Independent Predictive Ability of Procalcitonin of Acute Kidney Injury among Critically Ill Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants Selection
2.2. Measurements
2.3. Quantitative Measurement of Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Basic Characteristics and Clinical Variables of the Two Groups
3.2. The Association among Infection, Acute Kidney Injury and Impaired Renal Function
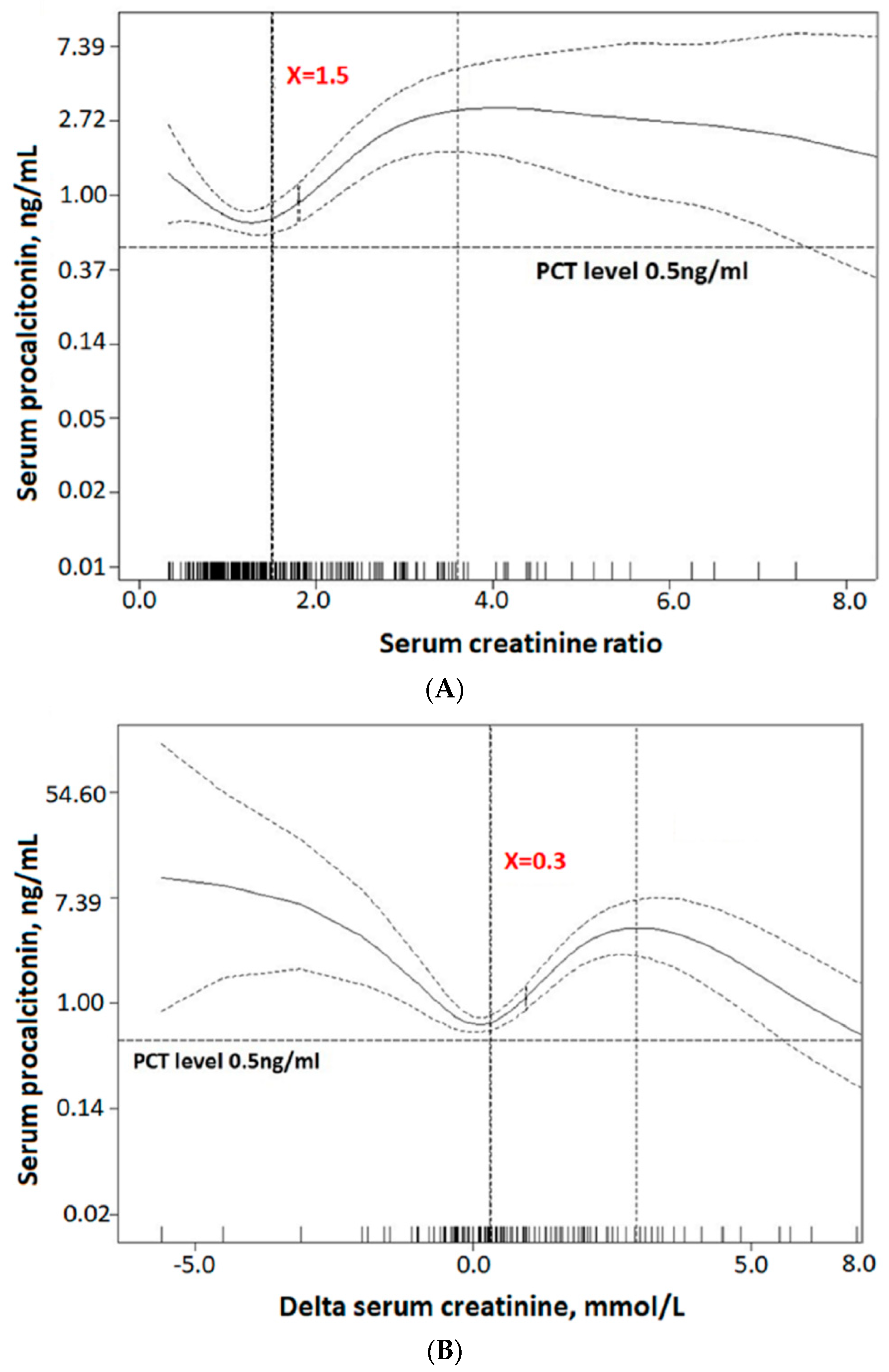
3.3. The Predictive Ability of Serum Procalcitonin for Acute Kidney Injury
4. Discussion
4.1. Influence on Procalcitonin: Infection, Residual Renal Function and Acute Kidney Injury
4.2. Influence on Procalcitonin: Acute Kidney Injury vs. Infection
4.3. Influence on Procalcitonin: Acute Kidney Injury vs. Chronic Kidney Disease
4.4. Serum Procalcitonin as a Predictor for Acute Kidney Injury
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostermann, M.; Joannidis, M. Acute kidney injury 2016: Diagnosis and diagnostic workup. Crit. Care 2016, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Mas-Font, S.; Ros-Martinez, J.; Perez-Calvo, C.; Villa-Diaz, P.; Aldunate-Calvo, S.; Moreno-Clari, E. Prevention of acute kidney injury in Intensive Care Units. Med. Intensiva 2017, 41, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.; Chung, W.; Kim, A.J.; Kim, H.; Ro, H.; Chang, J.H.; Lee, H.H.; Jung, J.Y. Association between acute kidney injury and serum procalcitonin levels and their diagnostic usefulness in critically ill patients. Sci. Rep. 2019, 9, 4777. [Google Scholar] [CrossRef] [PubMed]
- Jeeha, R.; Skinner, D.L.; De Vasconcellos, K.; Magula, N.P. Serum procalcitonin levels predict acute kidney injury in critically ill patients. Nephrology (Carlton) 2018, 23, 1090–1095. [Google Scholar] [CrossRef]
- Pannu, N.; James, M.; Hemmelgarn, B.; Klarenbach, S. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin. J. Am. Soc. Nephrol. 2013, 8, 194–202. [Google Scholar] [CrossRef]
- Druml, W. Systemic consequences of acute kidney injury. Curr. Opin. Crit. Care 2014, 20, 613–619. [Google Scholar] [CrossRef]
- Malhotra, R.; Siew, E.D. Biomarkers for the Early Detection and Prognosis of Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2017, 12, 149–173. [Google Scholar] [CrossRef]
- Kibe, S.; Adams, K.; Barlow, G. Diagnostic and prognostic biomarkers of sepsis in critical care. J. Antimicrob. Chemother. 2011, 66 (Suppl. 2), ii33–ii40. [Google Scholar] [CrossRef]
- Poddar, B.; Gurjar, M.; Singh, S.; Aggarwal, A.; Singh, R.; Azim, A.; Baronia, A. Procalcitonin kinetics as a prognostic marker in severe sepsis/septic shock. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2015, 19, 140–146. [Google Scholar]
- Assicot, M.; Gendrel, D.; Carsin, H.; Raymond, J.; Guilbaud, J.; Bohuon, C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef]
