Lifestyle, Inflammation, and Vascular Calcification in Kidney Transplant Recipients: Perspectives on Long-Term Outcomes
Abstract
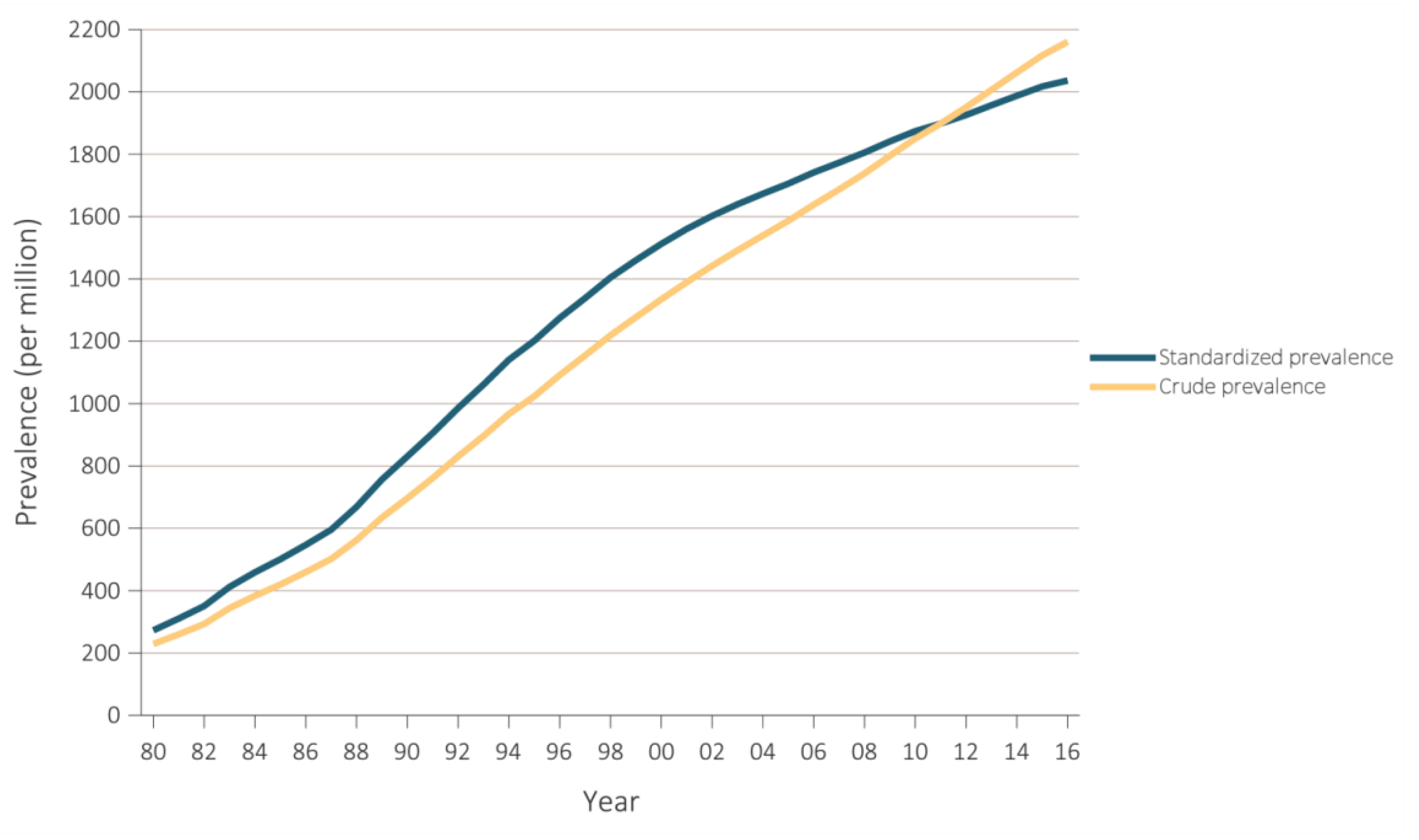
:1. Introduction
2. Lifestyle: Healthy Diet and Toxic Contaminants
- ≥200 g of fruit per day (2–3 servings).
- ≥200 g of vegetables per day (2–3 servings).
- Fish 1–2 times per week, one of which to be oily fish.
- Saturated fatty acids to account for <10% of total energy intake through replacement by polyunsaturated fatty acids.
- Trans unsaturated fatty acids: as little as possible, preferably no intake from processed food and <1% of total energy intake from natural origin.
- 30 g unsalted nuts per day.
- <5 g of salt per day.
- Consumption of alcoholic beverages should be limited to 2 glasses per day (20 g/d of alcohol) for men and 1 glass per day (10 g/d of alcohol) for women.
- Sugar-sweetened soft drinks and alcoholic beverages consumption must be discouraged.
