Recognizing Risks and Optimizing Perioperative Care to Reduce Respiratory Complications in the Pediatric Patient
Abstract
1. Introduction
2. Definition
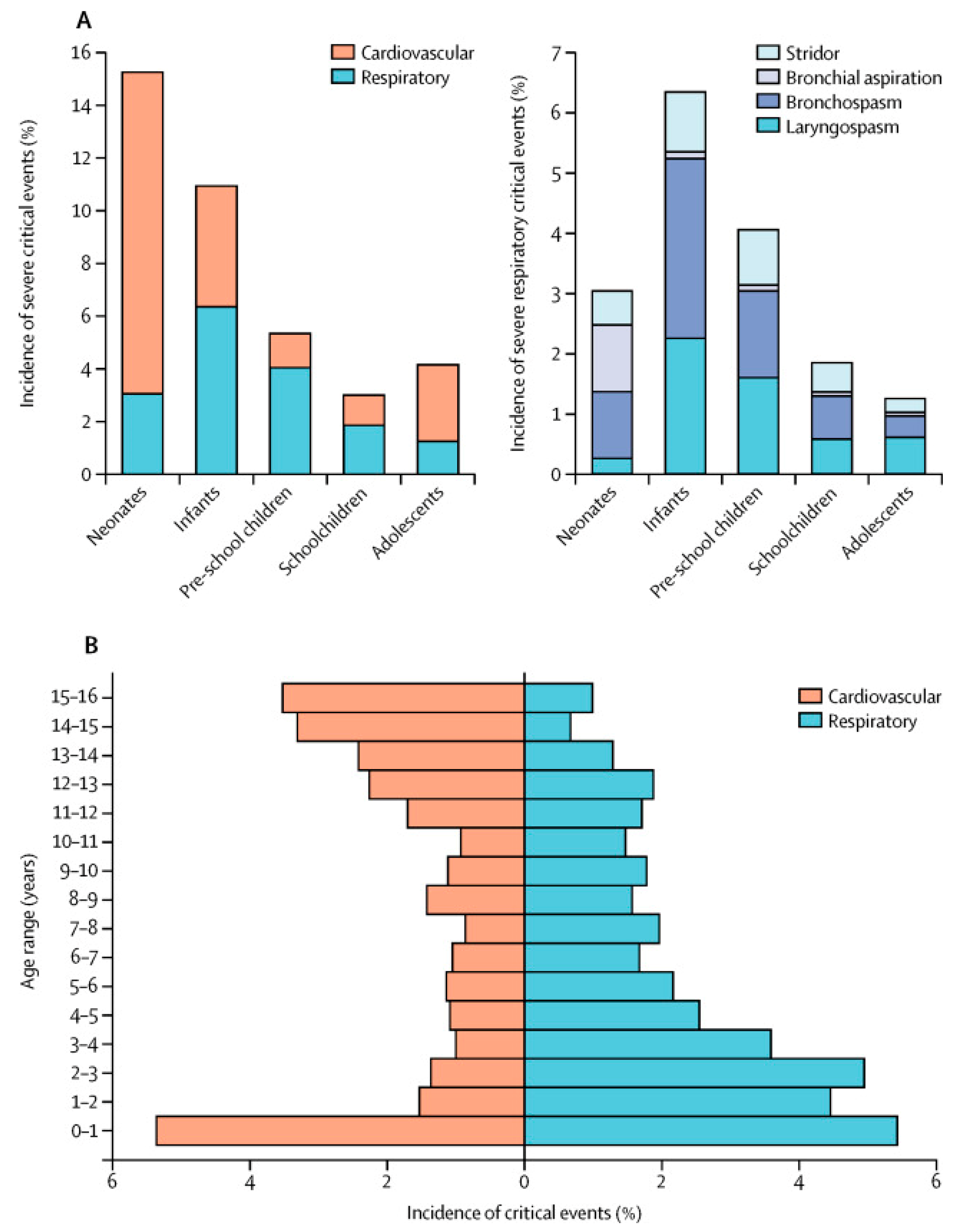
3. Incidence
4. Cost, Morbidity and Mortality
5. Preoperative Risk Assessment and Stratification
5.1. Pediatric Preoperative Risk Prediction Tools
5.1.1. Snoring, Trouble Breathing, and Un-Refreshed (STBUR) Questionnaire
5.1.2. Perioperative Respiratory Adverse Events in Pediatric Ambulatory Anesthesia: Risk Prediction Tool
5.1.3. The COLDS Score
5.2. Risk Factors for Perioperative Respiratory Complications
5.2.1. Non-Modifiable Risk Factors
Age
ASA Status
Type of Surgery
Preoperative Studies
The Pediatric Difficult Airway
5.2.2. Modifiable Risk Factors
Patient Factors
Procedure Factors
6. Postoperative Respiratory Concerns
6.1. Prediction Tool to Determine the Need for and the Duration of Use of Postoperative Oxygen Therapy
6.2. The Assess Respiratory Risk in Surgical Patients in Catalonia Score (ARISCAT)
6.3. GUPTA Risk Calculator Predicting Postoperative Respiratory Failure
6.4. Score for Prediction of Postoperative Respiratory Complications (SPORC)
6.5. Postoperative Pain Management
6.6. Physical Therapy: EARLY Mobilization and Chest Physiotherapy
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABG | arterial blood gases |
| ACS NSQIP | The American College of Surgeons National Surgical Quality Improvement Program |
| AHRQ | the Agency for Healthcare Research Quality |
| APRICOT | Anaesthesia Practice In Children Observational Trial |
| ARDS | acute respiratory distress syndrome |
| ARISCAT | Assess Respiratory Risk in Surgical Patients in Catalonia |
| ASA | American Society of Anesthesiologists |
| ASA-PS | American Society of Anesthesiologists Physical Status |
| BiPAP | bi-level positive airway pressure |
| CDH | congenital diaphragmatic hernia |
| CHD | congenital heart disease |
| CPAP | continuous positive airway pressure |
| CXR | chest x-ray |
| DL | direct laryngoscopy |
| ENT | Ear, Nose, and Throat |
| EPCO | European perioperative clinical outcomes |
| ETT | endotracheal tube |
| FEV1 | forced expiratory volume in 1 second |
| FiO2 | fraction of inspired oxygen |
| FVC | forced vital capacity |
| GA | general anesthesia |
| I COUGH | incentive spirometry, coughing and deep breathing, oral care, understanding, getting out of bed, and head of bed elevation |
| ICU | intensive care unit |
| LC | laparoscopic cholecystectomy |
| LMA | laryngeal mask airway |
| NEAR4kids | National Emergency Airway Registry for Children |
| NMBDs | neuromuscular blocking drugs |
| OC | open cholecystectomy |
| OOR | outside of the operating room |
| OR | odds ratio |
| OR | operating room |
| OSA | obstructive sleep apnea |
| PeDI | pediatric difficult airway |
| PEEP | positive end expiratory pressure |
| PFTs | pulmonary function tests |
| PIP | peak inspiratory pressure |
| POCA | pediatric perioperative cardiac arrest |
| PRAE | perioperative respiratory adverse event |
| PRC | perioperative respiratory complication |
| PROVHILO | protective ventilation using high versus low positive end-expiratory pressure |
| PSG | polysomnography |
| PSI | patient safety indicators |
| RM | recruitment maneuver |
| SAE | serious adverse event |
| SDB | sleep disordered breathing |