- Prkno, A.; Wacker, C.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—A systematic review and meta-analysis. Crit. Care 2013, 17, R291. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wu, B.; He, Y.; Huang, X.; Dai, Z.; Miao, Q.; Song, H.; Luo, T.; Gao, B.; Wang, L.; et al. Serum procalcitonin predicts development of acute kidney injury in patients with suspected infection. Clin. Chem. Lab. Med. 2013, 51, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lagunas, A.; Jimenez-Alvarez, L.; Ramirez, G.; Mendoza-Milla, C.; Garcia-Sancho, M.C.; Avila-Moreno, F.; Zamudio, P.; Urrea, F.; Ortiz-Quintero, B.; Campos-Toscuento, V.L.; et al. Obesity and pro-inflammatory mediators are associated with acute kidney injury in patients with A/H1N1 influenza and acute respiratory distress syndrome. Exp. Mol. Pathol. 2014, 97, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Reyes, L.F.; Monclou, J.; Suberviola, B.; Bodi, M.; Sirgo, G.; Sole-Violan, J.; Guardiola, J.; Barahona, D.; Diaz, E.; et al. Relationship between acute kidney injury and serum procalcitonin (PCT) concentration in critically ill patients with influenza infection. Med. Intensiva 2018, 42, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Liang, C.X.; Zhang, Y.L.; Hu, W.P. Elevated serum procalcitonin level in patients with chronic kidney disease without infection: A case-control study. J. Clin. Lab. Anal. 2020, 34, e23065. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef]
- Gómez, H.; Kellum, J.A. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 2016, 22, 546–553. [Google Scholar]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Palevsky, P.M.; Liu, K.D.; Brophy, P.D.; Chawla, L.S.; Parikh, C.R.; Thakar, C.V.; Tolwani, A.J.; Waikar, S.S.; Weisbord, S.D. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013, 61, 649–672. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute Dialysis Quality Initiative w: Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Henderson, A.R. Testing experimental data for univariate normality. Clin. Chim. Acta Int. J. Clin. Chem. 2006, 366, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Curran-Everett, D. Explorations in statistics: The log transformation. Adv. Physiol. Educ. 2018, 42, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F., Jr.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 8th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2019. [Google Scholar]
- Vijayan, A.L.; Vanimaya Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R.; Manoj, G. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; White, J.C.; Nylen, E.S.; Snider, R.H.; Becker, K.L.; Habener, J.F. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J. Clin. Endocrinol. Metab. 2001, 86, 396–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, Y.; Murai, A.; Mizunuma, M.; Ohta, D.; Kawano, Y.; Matsumoto, N.; Nishida, T.; Ishikura, H. Potential use of procalcitonin as biomarker for bacterial sepsis in patients with or without acute kidney injury. J. Infect. Chemother. 2015, 21, 257–263. [Google Scholar] [CrossRef]