2.1. Fruit and Vegetable Consumption Post-Kidney Transplantation
2.2. Fish Intake Post-Kidney Transplantation and Mercury Exposure
2.3. Cadmium Exposure and Nephrotoxicity in the Post-Kidney Transplant Setting
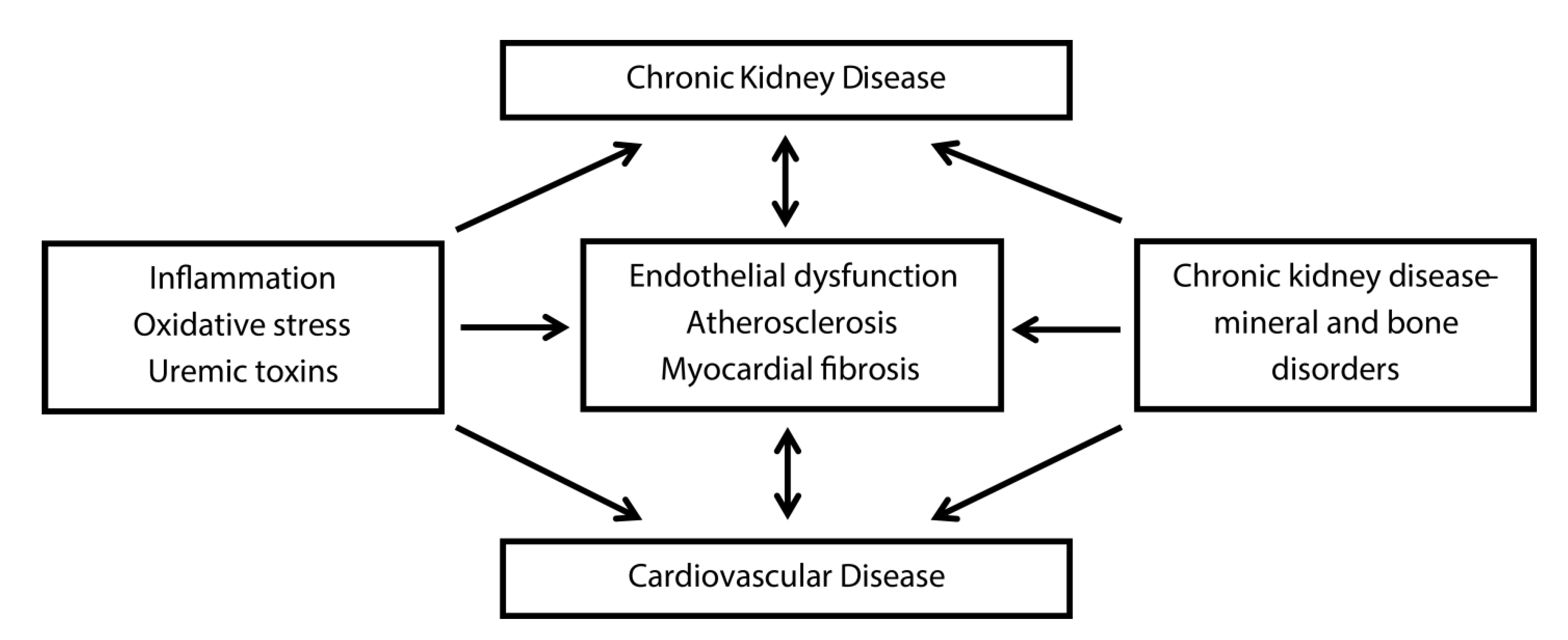
3. Inflammation and Oxidative Stress and Vascular Calcification
3.1. Inflammation and Oxidative Stress Post-Kidney Transplantation
3.1.1. Vitamin C as Anti-Inflammatory and Antioxidant Agent and Its Depletion Post-Kidney Transplant
3.1.2. Advanced Glycation End products as Amplifiers of Oxidative Stress and Inflammatory Responses
3.1.3. Inflammation, Galectin-3, and Fibrosis
3.2. Bone Disease and Vascular Calcification
3.3. Immunosuppressive Therapy and Traditional Risk Factors of Vascular Calcification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transpl. 2019, 34, 1803–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease-A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, B.O.; Ingebretsen, O.C. The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int. 2006, 69, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Renal Data System. USRDS 2018 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States; Bethesda, M., Ed.; National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018; Available online: https://www.usrds.org/Default.aspx (accessed on 8 September 2019).
- Cusumano, A.M.; Rosa-Diez, G.J.; Gonzalez-Bedat, M.C. Latin American Dialysis and Transplant Registry: Experience and contributions to end-stage renal disease epidemiology. World J. Nephrol. 2016, 5, 389. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, S.M.; Van Oosten, M.J.M.; Los, J.; Leegte, M.J.H.; Jager, K.J.; Hemmelder, M.H.; Logtenberg, S.J.J.; Stel, V.S.; Hakkaart-Van Roijen, L.; De Wit, G.A. Healthcare costs of patients on different renal replacement modalities – Analysis of Dutch health insurance claims data. PLoS ONE 2019, 14, e0220800. [Google Scholar] [CrossRef] [Green Version]
- Suthanthiran, M.; Strom, T.B. Renal Transplantation. N. Engl. J. Med. 1994, 331, 365–376. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.C.; Held, P.J.; Port, F.K. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [Green Version]
- Oniscu, G.C.; Brown, H.; Forsythe, J.L.R. Impact of Cadaveric Renal Transplantation on Survival in Patients Listed for Transplantation. J. Am. Soc. Nephrol. 2005, 16, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic Review: Kidney Transplantation Compared With Dialysis in Clinically Relevant Outcomes. Am. J. Transpl. 2011, 11, 2093–2109. [Google Scholar] [CrossRef]
- Lamb, K.E.; Lodhi, S.; Meier-Kriesche, H.U. Long-term renal allograft survival in the United States: A critical reappraisal. Am. J. Transpl. 2011, 11, 450–462. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.-S.; O’Connell, P.J.; Allen, R.D.M.; Chapman, J.R. The natural history of chronic allograft nephropathy. N. Engl. J. Med. 2003, 349, 2326–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nankivell, B.J.; Chapman, J.R. Chronic allograft nephropathy: Current concepts and future directions. Transplantation 2006, 81, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Nankivell, B.J.; Kuypers, D.R.J. Diagnosis and prevention of chronic kidney allograft loss. Lancet 2011, 378, 1428–1437. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.-S.; O’Connell, P.J.; Allen, R.D.M.; Chapman, J.R. Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation 2004, 78, 242–249. [Google Scholar] [CrossRef]
- Arend, S.M.; Mallat, M.J.K.; Westendorp, R.J.W.; Van Der Woude, F.J.; Van Es, L.A. Patient survival after renal transplantation; more than 25 years follow-up. Nephrol. Dial. Transpl. 1997, 12, 1672–1679. [Google Scholar]
- Oterdoom, L.H.; de Vries, A.P.J.; van Ree, R.M.; Gansevoort, R.T.; van Son, W.J.; van der Heide, J.J.H.; Navis, G.; de Jong, P.E.; Gans, R.O.B.; Bakker, S.J.L. N-Terminal Pro-B-Type Natriuretic Peptide and Mortality in Renal Transplant Recipients Versus the General Population. Transplantation 2009, 87, 1562–1570. [Google Scholar] [CrossRef]
- U.S. Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2010.