| SPORC | Score for Prediction of Postoperative Respiratory Complications |
| SRBD | sleep-related breathing disorder |
| SSI | surgical site infection |
| STUBR | snoring, trouble breathing, and un-refreshed |
| TBI | traumatic brain injury |
| TEF | tracheoesophageal fistula |
| URI | upper respiratory illness |
| WUS | wake-up safe |
References
- Kronman, M.P.; Hall, M.; Slonim, A.D.; Shah, S.S. Charges and lengths of stay attributable to adverse patient-care events using pediatric-specific quality indicators: A multicenter study of freestanding children’s hospitals. Pediatrics 2008, 121, e1653–e1659. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Zhan, C. Pediatric patient safety in hospitals: A national picture in 2000. Pediatrics 2004, 113, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Oofuvong, M.; Geater, A.F.; Chongsuvivatwong, V.; Chanchayanon, T.; Sriyanaluk, B.; Saefung, B.; Nuanjun, K. Excess costs and length of hospital stay attributable to perioperative respiratory events in children. Anesth. Analg. 2015, 120, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.K.; Nafiu, O.O.; Ghaferi, A.; Tremper, K.K.; Shanks, A.; Kheterpal, S. Independent predictors and outcomes of unanticipated early postoperative tracheal intubation after nonemergent, noncardiac surgery. Anesthesiology 2011, 115, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Davenport, D.L.; Henderson, W.G.; Khuri, S.F.; Mentzer, R.M., Jr. Preoperative risk factors and surgical complexity are more predictive of costs than postoperative complications: A case study using the National Surgical Quality Improvement Program (NSQIP) database. Ann. Surg. 2005, 242, 463–468. [Google Scholar] [CrossRef]
- Subramanyam, R.; Yeramaneni, S.; Hossain, M.M.; Anneken, A.M.; Varughese, A.M. Perioperative Respiratory Adverse Events in Pediatric Ambulatory Anesthesia: Development and Validation of a Risk Prediction Tool. Anesth. Analg. 2016, 122, 1578–1585. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Luhmann, S.J.; Furdock, R. Preoperative Variables Associated With Respiratory Complications After Pediatric Neuromuscular Spine Deformity Surgery. Spine Deform. 2019, 7, 107–111. [Google Scholar] [CrossRef]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: A statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol 2015, 32, 88–105. [Google Scholar] [CrossRef]
- Habre, W.; Disma, N.; Virag, K.; Becke, K.; Hansen, T.G.; Johr, M.; Leva, B.; Morton, N.S.; Vermeulen, P.M.; Zielinska, M.; et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): A prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir. Med. 2017, 5, 412–425. [Google Scholar] [CrossRef]
- Schleelein, L.E.; Vincent, A.M.; Jawad, A.F.; Pruitt, E.Y.; Kreher, G.D.; Rehman, M.A.; Goebel, T.K.; Cohen, D.E.; Cook-Sather, S.D. Pediatric perioperative adverse events requiring rapid response: A retrospective case-control study. Paediatr. Anaesth. 2016, 26, 734–741. [Google Scholar] [CrossRef] [PubMed]
- de Graaff, J.C.; Sarfo, M.C.; van Wolfswinkel, L.; van der Werff, D.B.; Schouten, A.N. Anesthesia-related critical incidents in the perioperative period in children; a proposal for an anesthesia-related reporting system for critical incidents in children. Paediatr. Anaesth. 2015, 25, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kurth, C.D.; Tyler, D.; Heitmiller, E.; Tosone, S.R.; Martin, L.; Deshpande, J.K. National pediatric anesthesia safety quality improvement program in the United States. Anesth. Analg. 2014, 119, 112–121. [Google Scholar] [CrossRef] [PubMed]
