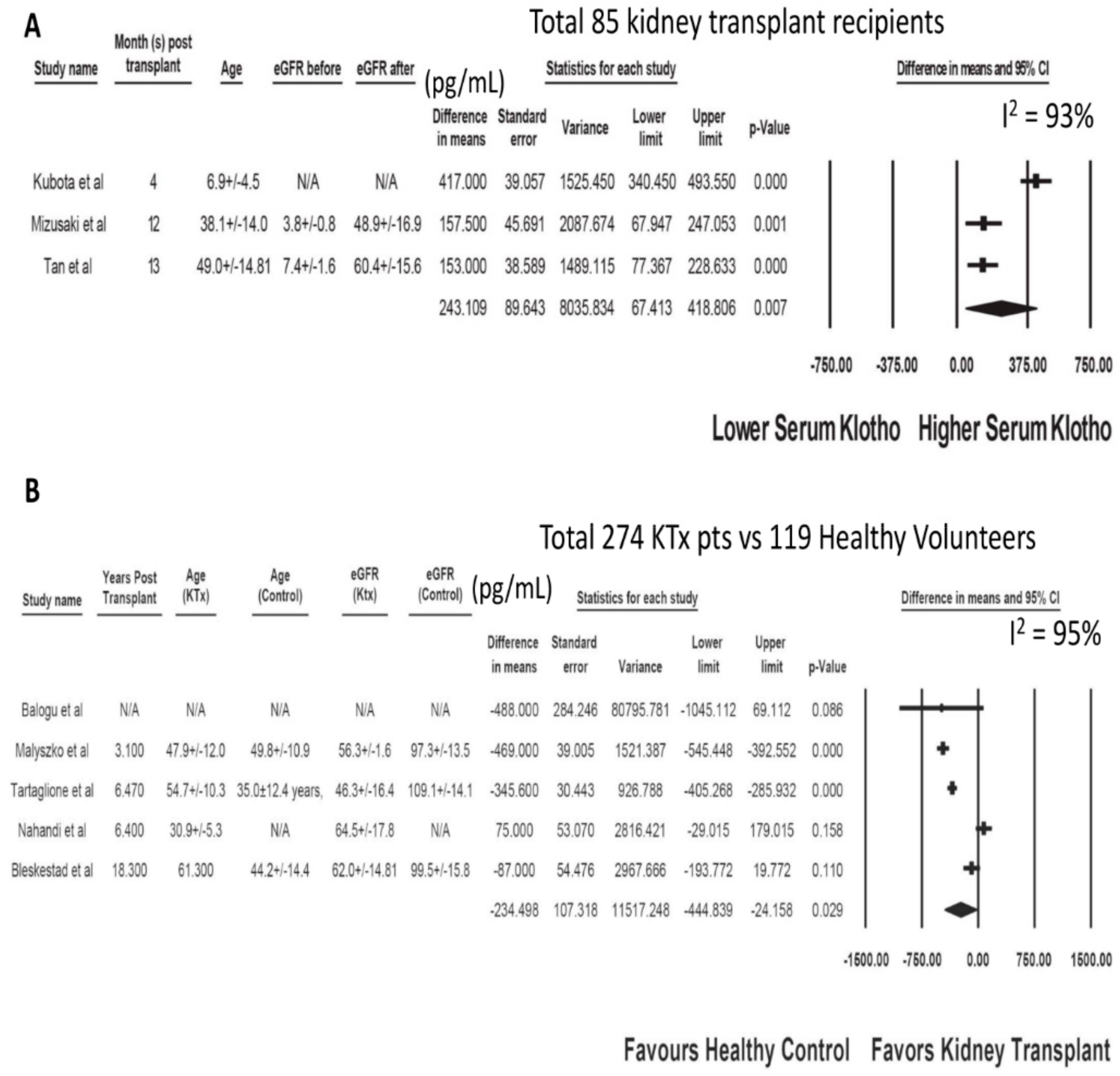
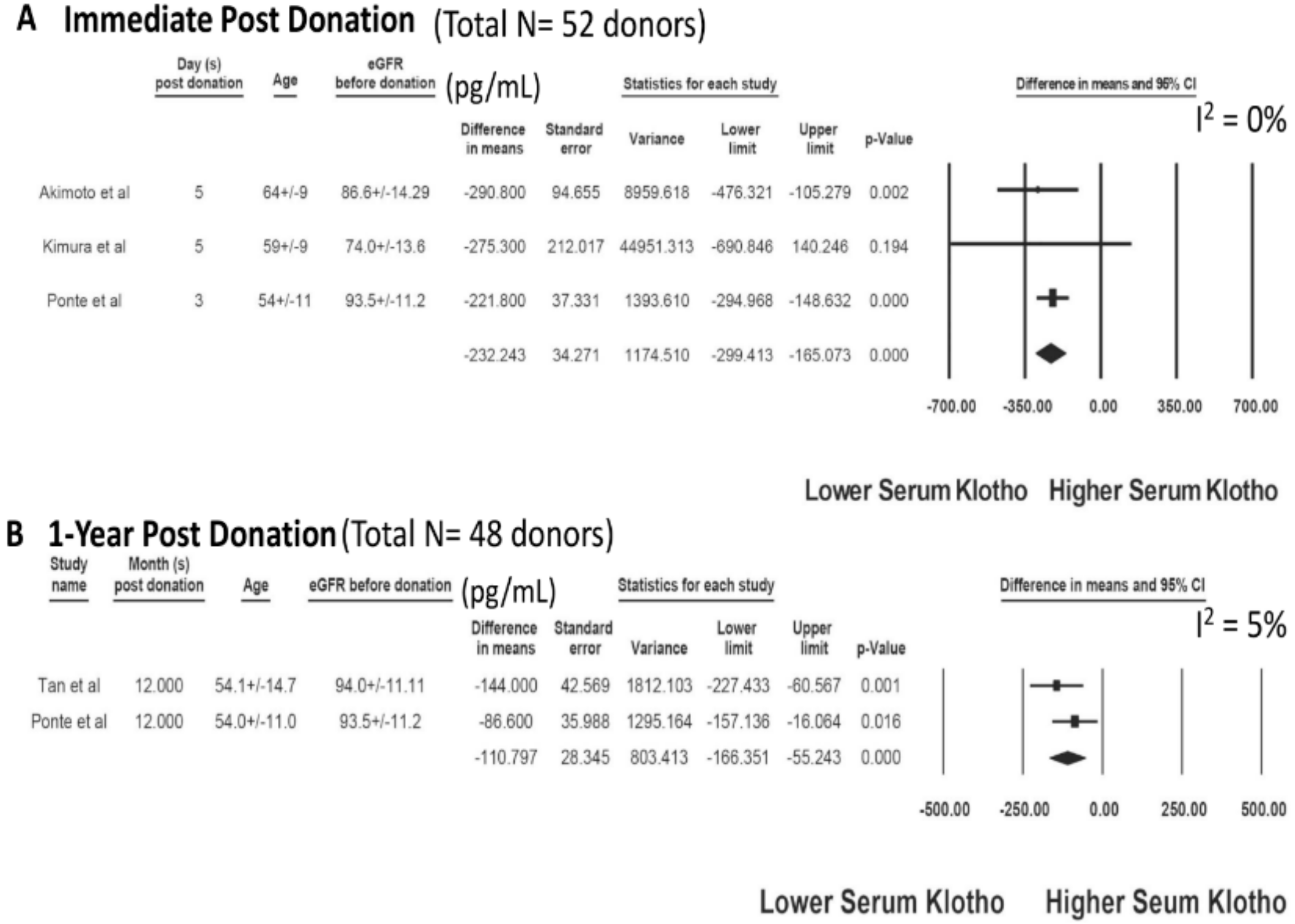
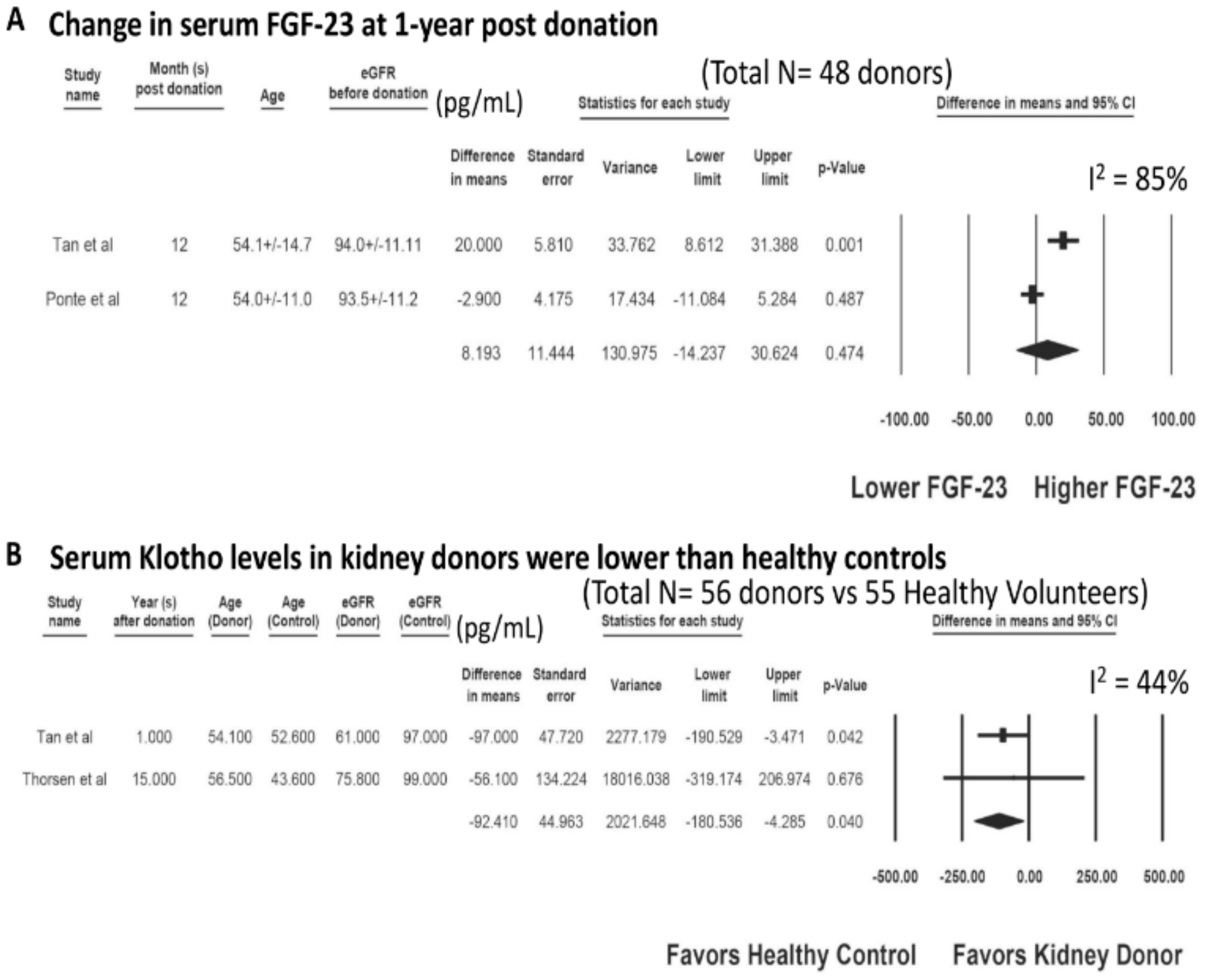
| Study | Year | Country | N-KTx | Characteristics-KTx | Klotho before KTx (pg/mL) | Other Markers before KTx | Kloth after KTx (pg/mL) | Other Markers after KTx |
|---|---|---|---|---|---|---|---|---|
| Kubota et al. | Japan | 20 | Age 6.9 ± 4.5 years | 988 ± 122 | FGF23 | At 4 months | N/A | |
| Male 12 (60%) | 5343 ± 1350 pg/mL | 1405 ± 125 | ||||||
| Tan et al. | 2017 | Australia | 29 | Age 49 (35–55) years | 307 (279–460) | iFGF23 | At 52 weeks | At 52 weeks |
| iFGF23 | ||||||||
| 2060 (825–5075) pg/mL | 64 (34–88) pg/mL | |||||||
| eGFR | 460 (311–525) | eGFR | ||||||
| Male 17 (59%) | 7.4 (6.5–8.7) mL/min/1.73 m2 | 60.4 (50.5–71.6) mL/min/1.73 m2 | ||||||
| Mizusaki et al. | 2019 | Japan | 36 | Age 38.1 ± 14 years | 211.8 | eGFR | At 1 year | At 1 year |
| eGFR | ||||||||
| Male 15 (42%) | 3.8 ± 0.8 mL/min/1.73 m2 | 369.3 | 49 ± 17 mL/min/1.73 m2 |
| Study | Year | Country | N-KTx | Characteristics-KTx | Klotho-KTx (pg/mL) | Other Markers-KTx | N-Control | Klotho-Control (pg/mL) | Other Markers-Control |
|---|---|---|---|---|---|---|---|---|---|
| Balogu et al. | Turkey | 40 | N/A | 153 ± 170 | FGF23 | 20 healthy subjects | 641 ± 1797 | FGF23 | |