- Meisner, M.; Lohs, T.; Huettemann, E.; Schmidt, J.; Hueller, M.; Reinhart, K. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur. J. Anaesthesiol. 2001, 18, 79–87. [Google Scholar] [CrossRef]
- Herget-Rosenthal, S.; Klein, T.; Marggraf, G.; Hirsch, T.; Jakob, H.G.; Philipp, T.; Kribben, A. Modulation and source of procalcitonin in reduced renal function and renal replacement therapy. Scand. J. Immunol. 2005, 61, 180–186. [Google Scholar] [CrossRef]
- Lavin-Gomez, B.A.; Palomar-Fontanet, R.; Gago-Fraile, M.; Quintanar-Lartundo, J.A.; Gomez-Palomo, E.; Gonzalez-Lamuno, D.; Garcia-Unzueta, M.T.; Arias-Rodriguez, M.A.; Gomez-Gerique, J.A. Inflammation markers, chronic kidney disease, and renal replacement therapy. Adv. Perit. Dial. 2011, 27, 33–37. [Google Scholar] [PubMed]
- Clementi, A.; Brocca, A.; Virzi, G.M.; de Cal, M.; Giavarina, D.; Carta, M.; Mucino-Bermejo, M.J.; Hinna Danesi, T.; Salvador, L.; Ronco, C. Procalcitonin and Interleukin-6 Levels: Are They Useful Biomarkers in Cardiac Surgery Patients? Blood Purif. 2017, 43, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Nie, X.; Cai, B.; Tang, J.T.; He, Y.; Miao, Q.; Song, H.L.; Luo, T.X.; Gao, B.X.; Wang, L.L.; et al. Procalcitonin levels predict acute kidney injury and prognosis in acute pancreatitis: A prospective study. PLoS ONE 2013, 8, e82250. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Doi, S.Q.; Palant, C.E.; Nylen, E.S.; Becker, K.L. Procalcitonin induced cytotoxicity and apoptosis in mesangial cells: Implications for septic renal injury. Inflamm. Res. 2013, 62, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, G.; Shibata, S.; Fukui, Y.; Okamura, Y.; Inoue, Y. Diagnostic accuracy of procalcitonin and presepsin for infectious disease in patients with acute kidney injury. Diagn. Microbiol. Infect. Dis. 2016, 86, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Itenov, T.S.; Jensen, J.U.; Ostrowski, S.R.; Johansson, P.I.; Thormar, K.M.; Lundgren, J.D.; Bestle, M.H.; “Procalcitonin and Survival Study” Study Group. Endothelial Damage Signals Refractory Acute Kidney Injury in Critically Ill Patients. Shock 2017, 47, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Ba Aqeel, S.H.; Sanchez, A.; Batlle, D. Angiotensinogen as a biomarker of acute kidney injury. Clin. Kidney J. 2017, 10, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Amour, J.; Birenbaum, A.; Langeron, O.; Le Manach, Y.; Bertrand, M.; Coriat, P.; Riou, B.; Bernard, M.; Hausfater, P. Influence of renal dysfunction on the accuracy of procalcitonin for the diagnosis of postoperative infection after vascular surgery. Crit. Care Med. 2008, 36, 1147–1154. [Google Scholar] [CrossRef]