- von Ungern-Sternberg, B.S.; Boda, K.; Chambers, N.A.; Rebmann, C.; Johnson, C.; Sly, P.D.; Habre, W. Risk assessment for respiratory complications in paediatric anaesthesia: A prospective cohort study. Lancet 2010, 376, 773–783. [Google Scholar] [CrossRef]
- Bhananker, S.M.; Ramamoorthy, C.; Geiduschek, J.M.; Posner, K.L.; Domino, K.B.; Haberkern, C.M.; Campos, J.S.; Morray, J.P. Anesthesia-related cardiac arrest in children: Update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth. Analg. 2007, 105, 344–350. [Google Scholar] [CrossRef]
- Mamie, C.; Habre, W.; Delhumeau, C.; Argiroffo, C.B.; Morabia, A. Incidence and risk factors of perioperative respiratory adverse events in children undergoing elective surgery. Paediatr. Anaesth. 2004, 14, 218–224. [Google Scholar] [CrossRef]
- Murat, I.; Constant, I.; Maud’huy, H. Perioperative anaesthetic morbidity in children: A database of 24,165 anaesthetics over a 30-month period. Paediatr. Anaesth. 2004, 14, 158–166. [Google Scholar] [CrossRef]
- Budic, I.; Simic, D. Risk factors for respiratory adverse events during general anesthesia in children. Med. Biol. 2004, 11521, 118–122. [Google Scholar]
- Tay, C.L.; Tan, G.M.; Ng, S.B. Critical incidents in paediatric anaesthesia: An audit of 10,000 anaesthetics in Singapore. Paediatr. Anaesth. 2001, 11, 711–718. [Google Scholar] [CrossRef]
- Morris, L.G.; Lieberman, S.M.; Reitzen, S.D.; Edelstein, D.R.; Ziff, D.J.; Katz, A.; Komisar, A. Characteristics and outcomes of malpractice claims after tonsillectomy. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2008, 138, 315–320. [Google Scholar] [CrossRef]
- Tait, A.R.; Voepel-Lewis, T.; Christensen, R.; O’Brien, L.M. The STBUR questionnaire for predicting perioperative respiratory adverse events in children at risk for sleep-disordered breathing. Paediatr. Anaesth. 2013, 23, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Bernardo, M.K.L.; Grogan, T.R.; Elashoff, D.A.; Ren, W.H.P. Perioperative respiratory adverse event risk assessment in children with upper respiratory tract infection: Validation of the COLDS score. Paediatr. Anaesth. 2018, 28, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Dones, F.; Foresta, G.; Russotto, V. Update on perioperative management of the child with asthma. Pediatr. Rep. 2012, 4, e19. [Google Scholar] [CrossRef] [PubMed]
- Kneyber, M.C.J.; de Luca, D.; Calderini, E.; Jarreau, P.H.; Javouhey, E.; Lopez-Herce, J.; Hammer, J.; Macrae, D.; Markhorst, D.G.; Medina, A.; et al. Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med. 2017, 43, 1764–1780. [Google Scholar] [CrossRef] [PubMed]
- Habre, W.; Peták, F. Perioperative use of oxygen: Variabilities across age. Br. J. Anaesth. 2014, 113 (Suppl. 2), ii26–ii36. [Google Scholar] [CrossRef]
- Grover, T.R.; Brozanski, B.S.; Barry, J.; Zaniletti, I.; Asselin, J.M.; Durand, D.J.; Short, B.L.; Pallotto, E.K.; Dykes, F.; Reber, K.M.; et al. High surgical burden for infants with severe chronic lung disease (sCLD). J. Pediatric Surg. 2014, 49, 1202–1205. [Google Scholar] [CrossRef]