| 47.4 ± 61 pg/mL | 1.6 ± 1.3 pg/mL | ||||||||
| Malyszko et al. | 2014 | Poland | 84 | Median time from KTx | 228 (161–384) | eGFR | 22 healthy subjects | 757 (632–839) | eGFR |
| 56.3 ± 1.6 | |||||||||
| 37 (13–72) months | ml/min/1.73 m2 | 97.3 ± 13.5 mL/min/1.73 m2 | |||||||
| Age 47.9 ± 12.0 years | FGF23 | FGF23 | |||||||
| Male 64 (76%) | 16.7 (13.8–21.2) pg/mL | 11.7 (10.8–17.2) pg/mL | |||||||
| Bleskestad et al. | 2015 | Norway | 40 | Median time from KTx | 605 (506–784) | eGFR | 39 GFR-matched controls | GFR-matched controls | GFR-matched control |
| eGFR | |||||||||
| 62 (57–73) mL/min/1.73 m2 | |||||||||
| iFGF23 | |||||||||
| 63 (52–87) pg/mL | |||||||||
| Healthy volunteer | |||||||||
| eGFR | |||||||||
| 99.5 (89.5–110.8) | |||||||||
| 18.3 (IQR 12.2–26.2) years | 62 (52–72) mL/min/1.73 m2 | 660 (536–847) | mL/min/1.73 m2 | ||||||
| Age 61.3 ± 11.8 years | iFGF23 | 20 healthy subjects | Healthy volunteers | iFGF23 | |||||
| Male 29 (73%) | 75 (53–108) pg/mL | 692 (618–866) | 51 (36–68) pg/mL | ||||||
| Tartaglione et al. | 2017 | Italy | 80 | Time for KTx | 449 (388–534) | eGFR | 30 healthy subjects | 795 (619–901) | eGFR |
| 77.6 (37.6–119.5) months | 46.3 (36.2–58.3) mL/min/1.73 m2 | 109.1 ± 14.1 mL/min/1.73 m2 | |||||||
| Age 54.7 ± 10.3 years | FGF23 | FGF23 | |||||||