- Ojo, A.O.; Hanson, J.A.; Wolfe, R.A.; Leichtman, A.B.; Agodoa, L.Y.; Port, F.K. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000, 57, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Shirali, A.C.; Bia, M.J. Management of cardiovascular disease in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2008, 3, 491–504. [Google Scholar] [CrossRef] [Green Version]
- Jardine, A.G.; Gaston, R.S.; Fellstrom, B.C.; Holdaas, H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet 2011, 378, 1419–1427. [Google Scholar] [CrossRef]
- Fry, K.; Patwardhan, A.; Ryan, C.; Trevillian, P.; Chadban, S.; Westgarth, F.; Chan, M. Development of Evidence-Based Guidelines for the Nutritional Management of Adult Kidney Transplant Recipients. J. Ren. Nutr. 2009, 19, 101–104. [Google Scholar] [CrossRef]
- Zelle, D.M.; Kok, T.; Dontje, M.L.; Danchell, E.I.; Navis, G.; van Son, W.J.; Bakker, S.J.L.; Corpeleijn, E. The role of diet and physical activity in post-transplant weight gain after renal transplantation. Clin. Transpl. 2013, 27, E484–E490. [Google Scholar] [CrossRef] [PubMed]
- Nolte Fong, J.V.; Moore, L.W. Nutrition Trends in Kidney Transplant Recipients: The Importance of Dietary Monitoring and Need for Evidence-Based Recommendations. Front. Med. 2018, 5, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaassen, G.; Zelle, D.M.; Navis, G.J.; Dijkema, D.; Bemelman, F.J.; Bakker, S.J.L.; Corpeleijn, E. Lifestyle intervention to improve quality of life and prevent weight gain after renal transplantation: Design of the Active Care after Transplantation (ACT) randomized controlled trial. BMC Nephrol. 2017, 18, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbatini, M.; Ferreri, L.; Pisani, A.; Capuano, I.; Morgillo, M.; Memoli, A.; Riccio, E.; Guida, B. Nutritional management in renal transplant recipients: A transplant team opportunity to improve graft survival. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Monier-Faugere, M.-C.; Mawad, H.; Qi, Q.; Friedler, R.M.; Malluche, H.H. High Prevalence of Low Bone Turnover and Occurrence of Osteomalacia after Kidney Transplantation. J. Am. Soc. Nephrol. 2000, 11, 1093–1099. [Google Scholar] [PubMed]
- Sprague, S.M.; Belozeroff, V.; Danese, M.D.; Martin, L.P.; Olgaard, K. Abnormal bone and mineral metabolism in kidney transplant patients—A review. Am. J. Nephrol. 2008, 28, 246–253. [Google Scholar] [CrossRef]
- Evenepoel, P.; Lerut, E.; Naesens, M.; Bammens, B.; Claes, K.; Kuypers, D.; Vermeersch, P.; Meijers, B.; Van Damme, B.; Vanrenterghem, Y. Localization, etiology and impact of calcium phosphate deposits in renal allografts. Am. J. Transpl. 2009, 9, 2470–2478. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Molnar, M.Z.; Kovesdy, C.P.; Mucsi, I.; Bunnapradist, S. Management of mineral and bone disorder after kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2012, 21, 389–403. [Google Scholar] [CrossRef]
- Lou, I.; Foley, D.; Odorico, S.K.; Leverson, G.; Schneider, D.F.; Sippel, R.; Chen, H. How Well Does Renal Transplantation Cure Hyperparathyroidism? Ann. Surg. 2015, 262, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.; Weir, M.R.; Kopyt, N.; Mannon, R.B.; Von Visger, J.; Deng, H.; Yue, S.; Vincenti, F. A Prospective Cohort Study of Mineral Metabolism After Kidney Transplantation. Transplantation 2016, 100, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Willett, W. Diet and health: What should we eat? Science 1994, 264, 532–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stampfer, M.J.; Hu, F.B.; Manson, J.E.; Rimm, E.B.; Willett, W.C. Primary Prevention of Coronary Heart Disease in Women through Diet and Lifestyle. N. Engl. J. Med. 2000, 343, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Chiuve, S.E.; McCullough, M.L.; Sacks, F.M.; Rimm, E.B. Healthy Lifestyle Factors in the Primary Prevention of Coronary Heart Disease Among Men. Circulation 2006, 114, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Chiuve, S.E.; Rexrode, K.M.; Spiegelman, D.; Logroscino, G.; Manson, J.E.; Rimm, E.B. Primary prevention of stroke by healthy lifestyle. Circulation 2008, 118, 947–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiuve, S.E.; Fung, T.T.; Rexrode, K.M.; Spiegelman, D.; Manson, J.E.; Stampfer, M.J.; Albert, C.M. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA 2011, 306, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.-J.; Campbell, K.L.; Strippoli, G.F.M. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017, 12, 272–279. [Google Scholar] [CrossRef]
- Chauveau, P. Nutrition in chronic kidney disease: Nephrology dialysis transplantation notable advances in 2018. Nephrol. Dial. Transpl. 2019, 34, 893–896. [Google Scholar] [CrossRef]
- Saglimbene, V.M.; Wong, G.; Ruospo, M.; Palmer, S.C.; Garcia-Larsen, V.; Natale, P.; Teixeira-Pinto, A.; Campbell, K.L.; Carrero, J.-J.; Stenvinkel, P.; et al. Article Fruit and Vegetable Intake and Mortality in Adults undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Notice. Kidney Int. Suppl. 2013, 3, 1. [Google Scholar] [CrossRef]
- Palmer, S.C.; Hanson, C.S.; Craig, J.C.; Strippoli, G.F.M.; Ruospo, M.; Campbell, K.; Johnson, D.W.; Tong, A. Dietary and fluid restrictions in CKD: A thematic synthesis of patient views from qualitative studies. Am. J. Kidney Dis. 2015, 65, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Mcmahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2015, 18, CD010070. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Su, G.; Guo, X.; Wu, Y.; Liu, X.; Zou, C.; Zhang, L.; Yang, Q.; Xu, Y.; Ma, W. Dietary interventions for mineral and bone disorder in people with chronic kidney disease. Cochrane Database Syst. Rev. 2015, 16, CD010350. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.J.; Prohaska, T.R.; Gallant, M.; Siminoff, L.A. Self-care strategies and barriers among kidney transplant recipients: A qualitative study. Chronic Illn. 2009, 5, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Stanfill, A.; Bloodworth, R.; Cashion, A. Lessons learned: Experiences of gaining weight by kidney transplant recipients. Prog. Transpl. 2012, 22, 71–78. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [Green Version]