- von Ungern-Sternberg, B.S.; Boda, K.; Schwab, C.; Sims, C.; Johnson, C.; Habre, W. Laryngeal mask airway is associated with an increased incidence of adverse respiratory events in children with recent upper respiratory tract infections. Anesthesiology 2007, 107, 714–719. [Google Scholar] [CrossRef]
- Rachel Homer, J.; Elwood, T.; Peterson, D.; Rampersad, S. Risk factors for adverse events in children with colds emerging from anesthesia: A logistic regression. Paediatr. Anaesth. 2007, 17, 154–161. [Google Scholar] [CrossRef]
- Tait, A.R.; Malviya, S.; Voepel-Lewis, T.; Munro, H.M.; Siewert, M.; Pandit, U.A. Risk Factors for Perioperative Adverse Respiratory Events in Children with Upper Respiratory Tract Infections. Anesth. J. Am. Soc. Anesth. 2001, 95, 299–306. [Google Scholar]
- Lee, S.; Reddington, E.; Koutsogiannaki, S.; Hernandez, M.R.; Odegard, K.C.; DiNardo, J.A.; Yuki, K. Incidence and Risk Factors for Perioperative Cardiovascular and Respiratory Adverse Events in Pediatric Patients With Congenital Heart Disease Undergoing Noncardiac Procedures. Anesth. Analg. 2018, 127, 724–729. [Google Scholar] [CrossRef]
- Parnis, S.J.; Barker, D.S.; Van Der Walt, J.H. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr. Anaesth. 2001, 11, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, E.T.; Vomvolakis, M.A.; Buck, K.A.; Mannino, S.F.; Sun, L.S. Exposure to environmental tobacco smoke and the risk of adverse respiratory events in children receiving general anesthesia. Anesthesiology 1998, 88, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, R.; Kattan, M.; Godbold, J.; Saltzberg, D.S.; Grimm, K.T.; Landrigan, P.J.; Lilienfeld, D.E. Childhood asthma and passive smoking. Urinary cotinine as a biomarker of exposure. Am. Rev. Respir. Dis. 1992, 145, 594–599. [Google Scholar] [CrossRef]
- Willers, S.; Svenonius, E.; Skarping, G. Passive smoking and childhood asthma. Urinary cotinine levels in children with asthma and in referents. Allergy 1991, 46, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.B.; Morrison, B.J. The effect of cigarette smoke from the mother on bronchial responsiveness and severity of symptoms in children with asthma. J. Allergy Clin. Immunol. 1986, 77, 575–581. [Google Scholar] [CrossRef]
- Mills, E.; Eyawo, O.; Lockhart, I.; Kelly, S.; Wu, P.; Ebbert, J.O. Smoking cessation reduces postoperative complications: A systematic review and meta-analysis. Am. J. Med. 2011, 124, 144–154. [Google Scholar] [CrossRef]
- Wong, J.; Lam, D.P.; Abrishami, A.; Chan, M.T.; Chung, F. Short-term preoperative smoking cessation and postoperative complications: A systematic review and meta-analysis. Can. J. Anaesth. J. Can. D’anesthesie 2012, 59, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Landry, J.; Jones, P.M.; Buhrmann, O.; Morley-Forster, P. Long-term quit rates after a perioperative smoking cessation randomized controlled trial. Anesth. Analg. 2015, 120, 582–587. [Google Scholar] [CrossRef]
- Shi, Y.; Warner, D.O. Surgery as a teachable moment for smoking cessation. Anesthesiology 2010, 112, 102–107. [Google Scholar] [CrossRef]
- McRobbie, H.; Bullen, C.; Hartmann-Boyce, J.; Hajek, P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst. Rev. 2014, Cd010216. [Google Scholar] [CrossRef]