| Male 49 (61%) | 41 (25–59) pg/mL | 34 (28–441) pg/mL | |||||||
| Nahandi et al. | 2017 | Iran | 30 | Time from KTx | 276 ± 241 | eGFR | 27 healthy subjects | N/A | N/A |
| 6.42 ± 2.44 years | 64.53 ± 17.83 mL/min/1.73 m2 | ||||||||
| Age 30.9 ± 5.3 years |
| Study | Year | Country | N-Donor | Characteristics-Donor | Klotho before Donor Nephrectomy (pg/mL) | Other Markers before Donor Nephrectomy | Klotho after Donor Nephrectomy (pg/mL) | Other Markers after Donor Nephrectomy |
|---|---|---|---|---|---|---|---|---|
| Akimoto et al. | 2013 | Japan | 10 | Age 64 ± 9 years | 910 (755–1132) | eGFR | At day 5 | N/A |
| Male 4 (40%) | 87 (72–92) mL/min/1.73 m2 | 619 (544.6–688.5) | ||||||
| Living donor | ||||||||
| Ponte et al. | 2014 | Switzerland | 27 | Age 54 ± 11 years | 526 (482–615) | eGFR | At day 3 | At day 3 |
| FGF23 | ||||||||
| 26.9 (22.1–38.0) pg/mL | ||||||||
| At day 360 | ||||||||
| eGFR | ||||||||
| 95 ± 11 mL/min/1.73 m2 | 304 (266–491) | 63 ± 13 mL/min/1.73 m2 | ||||||
| Male 15 (57%) | FGF23 | At day 360 | FGF23 | |||||
| Living donor | 48.1 (37.4–60.0) pg/mL | 440 (398–613) | 45.2 (37.7–56.4) pg/mL | |||||
| Kimura et al. | 2015 | Japan | 15 | Age 59 ± 9 years | 1084 (795–1638) | eGFR | At day 5 | N/A |
| Male 8 (53%) | 74 ± 14 mL/min/1.73 m2 | 809 (638–1357) | ||||||
| Living donor | ||||||||