- Crowe, F.L.; Roddam, A.W.; Key, T.J.; Appleby, P.N.; Overvad, K.; Jakobsen, M.U.; Tjonneland, A.; Hansen, L.; Boeing, H.; Weikert, C.; et al. Fruit and vegetable intake and mortality from ischaemic heart disease: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heart study. Eur. Heart J. 2011, 32, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [Green Version]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Total and specific fruit and vegetable consumption and risk of stroke: A prospective study. Atherosclerosis 2013, 227, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, X.-O.; Xiang, Y.-B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.-T.; Zheng, W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am. J. Clin. Nutr. 2011, 94, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Cases, A.; Cigarrán-Guldrís, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients 2019, 11, 1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.M.; Cohen, A.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef] [Green Version]
- Sotomayor, C.G.; Gomes-Neto, A.W.; Eisenga, M.F.; Nolte, I.M.; Anderson, J.L.; de Borst, M.H.; Osté, M.C.; Rodrigo, R.; Gans, R.O.; Berger, S.P.; et al. Consumption of fruits and vegetables and cardiovascular mortality in renal transplant recipients: A prospective cohort study. Nephrol. Dial. Transpl. 2020, 35, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes-Neto, A.W.; Osté, M.C.; Sotomayor, C.; Berg, E.V.D.; Geleijnse, J.M.; Gans, R.O.; Navis, G.J.; Bakker, S.J. Fruit and Vegetable Intake and Risk of Posttransplantation Diabetes in Renal Transplant Recipients. Diabetes Care 2019, 42, 1644–1652. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Gottdiener, J.S.; Siscovick, D.S. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am. J. Cardiol. 2006, 97, 216–222. [Google Scholar] [CrossRef]
- Chin, J.P.; Gust, A.P.; Nestel, P.J.; Dart, A.M. Marine oils dose-dependently inhibit vasoconstriction of forearm resistance vessels in humans. Hypertension 1993, 21, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Nestel, P.J. Fish oil and cardiovascular disease: Lipids and arterial function. Am. J. Clin. Nutr. 2000, 71, 228S–231S. [Google Scholar] [CrossRef]
- Kristensen, S.D.; Bach Iversen, A.M.; Schmidt, E.B. n−3 polyunsaturated fatty acids and coronary thrombosis. Lipids 2001, 36, S79–S82. [Google Scholar] [CrossRef]
- Bar-Or, A.; Bashinskaya, V.V.; Kulakova, O.G.; Boyko, A.N.; Favorov, A.V.; Favorova, O.O.; Medicina, F.D.E.; Bos, S.D.; Berge, T.; Celius, E.G.; et al. The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. PLoS ONE 2012, 7, 457. [Google Scholar] [CrossRef]
- Christensen, J.H. Omega-3 polyunsaturated Fatty acids and heart rate variability. Front. Med. 2011, 2, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, S.; Marchioli, R.; Mozaffarian, D.; Bernasconi, R.; Milani, V.; Dragani, L.; Tacconi, M.; Marfisi, R.M.; Borgese, L.; Cirrincione, V.; et al. Plasma n-3 polyunsaturated fatty acids in chronic heart failure in the GISSI-heart failure trial: Relation with fish intake, circulating biomarkers, and mortality. Am. Heart J. 2013, 165, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eide, I.A.; Jenssen, T.; Hartmann, A.; Diep, L.M.; Dahle, D.O.; Reisæter, A.V.; Bjerve, K.S.; Christensen, J.H.; Schmidt, E.B.; Svensson, M. The association between marine n-3 polyunsaturated fatty acid levels and survival after renal transplantation. Clin. J. Am. Soc. Nephrol. 2015, 10, 1246–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes-Neto, A.W.; Sotomayor, C.G.; Pranger, I.; van den Berg, E.; Gans, R.O.; Soedamah-Muthu, S.; Navis, G.J.; Bakker, S.J. Intake of Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Mortality in Renal Transplant Recipients. Nutrients 2017, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Tatsioni, A.; Chung, M.; Sun, Y.; Kupelnick, B.; Lichtenstein, A.H.; Perrone, R.; Chew, P.; Lau, J.; Bonis, P.A. Effects of fish oil supplementation on kidney transplantation: A systematic review and meta-analysis of randomized, controlled trials. J. Am. Soc. Nephrol. 2005, 16, 2462–2670. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.K.; Manley, K.J.; Roberts, M.A.; Fraenkel, M.B. Fish oil for kidney transplant recipients. Cochrane Database Syst. Rev. 2016, 8, CD005282. [Google Scholar] [CrossRef]
- Kromhout, D.; Feskens, E.J.; Bowles, C.H. The protective effect of a small amount of fish on coronary heart disease mortality in an elderly population. Int. J. Epidemiol. 1995, 24, 340–345. [Google Scholar] [CrossRef]
- Stone, N.J. Fish consumption, fish oil, lipids, and coronary heart disease. Circulation 1996, 94, 2337–2340. [Google Scholar] [CrossRef]
- Krauss, R.M.; Eckel, R.H.; Howard, B.; Appel, L.J.; Daniels, S.R.; Deckelbaum, R.J.; Erdman, J.W.; Kris-Etherton, P.; Goldberg, I.J.; Kotchen, T.A.; et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000, 102, 2284–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psota, T.L.; Gebauer, S.K.; Kris-Etherton, P. Dietary Omega-3 Fatty Acid Intake and Cardiovascular Risk. Am. J. Cardiol. 2006, 98, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Harris, W.S.; Chung, M.; Lichtenstein, A.H.; Balk, E.M.; Kupelnick, B.; Jordan, H.S.; Lau, J. n-3 Fatty acids from fish or fish-oil supplements, but not α-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am. J. Clin. Nutr. 2006, 84, 5–17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preventing Disease through Healthy Environment—Exposure to Mercury: A Major Public Health Concern; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Hightower, J.M.; Moore, D. Mercury levels in high-end consumers of fish. Environ. Health Perspect. 