- Miech, R.A.; Johnston, L.D.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E.; Patrick, M.E. Monitoring the Future National Survey Results on Drug Use, 1975–2017. Volume I, Secondary School Students. Inst. Soc. Res. 2018, 110–300. [Google Scholar] [CrossRef]
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.W.; Navon, L.; Hoots, B.; Salvatore, P.P.; Elderbrook, M.; et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin-Final Report. N. Engl. J. Med. 2020, 382, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef]
- Cook-Sather, S.D.; Gallagher, P.R.; Kruge, L.E.; Beus, J.M.; Ciampa, B.P.; Welch, K.C.; Shah-Hosseini, S.; Choi, J.S.; Pachikara, R.; Minger, K.; et al. Overweight/obesity and gastric fluid characteristics in pediatric day surgery: Implications for fasting guidelines and pulmonary aspiration risk. Anesth. Analg. 2009, 109, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Nafiu, O.O.; Curcio, C. Simplified table to identify overweight and obese children undergoing anesthesia. Paediatr. Anaesth. 2013, 23, 964–966. [Google Scholar] [CrossRef]
- Mortensen, A.; Lenz, K.; Abildstrøm, H.; Lauritsen, T.L. Anesthetizing the obese child. Paediatr. Anaesth. 2011, 21, 623–629. [Google Scholar] [CrossRef]
- Bandla, P.; Brooks, L.J.; Trimarchi, T.; Helfaer, M. Obstructive sleep apnea syndrome in children. Anesth. Clin. North Am. 2005, 23, 535–549. [Google Scholar] [CrossRef]
- Sorof, J.M.; Turner, J.; Martin, D.S.; Garcia, K.; Garami, Z.; Alexandrov, A.V.; Wan, F.; Portman, R.J. Cardiovascular risk factors and sequelae in hypertensive children identified by referral versus school-based screening. Hypertension 2004, 43, 214–218. [Google Scholar] [CrossRef]
- Hanevold, C.; Waller, J.; Daniels, S.; Portman, R.; Sorof, J. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: A collaborative study of the International Pediatric Hypertension Association. Pediatrics 2004, 113, 328–333. [Google Scholar] [CrossRef]
- Mannino, D.M.; Mott, J.; Ferdinands, J.M.; Camargo, C.A.; Friedman, M.; Greves, H.M.; Redd, S.C. Boys with high body masses have an increased risk of developing asthma: Findings from the National Longitudinal Survey of Youth (NLSY). Int. J. Obes. (Lond) 2006, 30, 6–13. [Google Scholar] [CrossRef]
- Rosen, C.L.; Wang, R.; Taylor, H.G.; Marcus, C.L.; Katz, E.S.; Paruthi, S.; Arens, R.; Muzumdar, H.; Garetz, S.L.; Mitchell, R.B.; et al. Utility of symptoms to predict treatment outcomes in obstructive sleep apnea syndrome. Pediatrics 2015, 135, e662–e671. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.E.; Holguin, F.; Sood, A.; Salome, C.M.; Pratley, R.E.; Beuther, D.A.; Celedón, J.C.; Shore, S.A. An official American Thoracic Society Workshop report: Obesity and asthma. Proc. Am. Thorac. Soc. 2010, 7, 325–335. [Google Scholar] [CrossRef] [PubMed]
- El-Metainy, S.; Ghoneim, T.; Aridae, E.; Abdel Wahab, M. Incidence of perioperative adverse events in obese children undergoing elective general surgery. Br. J. Anaesth. 2011, 106, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Arens, R.; Muzumdar, H. Childhood obesity and obstructive sleep apnea syndrome. J. Appl. Physiol. (1985) 2010, 108, 436–444. [Google Scholar] [CrossRef]
- Nafiu, O.O.; Reynolds, P.I.; Bamgbade, O.A.; Tremper, K.K.; Welch, K.; Kasa-Vubu, J.Z. Childhood body mass index and perioperative complications. Paediatr. Anaesth. 2007, 17, 426–430. [Google Scholar] [CrossRef]