| Tan et al. | 2017 | Australia | 21 | Age 54.1 ± 14.7 | 564 (468–663) | eGFR | At 12 months | At 12 months |
| eGFR | ||||||||
| 94 (82–97) mL/min/1.73 m2 | 61 (49–69) ml/min/1.73 m2 | |||||||
| Male 13 (62%) | iFGF23 | 420 (378–555) | iFGF23 | |||||
| Living donor | 52 ± 15 pg/mL | 72 ± 22 pg/mL |
| Study | Year | Country | N-Donor | Characteristics-Donor | Klotho-Donor (pg/mL) | Other Markers-Donor | N-Control | Klotho-Control (pg/mL) | Other Markers-Control |
|---|---|---|---|---|---|---|---|---|---|
| Thorsen et al. | 2016 | Norway | 35 | Age 56.5 ± 9.4 years | 669 (409–1161) | FGF23 | 35 healthy subjects | 725 (458–1222) | FGF23 |
| Male 21 (60%) | 62.6 (6.6–112) pg/mL | 51.8 (25.9–90) pg/mL | |||||||
| Living donor | |||||||||
| Time from donor nephrectomy | eGFR | eGFR | |||||||
| Median 15 years | 75.8 ± 12.3 mL/min/1.73 m2 | 99.0 ± 13.1 mL/min/1.73 m2 | |||||||
| Tan et al. | 2017 | Australia | 21 | Age 54.1 ± 14.7 years | 420 (378–555) | eGFR | 20 healthy subjects | 517 (434–667) | eGFR |
| Male 13 (62%) | 61 (49–69) mL/min/1.73 m2 | 97 (89–102) mL/min/1.73 m2 | |||||||
| Living donor | iFGF23 | iFGF | |||||||