2003, 111, 604–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.; Douglass, C.W.; Kim, H.D.; Joshipura, K.J.; Park, M.C.; Rimm, E.B.; Carino, M.J.; Garcia, R.I.; Morris, J.S.; Willett, W.C. The relationship between amalgam restorations and mercury levels in male dentists and nondental health professionals. J. Public Health Dent. 2003, 63, 52–60. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Gomes-Neto, A.W.; Gans, R.O.; de Borst, M.; Berger, S.P.; Rodrigo, R.; Navis, G.J.; Touw, D.J.; Bakker, S.J.; Sotomayor, C.G.; et al. Fish Intake, Circulating Mercury and Mortality in Renal Transplant Recipients. Nutrients 2018, 10, 1419. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, J.C.; DiNatale, B.C.; Murray, I.A.; Flaveny, C.A.; Liu, Q.; Laurenzana, E.M.; Lin, J.M.; Strom, S.C.; Omiecinski, C.J.; Amin, S.; et al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 2010, 49, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Tellez-Plaza, M.; Navas-Acien, A.; Crainiceanu, C.M.; Guallar, E. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES). Environ. Health Perspect. 2008, 116, 51–56. [Google Scholar] [CrossRef]
- Mange, K.C.; Cizman, B.; Joffe, M.; Feldman, H.I. Arterial hypertension and renal allograft survival. JAMA 2000, 283, 633–638. [Google Scholar] [CrossRef] [Green Version]
- Mange, K.C.; Feldman, H.I.; Joffe, M.M.; Fa, K.; Bloom, R.D. Blood Pressure and the Survival of Renal Allografts from Living Donors. J. Am. Soc. Nephrol. 2004, 15, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Weir, M.R.; Burgess, E.D.; Cooper, J.E.; Fenves, A.Z.; Goldsmith, D.; McKay, D.; Mehrotra, A.; Mitsnefes, M.M.; Sica, D.A.; Taler, S.J. Assessment and management of hypertension in transplant patients. J. Am. Soc. Nephrol. 2015, 26, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Lundh, T.; Vahter, M.; Bjellerup, P.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Strömberg, U.; Skerfving, S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ. Health Perspect. 2005, 113, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Buser, M.C.; Ingber, S.Z.; Raines, N.; Fowler, D.A.; Scinicariello, F. Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012. Int. J. Hyg. Environ. Health 2016, 219, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Choi, S.J.; Kim, D.W.; Kim, N.Y.; Park, C.H.; Do Yu, S.; Kim, D.S.; Park, K.S.; Song, J.S.; Kim, H.; et al. Risk assessment of low-level cadmium and arsenic on the kidney. J. Toxicol. Environ. Health A 2009, 72, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Geeth Gunawardana, C.; Martinez, R.E.; Xiao, W.; Templeton, D.M.; Geeth, C. Cadmium inhibits both intrinsic and extrinsic apoptotic pathways in renal mesangial cells. Am. J. Physiol. Ren. Physiol. 2006, 290, 1074–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharm. Exp. 2012, 343, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, Z.A.; Vu, T.T.; Zaman, K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharm. 1999, 154, 256–263. [Google Scholar] [CrossRef]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. BioMetals 2010, 23, 783–792. [Google Scholar] [CrossRef]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharm. 2009, 238, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Andersen, O. Chelation of cadmium. Environ. Health Perspect. 1984, 54, 249–266. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Guijarro, C.; Massy, Z.A.; Wiederkehr, M.R.; Ma, J.Z. Cardiovascular disease after renal transplantation. J. Am. Coll. Cardiol. 1996, 7, 158–165. [Google Scholar]
- Kasiske, B.L.; Vazquez, M.A.; Harmon, W.E.; Brown, R.S.; Danovitch, G.M.; Gaston, R.S.; Roth, D.; Scandling, J.D.; Singer, G.G.; The American Society of Transplantation. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J. Am. Soc. Nephrol. 2000, 11, S1–S86. [Google Scholar] [PubMed]
- Ducloux, D.; Kazory, A.; Chalopin, J.M. Predicting coronary heart disease in renal transplant recipients: A prospective study. Kidney Int. 2004, 66, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiberd, B.; Panek, R. Cardiovascular outcomes in the outpatient kidney transplant clinic: The Framingham risk score revisited. Clin. J. Am. Soc. Nephrol. 2008, 3, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Winkelmayer, W.C.; Lorenz, M.; Kramar, R.; Födinger, M.; Hörl, W.H.; Sunder-Plassmann, G. C-reactive protein and body mass index independently predict mortality in kidney transplant recipients. Am. J. Transpl. 2004, 4, 1148–1154. [Google Scholar] [CrossRef]
- Hartog, J.W.L. Risk factors for chronic transplant dysfunction and cardiovascular disease are related to accumulation of advanced glycation end-products in renal transplant recipients. Nephrol. Dial. Transpl. 2006, 21, 2263–2269. [Google Scholar] [CrossRef] [Green Version]
- Abedini, S.; Holme, I.; März, W.; Weihrauch, G.; Fellström, B.; Jardine, A.; Cole, E.; Maes, B.; Neumayer, H.H.; Grønhagen-Riska, C.; et al. Inflammation in renal transplantation. Clin. J. Am. Soc. Nephrol. 2009, 4, 1246–1254. [Google Scholar] [CrossRef] [Green Version]
- Turkmen, K.; Tonbul, H.Z.; Toker, A.; Gaipov, A.; Erdur, F.M.; Cicekler, H.; Anil, M.; Ozbek, O.; Selcuk, N.Y.; Yeksan, M.; et al. The relationship between oxidative stress, inflammation, and atherosclerosis in renal transplant and end-stage renal disease patients. Ren. Fail. 2012, 34, 1229–1237. [Google Scholar] [CrossRef]
- Ocak, N.; Dirican, M.; Ersoy, A.; Sarandol, E. Adiponectin, leptin, nitric oxide, and C-reactive protein levels in kidney transplant recipients: Comparison with the hemodialysis and chronic renal failure. Ren. Fail. 2016, 38, 1639–1646. [Google Scholar] [CrossRef]
- van Gennip, A.C.E.; Broers, N.J.H.; ter Meulen, K.J.; Canaud, B.; Christiaans, M.H.L.; Cornelis, T.; Gelens, M.A.C.J.; Hermans, M.M.H.; Konings, C.J.A.M.; van der Net, J.B.; et al. Endothelial dysfunction and low-grade inflammation in the transition to renal replacement therapy. PLoS ONE 2019, 14, e0222547. [Google Scholar] [CrossRef] [Green Version]