- Goldstein, D.J. Beneficial health effects of modest weight loss. Int. J. Obes. Relat. Metab Disord. 1992, 16, 397–415. [Google Scholar]
- Alzahrani, A.; Othman, N.; Bin-Ali, T.; Elfaraidi, H.; Al Mussaed, E.; Alabbas, F.; Sedick, Q.; Albatniji, F.; Alshahrani, Z.; Asiri, M.; et al. Routine Preoperative Coagulation Tests in Children Undergoing Elective Surgery or Invasive Procedures: Are They Still Necessary? Clin. Med. Insights Blood Disord. 2019, 12, 1179545x18821158. [Google Scholar] [CrossRef]
- Almesbah, F.; Mandiwanza, T.; Kaliaperumal, C.; Caird, J.; Crimmins, D. Routine preoperative blood testing in pediatric neurosurgery. J. Neurosurg. Pediatrics 2013, 12, 615–621. [Google Scholar] [CrossRef]
- Nieto, R.M.; De Leon, L.E.; Diaz, D.T.; Krauklis, K.A.; Fraser, C.D., Jr. Routine preoperative laboratory testing in elective pediatric cardiothoracic surgery is largely unnecessary. J. Thoracic Cardiovasc. Surg. 2017, 153, 678–685. [Google Scholar] [CrossRef]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef]
- O’Connor, M.E.; Drasner, K. Preoperative laboratory testing of children undergoing elective surgery. Anesth. Analg. 1990, 70, 176–180. [Google Scholar] [PubMed]
- Faraoni, D.; DiNardo, J.A.; Goobie, S.M. Relationship Between Preoperative Anemia and In-Hospital Mortality in Children Undergoing Noncardiac Surgery. Anesth. Analg. 2016, 123, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Goobie, S.M.; Faraoni, D.; Zurakowski, D.; DiNardo, J.A. Association of Preoperative Anemia with Postoperative Mortality in Neonates. JAMA Pediatr. 2016, 170, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Kedir, H.; Miller, R.; Syed, F.; Hakim, M.; Walia, H.; Tumin, D.; McKee, C.; Tobias, J.D. Association between anemia and postoperative complications in infants undergoing pyloromyotomy. J. Pediatric Surg. 2019, 54, 2075–2079. [Google Scholar] [CrossRef]
- Mazo, V.; Sabaté, S.; Canet, J.; Gallart, L.; de Abreu, M.G.; Belda, J.; Langeron, O.; Hoeft, A.; Pelosi, P. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014, 121, 219–231. [Google Scholar] [CrossRef]
- McAlister, F.A.; Bertsch, K.; Man, J.; Bradley, J.; Jacka, M. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am. J. Respir. Crit. Care Med. 2005, 171, 514–517. [Google Scholar] [CrossRef]
- Kovacevic, M.; Goranovic, T.; Markic, A.; Jelisavac, M.; Zuric, I.; Tonkovic, D. Usefulness of routine chest X-ray in preoperative evaluation of patients undergoing non-cardiopulmonary surgery: A prospective observational study: 1AP5-5. Eur. J. Anaesthesiol (EJA) 2012, 29, 16. [Google Scholar] [CrossRef]
- Archer, C.; Levy, A.R.; McGregor, M. Value of routine preoperative chest x-rays: A meta-analysis. Can. J. Anaesth. J. Can. D’Anesthesie 1993, 40, 1022–1027. [Google Scholar] [CrossRef]
- Tape TG, M.A. Diagnostic Decision: The Utility of Routine Chest Radiographs. Ann. Intern. Med. 1986, 104, 663–670. [Google Scholar] [CrossRef]
- Kerr, I.H. The preoperative chest X-ray. Br. J. Anaesth. 1974, 46, 558–563. [Google Scholar] [CrossRef][Green Version]