| Time from donor nephrectomy 1 year | 72 ± 22 pg/mL | 52 ± 15 pg/mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongprayoon, C.; Neyra, J.A.; Hansrivijit, P.; Medaura, J.; Leeaphorn, N.; Davis, P.W.; Kaewput, W.; Bathini, T.; Salim, S.A.; Chewcharat, A.; et al. Serum Klotho in Living Kidney Donors and Kidney Transplant Recipients: A Meta-Analysis. J. Clin. Med. 2020, 9, 1834. https://doi.org/10.3390/jcm9061834
Thongprayoon C, Neyra JA, Hansrivijit P, Medaura J, Leeaphorn N, Davis PW, Kaewput W, Bathini T, Salim SA, Chewcharat A, et al. Serum Klotho in Living Kidney Donors and Kidney Transplant Recipients: A Meta-Analysis. Journal of Clinical Medicine. 2020; 9(6):1834. https://doi.org/10.3390/jcm9061834
Chicago/Turabian StyleThongprayoon, Charat, Javier A. Neyra, Panupong Hansrivijit, Juan Medaura, Napat Leeaphorn, Paul W. Davis, Wisit Kaewput, Tarun Bathini, Sohail Abdul Salim, Api Chewcharat, and et al. 2020. "Serum Klotho in Living Kidney Donors and Kidney Transplant Recipients: A Meta-Analysis" Journal of Clinical Medicine 9, no. 6: 1834. https://doi.org/10.3390/jcm9061834
APA StyleThongprayoon, C., Neyra, J. A., Hansrivijit, P., Medaura, J., Leeaphorn, N., Davis, P. W., Kaewput, W., Bathini, T., Salim, S. A., Chewcharat, A., Aeddula, N. R., Vallabhajosyula, S., Mao, M. A., & Cheungpasitporn, W. (2020). Serum Klotho in Living Kidney Donors and Kidney Transplant Recipients: A Meta-Analysis. Journal of Clinical Medicine, 9(6), 1834. https://doi.org/10.3390/jcm9061834