- Mazzaferro, S.; Pasquali, M.; Taggi, F.; Baldinelli, M.; Conte, C.; Muci, M.L.; Pirozzi, N.; Carbone, I.; Francone, M.; Pugliese, F. Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin. J. Am. Soc. Nephrol. 2009, 4, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Tian, J.; Chaudhry, M.; Maxwell, K.; Yan, Y.; Wang, X.; Shah, P.T.; Khawaja, A.A.; Martin, R.; Robinette, T.J.; et al. Attenuation of Na/K-ATPase Mediated Oxidant Amplification with pNaKtide Ameliorates Experimental Uremic Cardiomyopathy. Sci. Rep. 2016, 6, 34592. [Google Scholar] [CrossRef]
- Jerotic, D.; Matic, M.; Suvakov, S.; Vucicevic, K.; Damjanovic, T.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Coric, V.; Stefanovic, A.; Ivanisevic, J.; et al. Association of Nrf2, SOD2 and GPX1 Polymorphisms with Biomarkers of Oxidative Distress and Survival in End-Stage Renal Disease Patients. Toxins 2019, 11, 431. [Google Scholar] [CrossRef] [Green Version]
- La Russa, D.; Pellegrino, D.; Montesanto, A.; Gigliotti, P.; Perri, A.; La Russa, A.; Bonofiglio, R. Oxidative Balance and Inflammation in Hemodialysis Patients: Biomarkers of Cardiovascular Risk? Oxid. Med. Cell Longev. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Sies, H. (Ed.) Oxidative Stress II: Oxidants and Antioxidants; Academic Press: New York, NY, USA, 1991. [Google Scholar]
- Langlois, M.; Duprez, D.; Delanghe, J.; De Buyzere, M.; Clement, D.L. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation 2001, 103, 1863–1868. [Google Scholar] [CrossRef] [Green Version]
- Korantzopoulos, P.; Kolettis, T.M.; Kountouris, E.; Dimitroula, V.; Karanikis, P.; Pappa, E.; Siogas, K.; Goudevenos, J.A. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int. J. Cardiol. 2005, 102, 321–326. [Google Scholar] [CrossRef]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J. Transl. Med. 2012, 10. [Google Scholar] [CrossRef] [Green Version]
- Mikirova, N.; Casciari, J.; Riordan, N.; Hunninghake, R. Clinical experience with intravenous administration of ascorbic acid: Achievable levels in blood for different states of inflammation and disease in cancer patients. J. Transl. Med. 2013, 11, 191. [Google Scholar] [CrossRef] [Green Version]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Eide, T.C.; Sogn, E.M.; Berg, K.J.; Sund, R.B. Plasma ascorbic acid in patients undergoing chronic haemodialysis. Eur. J. Clin. Pharm. 1999, 55, 527–532. [Google Scholar] [CrossRef]
- Morena, M.; Cristol, J.P.; Bosc, J.Y.; Tetta, C.; Forret, G.; Leger, C.L.; Delcourt, C.; Papoz, L.; Descomps, B.; Canaud, B. Convective and diffusive losses of vitamin C during haemodiafiltration session: A contributive factor to oxidative stress in haemodialysis patients. Nephrol. Dial. Transpl. 2002, 17, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, J.F.; Eisenstein, A.B. Ascorbic acid depletion during hemodialysis. JAMA 1972, 220, 1697–1699. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.A. Assessment of Human Vitamin C Status. J. Nutr. 1990, 120, 1480–1485. [Google Scholar] [CrossRef]
- Johnston, C.S.; Solomon, R.E.; Corte, C. Vitamin C depletion is associated with alterations in blood histamine and plasma free carnitine in adults. J. Am. Coll. Nutr. 1996, 15, 586–591. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Cheng, X.; Liu, L.; Bai, W.; Guo, W.; Wu, L.; Zuo, L. Cross-over study of influence of oral vitamin C supplementation on inflammatory status in maintenance hemodialysis patients. BMC Nephrol. 2013, 14, 252. [Google Scholar] [CrossRef] [Green Version]
- Attallah, N.; Osman-Malik, Y.; Frinak, S.; Besarab, A. Effect of intravenous ascorbic acid in hemodialysis patients with EPO-hyporesponsive anemia and hyperferritinemia. Am. J. Kidney Dis. 2006, 47, 644–654. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef] [Green Version]
- Sotomayor, C.G.; Eisenga, M.F.; Gomes Neto, A.W.; Ozyilmaz, A.; Gans, R.O.B.; de Jong, W.H.A.; Zelle, D.M.; Berger, S.P.; Gaillard, C.A.J.M.; Navis, G.J.; et al. Vitamin C Depletion and All-Cause Mortality in Renal Transplant Recipients. Nutrients 2017, 9, 568. [Google Scholar] [CrossRef] [Green Version]
- Gacitúa, T.A.; Sotomayor, C.G.; Groothof, D.; Eisenga, M.F.; Pol, R.A.; de Borst, M.H.; Gans, R.O.B.; Berger, S.P.; Rodrigo, R.; Navis, G.J.; et al. Plasma Vitamin C and Cancer Mortality in Kidney Transplant Recipients. J. Clin. Med. 2019, 8, 2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Yan, S.D.; Schmidt, A.M.; Anderson, G.M.; Zhang, J.; Brett, J.; Zou, Y.S.; Pinsky, D.; Stern, D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 1994, 269, 9889–9897. [Google Scholar] [PubMed]
- Linden, E.; Cai, W.; He, J.C.; Xue, C.; Li, Z.; Winston, J.; Vlassara, H.; Uribarri, J. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin. J. Am. Soc. Nephrol. 2008, 3, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Stinghen, A.E.M.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stam, F.; van Guldener, C.; Becker, A.; Dekker, J.M.; Heine, R.J.; Bouter, L.M.; Stehouwer, C. DA Endothelial Dysfunction Contributes to Renal Function-Associated Cardiovascular Mortality in a Population with Mild Renal Insufficiency: The Hoorn Study. J. Am. Soc. Nephrol. 2006, 17, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Calviño, J.; Cigarran, S.; Gonzalez-Tabares, L.; Menendez, N.; Latorre, J.; Cillero, S.; Millan, B.; Cobelo, C.; Sanjurjo-Amado, A.; Quispe, J.; et al. Advanced glycation end products (AGEs) estimated by skin autofluorescence are related with cardiovascular risk in renal transplant. PLoS ONE 2018, 13, e0201118. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.S.; Shin, M.J.; Shin, S.J.; Kim, Y.S.; Choi, Y.J.; Kim, Y.-S.; Moon, I.S.; Kim, S.Y.; Koh, Y.B.; Bang, B.K.; et al. Clinical significance of an early protocol biopsy in living-donor renal transplantation: Ten-year experience at a single center. Am. J. Transpl. 2005, 5, 1354–1360. [Google Scholar] [CrossRef]