- Burjek, N.E.; Rao, K.E.; Wieser, J.P.; Evans, M.A.; Toaz, E.E.; Balmert, L.C.; Sarwark, J.F.; Jagannathan, N. Preoperative Pulmonary Function Test Results Are Not Associated With Postoperative Intubation in Children Undergoing Posterior Spinal Fusion for Scoliosis: A Retrospective Observational Study. Anesth. Analg. 2019, 129, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.W.; Majumdar, S.R.; McAlister, F.A. Predicting pulmonary complications after nonthoracic surgery: A systematic review of blinded studies. Am. J. Med. 2002, 112, 219–225. [Google Scholar] [CrossRef]
- Paterson, N.; Waterhouse, P. Risk in pediatric anesthesia. Pediatric Anesth. 2011, 21, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Fiadjoe, J.E.; Nishisaki, A.; Jagannathan, N.; Hunyady, A.I.; Greenberg, R.S.; Reynolds, P.I.; Matuszczak, M.E.; Rehman, M.A.; Polaner, D.M.; Szmuk, P.; et al. Airway management complications in children with difficult tracheal intubation from the Pediatric Difficult Intubation (PeDI) registry: A prospective cohort analysis. Lancet Respir. Med. 2016, 4, 37–48. [Google Scholar] [CrossRef]
- Auroy, Y.; Ecoffey, C.; Messiah, A.; Rouvier, B. Relationship between complications of pediatric anesthesia and volume of pediatric anesthetics. Anesth. Analg. 1997, 84, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Arozullah, A.M.; Khuri, S.F.; Henderson, W.G.; Daley, J. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann. Int. Med. 2001, 135, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Guay, J.; Choi, P.; Suresh, S.; Albert, N.; Kopp, S.; Pace, N.L. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2014, 2014, Cd010108. [Google Scholar] [CrossRef]
- Cheng, H.; Clymer, J.W.; Po-Han Chen, B.; Sadeghirad, B.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration is associated with complications: A systematic review and meta-analysis. J. Surg. Res. 2018, 229, 134–144. [Google Scholar] [CrossRef]
- Brooks-Brunn, J.A. Predictors of postoperative pulmonary complications following abdominal surgery. Chest 1997, 111, 564–571. [Google Scholar] [CrossRef]
- McAlister, F.A.; Khan, N.A.; Straus, S.E.; Papaioakim, M.; Fisher, B.W.; Majumdar, S.R.; Gajic, O.; Daniel, M.; Tomlinson, G. Accuracy of the preoperative assessment in predicting pulmonary risk after nonthoracic surgery. Am. J. Respir. Crit. Care Med. 2003, 167, 741–744. [Google Scholar] [CrossRef]
- Grosse-Sundrup, M.; Henneman, J.P.; Sandberg, W.S.; Bateman, B.T.; Uribe, J.V.; Nguyen, N.T.; Ehrenfeld, J.M.; Martinez, E.A.; Kurth, T.; Eikermann, M. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: Prospective propensity score matched cohort study. BMJ 2012, 345, e6329. [Google Scholar] [CrossRef] [PubMed]
- Suy, K.; Morias, K.; Cammu, G.; Hans, P.; van Duijnhoven, W.G.; Heeringa, M.; Demeyer, I. Effective reversal of moderate rocuronium- or vecuronium-induced neuromuscular block with sugammadex, a selective relaxant binding agent. Anesthesiology 2007, 106, 283–288. [Google Scholar] [CrossRef]
- Rex, C.; Bergner, U.A.; Pühringer, F.K. Sugammadex: A selective relaxant-binding agent providing rapid reversal. Curr. Opin. Anaesthesiol 2010, 23, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, R.; Yan, Y.; Fan, L.; Xue, J.; Wang, T. The efficacy and safety of sugammadex for reversing postoperative residual neuromuscular blockade in pediatric patients: A systematic review. Sci. Rep. 2017, 7, 5724. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Meyer, M.J.; Malviya, S.A.; Stanislaus, A.B.; MacDonald, T.; Doran, M.E.; Igumenshcheva, A.; Hoang, A.H.; Eikermann, M. Effects of neostigmine reversal of nondepolarizing neuromuscular blocking agents on postoperative respiratory outcomes: A prospective study. Anesthesiology 2014, 121, 959–968. [Google Scholar] [CrossRef]