- Heredia-Rodriguez, M.; Bustamante-Munguira, J.; Fierro, I.; Lorenzo, M.; Jorge-Monjas, P.; Gomez-Sanchez, E.; Alvarez, F.J.; Bergese, S.D.; Eiros, J.M.; Bermejo-Martin, J.F.; et al. Procalcitonin cannot be used as a biomarker of infection in heart surgery patients with acute kidney injury. J. Crit. Care 2016, 33, 233–239. [Google Scholar] [CrossRef]


| Total (n = 330) | Non-AKI Group (n = 203) | AKI Group (n = 127) | p-Value | |
|---|---|---|---|---|
| Basic demographic data | ||||
| Age | 70.5 ± 16.4 | 70.5 ± 16.5 | 70.6 ± 16.3 | 0.933 |
| Gender, male | 188 (57.0%) | 119 (58.6%) | 69 (54.3%) | 0.444 |
| Smoker | 72 (21.8%) | 42 (20.7%) | 30 (23.6%) | 0.752 |
| Undertaking oral antibiotics | 19 (5.8%) | 13 (6.4%) | 6 (4.7%) | 0.524 |
| Body mass index | 21.9 ± 5.7 | 22.1 ± 5.5 | 21.5 ± 5.9 | 0.351 |
| Comorbidities | ||||
| Coronal artery disease | 73 (22.1%) | 48 (23.6%) | 25 (19.7%) | 0.399 |
| Congestive heart failure | 47 (14.2%) | 29 (14.3%) | 18 (14.2%) | 0.977 |
| Peripheral artery occlusive disease | 8 (2.4%) | 5 (2.5%) | 3 (2.4%) | 0.954 |
| Cerebral vascular accident | 104 (31.5%) | 61 (30%) | 43 (33.9%) | 0.469 |
| Chronic lung disease | 82 (24.8%) | 51 (25.1%) | 31 (24.4%) | 0.884 |
| Chronic kidney disease | 98 (29.7%) | 56 (27.6%) | 42 (33.1%) | 0.289 |
| Diabetes mellitus | 131(39.7%) | 80 (39.4%) | 51 (40.2%) | 0.892 |
| Cancer | 44 (13.3%) | 24 (11.8%) | 20 (15.7%) | 0.307 |
| Liver cirrhosis | 28 (8.5%) | 16 (7.9%) | 12 (9.4%) | 0.619 |
| Hypertension | 189 (57.3%) | 115 (56.7%) | 74 (58.3%) | 0.773 |
| Charlson’s score, points | 3.8 ± 2.6 | 3.7 ± 2.6 | 3.9 ± 2.6 | 0.460 |
| Culture-proven infection | 173 (52.4%) | 103 (50.7%) | 70 (55.1%) | 0.438 |
| Infection source | ||||
| Pneumonia | 52 (15.8%) | 25 (12.3%) | 27 (21.3%) | 0.030 |
| Urinary tract infection | 65 (19.7%) | 40 (19.7%) | 25 (19.7%) | 0.997 |
| Bloodstream infection | 72 (21.8%) | 38 (18.7%) | 34 (26.8%) | 0.085 |
| Skin infection | 13 (3.9%) | 9 (4.4%) | 4 (3.1%) | 0.560 |
| Other source | 45 (13.6%) | 28 (13.8%) | 17 (13.4%) | 0.916 |
| Clinical variables at ICU admission | ||||
| Body temperature, °C | 36.5 ± 1.2 | 36.6 ± 1.1 | 36.4 ± 1.2 | 0.135 |
| Heart rate, beat/min | 103 ± 24.4 | 101.9 ± 24.9 | 104.9 ± 23.5 | 0.282 |
| Respiratory rate, breath/min | 25.1 ± 9.1 | 24.7 ± 9.5 | 25.6 ± 8.6 | 0.363 |
| Mean arterial pressure, mmHg | 89.0 ± 25.5 | 89.9 ± 25.5 | 87.5 ± 25.6 | 0.415 |
| Glasgow coma scale, points | 10.3 ± 4.5 | 10.1 ± 4.6 | 10.7 ± 4.4 | 0.280 |
| APACHE II, points | 20.8 ± 8.2 | 20.1 ± 8.4 | 21.9 ± 7.9 | 0.051 |
| SOFA score, points | 6.9 ± 3.8 | 6.2 ± 3.8 | 8.1 ± 3.6 | <0.001 |
| With ventilator | 99 (30.0%) | 63 (31.0%) | 36(28.3%) | 0.604 |
| With NIPPV | 88 (26.7%) | 53 (26.1%) | 35(27.6%) | 0.772 |
| With vasopressor | 112 (33.9%) | 61 (30.0%) | 51(40.2%) | 0.059 |
| Underwent CPR | 29 (8.8%) | 17(8.4%) | 12(9.4%) | 0.737 |
| 30-days mortality | 81 (24.5%) | 41 (20.2%) | 40 (31.5%) | 0.020 |
| Total (n = 330) | Non-AKI Group (n = 203) | AKI Group (n = 127) | p-Value | |
|---|---|---|---|---|