- Heilman, R.L.; Devarapalli, Y.; Chakkera, H.A.; Mekeel, K.L.; Moss, A.A.; Mulligan, D.C.; Mazur, M.J.; Hamawi, K.; Williams, J.W.; Reddy, K.S. Impact of Subclinical Inflammation on the Development of Interstitial Fibrosis and Tubular Atrophy in Kidney Transplant Recipients. Am. J. Transpl. 2010, 10, 563–570. [Google Scholar] [CrossRef]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.T.; Hughes, J.; Sethi, T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Kolatsi-Joannou, M.; Price, K.L.; Winyard, P.J.; Long, D.A. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS ONE 2011, 6, 18683. [Google Scholar] [CrossRef] [Green Version]
- Frenay, A.-R.S.; Yu, L.; van der Velde, A.R.; Vreeswijk-Baudoin, I.; López-Andrés, N.; van Goor, H.; Silljé, H.H.; Ruifrok, W.P.; de Boer, R.A. Pharmacological inhibition of galectin-3 protects against hypertensive nephropathy. Am. J. Physiol. Ren. Physiol. 2015, 308, F500–F509. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Martinez, E.; Ibarrola, J.; Calvier, L.; Fernandez-Celis, A.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. Galectin-3 blockade reduces renal fibrosis in two normotensive experimental models of renal damage. PLoS ONE 2016, 11, e0166272. [Google Scholar] [CrossRef]
- Dang, Z.; Mackinnon, A.; Marson, L.P.; Sethi, T. Tubular Atrophy and Interstitial Fibrosis After Renal Transplantation Is Dependent on Galectin-3. Transplantation 2012, 93, 477–484. [Google Scholar] [CrossRef]
- O’Seaghdha, C.; Hwang, S.; Ho, J.; Vasan, R.; Levy, D.; Fox, C. Elevated Galectin-3 Precedes the Development of CKD. J. Am. Soc. Nephrol. 2013, 24, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- Rebholz, C.M.; Selvin, E.; Liang, M.; Ballantyne, C.M.; Hoogeveen, R.C.; Aguilar, D.; McEvoy, J.W.; Grams, M.E.; Coresh, J. Plasma galectin-3 levels are associated with the risk of incident chronic kidney disease. Kidney Int. 2018, 93, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.L.; Katz, R.; Bellovich, K.A.; Bhat, Z.Y.; Brosius, F.C.; de Boer, I.H.; Gadegbeku, C.A.; Gipson, D.S.; Hawkins, J.J.; Himmelfarb, J.; et al. Soluble ST2 and Galectin-3 and Progression of CKD. Kidney Int. Rep. 2018, 4, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Moe, S.M.; Drüeke, T.; Lameire, N.; Eknoyan, G. Chronic Kidney Disease-Mineral-Bone Disorder: A New Paradigm. Adv. Chronic. Kidney Dis. 2007, 14, 3–12. [Google Scholar] [CrossRef]
- Simmons, E.M.; Langone, A.; Sezer, M.T.; Vella, J.P.; Recupero, P.; Morrow, J.D.; Ikizler, T.A.; Himmelfarb, J. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation 2005, 79, 914–919. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Sonmez, A.; Saglam, M.; Cayci, T.; Kilic, S.; Unal, H.U.; Karaman, M.; Cetinkaya, H.; Eyileten, T.; Gok, M.; et al. A longitudinal study of inflammation, CKD-mineral bone disorder, and carotid atherosclerosis after renal transplantation. Clin. J. Am. Soc. Nephrol. 2015, 10, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transpl. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- DeLoach, S.S.; Joffe, M.M.; Mai, X.; Goral, S.; Rosas, S.E. Aortic calcification predicts cardiovascular events and all-cause mortality in renal transplantation. Nephrol. Dial. Transpl. 2009, 24, 1314–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.T.; Henrard, S.; Coche, E.; Goffin, E.; Devuyst, O.; Jadoul, M. Coronary artery calcification: A strong predictor of cardiovascular events in renal transplant recipients. Nephrol. Dial. Transpl. 2010, 25, 3773–3778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roe, P.; Wolfe, M.; Joffe, M.; Rosas, S.E. Inflammation, coronary artery calcification and cardiovascular events in incident renal transplant recipients. Atherosclerosis 2010, 212, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Claes, K.J.; Heye, S.; Bammens, B.; Kuypers, D.R.; Meijers, B.; Naesens, M.; Vanrenterghem, Y.; Evenepoel, P. Aortic calcifications and arterial stiffness as predictors of cardiovascular events in incident renal transplant recipients. Transpl. Int. 2013, 26, 973–981. [Google Scholar] [CrossRef]
- Davis, B.; Marin, D.; Hurwitz, L.M.; Ronald, J.; Ellis, M.J.; Ravindra, K.V.; Collins, B.H.; Kim, C.Y. Application of a Novel CT-Based Iliac Artery Calcification Scoring System for Predicting Renal Transplant Outcomes. Am. J. Roent 2016, 206, 436–441. [Google Scholar] [CrossRef]
- Benjamens, S.; Pol, R.A.; Glaudemans, A.W.J.M.; Wieringa, I.; Berger, S.P.; Bakker, S.J.L.; Slart, R.H.J.A. A high abdominal aortic calcification score by dual X-ray absorptiometry is associated with cardiovascular events after kidney transplantation. Nephrol. Dial. Transpl. 2018, 33, 2253–2259. [Google Scholar] [CrossRef]
- Braun, J.; Oldendorf, M.; Moshage, W.; Heidler, R.; Zeitler, E.; Luft, F.C. Electron beam computed tomography in the evaluation of cardiac calcifications in chronic dialysis patients. Am. J. Kidney Dis. 1996, 27, 394–401. [Google Scholar] [CrossRef]
- London, G.; Marty, C.; Marchais, S.J.; Guerin, A.P.; Metivier, F.; de Vernejoul, M. Arterial Calcifications and Bone Histomorphometry in End-Stage Renal Disease. J. Am. Soc. Nephrol. 2004, 15, 1943–1951. [Google Scholar] [CrossRef]
- Seifert, M.E.; Hruska, K.A. The Kidney-Vascular-Bone Axis in the Chronic Kidney Disease-Mineral Bone Disorder. Transplantation 2016, 100, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Adragao, T.; Herberth, J.; Monier-Faugere, M.-C.; Branscum, A.J.; Ferreira, A.; Frazao, J.M.; Dias Curto, J.; Malluche, H.H. Low Bone Volume-A Risk Factor for Coronary Calcifications in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 450–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asci, G.; Ok, E.; Savas, R.; Ozkahya, M.; Duman, S.; Toz, H.; Kayikcioglu, M.; Branscum, A.J.; Monier-Faugere, M.C.; Herberth, J.; et al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol. Dial. Transpl. 2011, 26, 1010–1015. [Google Scholar] [CrossRef] [Green Version]
- Bouquegneau, A.; Salam, S.; Delanaye, P.; Eastell, R.; Khwaja, A. Mini-Review Bone Disease after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2016, 11, 1282–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malluche, H.H.; Monier-Faugere, M.-C.; Herberth, J. Bone disease after renal transplantation. Nat. Rev. Neprhol. 2010, 6, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drüeke, T.B.; Evenepoel, P. The Bone after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2019, 14, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Neves, C.L.; Dos Reis, L.M.; Batista, D.G.; Custodio, M.R.; Graciolli, F.G.; Martin, R.D.C.T.; Neves, K.R.; Dominguez, W.V.; Moyses, R.M.; Jorgetti, V. Persistence of bone and mineral disorders 2 years after successful kidney transplantation. Transplantation 2013, 96, 290–296. [Google Scholar] [CrossRef]
- Iyer, S.P.; Nikkel, L.E.; Nishiyama, K.K.; Dworakowski, E.; Cremers, S.; Zhang, C.; Mcmahon, D.J.; Boutroy, S.; Liu, X.S.; Ratner, L.E.; et al. Kidney Transplantation with Early Corticosteroid Withdrawal: Paradoxical Effects at the Central and Peripheral Skeleton. J. Am. Soc. Nephrol. 2014, 25, 1331–1341. [Google Scholar] [CrossRef] [Green Version]
- Keronen, S.; Martola, L.; Finne, P.; Burton, I.S.; Kröger, H.; Honkanen, E. Changes in Bone Histomorphometry after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2019, 14, 894–903. [Google Scholar] [CrossRef] [Green Version]
- Moe, S.M. Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. Eur. J. Clin. Investig. 2006, 36, 51–62. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Benjamens, S.; Gomes-Neto, A.W.; Pol, R.A.; Groothof, D.; te Velde-Keyzer, C.A.; Chong, G.; Glaudemans, A.W.J.M.; Berger, S.P.; Bakker, S.J.L.; et al. Bone Mineral Density and Aortic Calcification. Transplantation 2020. [Google Scholar] [CrossRef]
- Czaya, B.; Faul, C. FGF23 and inflammation—a vicious coalition in CKD. Kidney Int. 2019, 96, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Grabner, A.; Yanucil, C.; Schramm, K.; Czaya, B.; Krick, S.; Czaja, M.J.; Bartz, R.; Abraham, R.; Di Marco, G.S.; et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016, 90, 985–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egli-Spichtig, D.; Imenez Silva, P.H.; Glaudemans, B.; Gehring, N.; Bettoni, C.; Zhang, M.Y.H.; Pastor-Arroyo, E.M.; Schönenberger, D.; Rajski, M.; Hoogewijs, D.; et al. Tumor necrosis factor stimulates fibroblast growth factor 23 levels in chronic kidney disease and non-renal inflammation. Kidney Int. 2019, 96, 890–905. [Google Scholar] [CrossRef] [PubMed]
- Hamano, T.; Matsui, I.; Mikami, S.; Tomida, K.; Fujii, N.; Imai, E.; Rakugi, H.; Isaka, Y. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J. Am. Soc. Nephrol. 2010, 21, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Kuro-O, M. Klotho and endocrine fibroblast growth factors: markers of chronic kidney disease progression and cardiovascular complications? Nephrol. Dial. Transpl. 2019, 34, 15–21. [Google Scholar] [CrossRef]
- Cianciolo, G.; Capelli, I.; Angelini, M.L.; Valentini, C.; Baraldi, O.; Scolari, M.P.; Stefoni, S. Importance of vascular calcification in kidney transplant recipients. Am. J. Nephrol. 2014, 39, 418–426. [Google Scholar] [CrossRef]
- Van Ballegooijen, A.J.; Cepelis, A.; Visser, M.; Brouwer, I.A.; Van Schoor, N.M.; Beulens, J.W. Joint Association of Low Vitamin D and Vitamin K Status with Blood Pressure and Hypertension. Hypertension 2017, 69, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Lu, C.-L.; Zheng, C.-M.; Chen, R.-M.; Lin, Y.-F.; Liu, W.-C.; Yen, T.-H.; Chen, R.; Lu, K.-C. Correction: Hou et al. Emerging Role of Vitamins D and K in Modulating Uremic Vascular Calcification: The Aspect of Passive Calcification. Nutrients 2019, 11, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ballegooijen, A.J.; Beulens, J.W.J.; Keyzer, C.A.; Navis, G.J.; Berger, S.P.; de Borst, M.H.; Vervloet, M.G.; Bakker, S.J.L. Joint association of vitamins D and K status with long-term outcomes in stable kidney transplant recipients. Nephrol. Dial. Transpl. 2020, 35, 706–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.T.H.; Coche, E.; Goffin, E.; Beguin, C.; Vlassenbroek, A.; Devuyst, O.; Robert, A.; Jadoul, M. Prevalence and determinants of coronary and aortic calcifications assessed by chest CT in renal transplant recipients. Am. J. Nephrol. 2007, 27, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Takahashi, M.; Takeda, S.I.; Inoue, S.; Fujishiro, J.; Hakamata, Y.; Kaneko, T.; Murakami, T.; Takeuchi, K.; Takeyoshi, I.; et al. Mycophenolate mofetil prevents transplant arteriosclerosis by direct inhibition of vascular smooth muscle cell proliferation. Transplantation 2004, 77, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; Gomes-Neto, A.W.; Van Londen, M.; Ziengs, A.L.; Douwes, R.M.; Stam, S.P.; Osté, M.C.J.; Knobbe, T.J.; Hessels, N.R.; Buunk, A.M.; et al. Rationale and design of TransplantLines: A prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018, 8, 24502. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotomayor, C.G.; te Velde-Keyzer, C.A.; de Borst, M.H.; Navis, G.J.; Bakker, S.J.L. Lifestyle, Inflammation, and Vascular Calcification in Kidney Transplant Recipients: Perspectives on Long-Term Outcomes. J. Clin. Med. 2020, 9, 1911. https://doi.org/10.3390/jcm9061911
Sotomayor CG, te Velde-Keyzer CA, de Borst MH, Navis GJ, Bakker SJL. Lifestyle, Inflammation, and Vascular Calcification in Kidney Transplant Recipients: Perspectives on Long-Term Outcomes. Journal of Clinical Medicine. 2020; 9(6):1911. https://doi.org/10.3390/jcm9061911
Chicago/Turabian StyleSotomayor, Camilo G., Charlotte A. te Velde-Keyzer, Martin H. de Borst, Gerjan J. Navis, and Stephan J.L. Bakker. 2020. "Lifestyle, Inflammation, and Vascular Calcification in Kidney Transplant Recipients: Perspectives on Long-Term Outcomes" Journal of Clinical Medicine 9, no. 6: 1911. https://doi.org/10.3390/jcm9061911
APA StyleSotomayor, C. G., te Velde-Keyzer, C. A., de Borst, M. H., Navis, G. J., & Bakker, S. J. L. (2020). Lifestyle, Inflammation, and Vascular Calcification in Kidney Transplant Recipients: Perspectives on Long-Term Outcomes. Journal of Clinical Medicine, 9(6), 1911. https://doi.org/10.3390/jcm9061911