| Procalcitonin, ng/mL | 0.8 (0.02, 242.8) | 0.5 (0.02, 242.8) | 2.3 (0.05, 234.6) | <0.001 |
| White blood cell, ×103/mL | 13.5 ± 8.7 | 12.8 ± 7.4 | 14.6 ± 10.4 | 0.057 |
| Neutrophil/ Lymphocyte ratio | 8.3 (0.2, 95.8) | 7.0 (0.2, 95.8) | 10.4 (0.2, 91.8) | 0.068 |
| Hemoglobin, g/dL | 11.1 ± 2.9 | 11.2 ± 3.0 | 10.8 ± 2.8 | 0.321 |
| Platelet, ×103/mL | 216.7 ± 114.0 | 219.8 ± 116.6 | 211.6 ± 110.1 | 0.527 |
| Blood urea nitrogen, mmol/L | 32.2 (5.3, 210.7) | 23.4 (5.3, 210.7) | 54.4 (11.0, 205.0) | <0.001 |
| sCr, mmol/L | 1.5 (0.3, 18.2) | 1.0 (0.3, 15.9) | 2.6 (0.4, 18.2) | <0.001 |
| eGFR, ml/min/1.73 m2 | 44.2 (1.3, 557.8) | 65.9 (1.3, 382.3) | 23.5 (1.8, 557.8) | <0.001 |
| AST, units/L | 32.0 (3.4, 2236.0) | 29.0 (9.0, 2236.0) | 41.0 (3.4, 1027.0) | 0.067 |
| ALT, units/L | 25.0 (1.0, 1891.0) | 21.0 (1.0, 709.0) | 36.0 (3.0, 1891.0) | 0.002 |
| Sodium, mmol/L | 136.8 ± 9.2 | 136.7 ± 8.3 | 137.0 ± 10.6 | 0.806 |
| Potassium, mEq/L | 4.2 ± 1.1 | 4.1 ± 1.0 | 4.4 ± 1.2 | 0.031 |
| Calcium, mEq/L | 8.3 ± 1.0 | 8.3 ± 1.0 | 8.3 ± 1.1 | 0.967 |
| PH | 7.3 ± 0.1 | 7.4 ± 0.1 | 7.3 ± 0.1 | 0.512 |
| HCO3, mEq/L | 19.8 ± 7.8 | 21.2 ± 7.8 | 17.5 ± 7.4 | <0.001 |
| Glucose, mg/dl | 225.1 ± 170.9 | 209.6 ± 132.9 | 249.8 ± 216.7 | 0.062 |
| Albumin, mg/dl | 3.1 ± 0.6 | 3.1 ± 0.6 | 3.0 ± 0.6 | 0.144 |
| Bililubin (total), mg/dl | 0.9 (0.0, 42.5) | 0.9 (0.1, 15.0) | 0.9 (0.0, 42.5) | 0.210 |
| Baseline SCr, mmol/L | 1.0 (0.2, 11.2) | 1.0 (0.2, 11.2) | 1.0 (0.2, 10.6) | 0.056 |
| Delta SCr, mmol/L | 0.9 ± 2.2 | 0.0 ± 0.9 | 2.4 ± 2.7 | <0.001 |
| Ratio of SCr | 1.8 ± 1.6 | 1.0 ± 0.3 | 3.0 ± 2.1 | <0.001 |
| Multivariate Logistic Regression | |||
|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | p-Value | |
| Total cohort (n = 330) | 1.27 | 1.12–1.43 | <0.001 |
| Non-infection group (n = 157) | 1.38 | 1.12–1.71 | 0.003 |
| Infection group (n = 173) | 1.23 | 1.03–1.46 | 0.020 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-T.; Lai, M.-Y.; Kan, W.-C.; Shiao, C.-C. Independent Predictive Ability of Procalcitonin of Acute Kidney Injury among Critically Ill Patients. J. Clin. Med. 2020, 9, 1939. https://doi.org/10.3390/jcm9061939
Huang Y-T, Lai M-Y, Kan W-C, Shiao C-C. Independent Predictive Ability of Procalcitonin of Acute Kidney Injury among Critically Ill Patients. Journal of Clinical Medicine. 2020; 9(6):1939. https://doi.org/10.3390/jcm9061939
Chicago/Turabian StyleHuang, Ya-Ting, Min-Yu Lai, Wei-Chih Kan, and Chih-Chung Shiao. 2020. "Independent Predictive Ability of Procalcitonin of Acute Kidney Injury among Critically Ill Patients" Journal of Clinical Medicine 9, no. 6: 1939. https://doi.org/10.3390/jcm9061939
APA StyleHuang, Y.-T., Lai, M.-Y., Kan, W.-C., & Shiao, C.-C. (2020). Independent Predictive Ability of Procalcitonin of Acute Kidney Injury among Critically Ill Patients. Journal of Clinical Medicine, 9(6), 1939. https://doi.org/10.3390/jcm9061939