- Todd, M.M.; Hindman, B.J. The Implementation of Quantitative Electromyographic Neuromuscular Monitoring in an Academic Anesthesia Department: Follow-Up Observations. Anesth. Analg. 2015, 121, 836–838. [Google Scholar] [CrossRef]
- Brull, S.J.; Kopman, A.F. Current Status of Neuromuscular Reversal and Monitoring: Challenges and Opportunities. Anesthesiology 2017, 126, 173–190. [Google Scholar] [CrossRef]
- Bordet, F.; Allaouchiche, B.; Lansiaux, S.; Combet, S.; Pouyau, A.; Taylor, P.; Bonnard, C.; Chassard, D. Risk factors for airway complications during general anaesthesia in paediatric patients. Paediatr. Anaesth. 2002, 12, 762–769. [Google Scholar] [CrossRef]
- Frazee, R.C.; Roberts, J.W.; Okeson, G.C.; Symmonds, R.E.; Snyder, S.K.; Hendricks, J.C.; Smith, R.W. Open versus laparoscopic cholecystectomy. A comparison of postoperative pulmonary function. Ann. Surg. 1991, 213, 651–653. [Google Scholar] [CrossRef]
- Memon, M.A.; Cooper, N.J.; Memon, B.; Memon, M.I.; Abrams, K.R. Meta-analysis of randomized clinical trials comparing open and laparoscopic inguinal hernia repair. Br. J. Surg. 2003, 90, 1479–1492. [Google Scholar] [CrossRef]
- Winslow, E.R.; Brunt, L.M. Perioperative outcomes of laparoscopic versus open splenectomy: A meta-analysis with an emphasis on complications. Surgery 2003, 134, 647–653. [Google Scholar] [CrossRef]
- Kauffman, J.D.; Snyder, C.W.; Danielson, P.D.; Chandler, N.M. 30-Day Outcomes of Laparoscopic Versus Open Total Proctocolectomy with Ileoanal Anastomosis in Children and Young Adults: A Combined Analysis of the National Surgical Quality Improvement Project Pediatric and Adult Databases. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nafiu, O.O.; Ramachandran, S.K.; Ackwerh, R.; Tremper, K.K.; Campbell, D.A., Jr.; Stanley, J.C. Factors associated with and consequences of unplanned post-operative intubation in elderly vascular and general surgery patients. Eur. J. Anaesthesiol 2011, 28, 220–224. [Google Scholar] [CrossRef]
- Holst, L.B.; Petersen, M.W.; Haase, N.; Perner, A.; Wetterslev, J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: Systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015, 350, h1354. [Google Scholar] [CrossRef]
- Clevenger, B.; Richards, T. Pre-operative anaemia. Anaesthesia 2015, 70 (Suppl. 1), e26–e28. [Google Scholar] [CrossRef]
- Kotzé, A.; Harris, A.; Baker, C.; Iqbal, T.; Lavies, N.; Richards, T.; Ryan, K.; Taylor, C.; Thomas, D. British Committee for Standards in Haematology Guidelines on the Identification and Management of Pre-Operative Anaemia. Br. J. Haematol. 2015, 171, 322–331. [Google Scholar] [CrossRef]
- Wanderer, J.P.; Ehrenfeld, J.M.; Epstein, R.H.; Kor, D.J.; Bartz, R.R.; Fernandez-Bustamante, A.; Vidal Melo, M.F.; Blum, J.M. Temporal trends and current practice patterns for intraoperative ventilation at U.S. academic medical centers: A retrospective study. BMC Anesth. 2015, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.P.; Paganelli, W.C.; Gerety, L.P.; Tharp, W.G.; Shanks, A.M.; Housey, M.; Blank, R.S.; Colquhoun, D.A.; Fernandez-Bustamante, A.; Jameson, L.C.; et al. Intraoperative Lung-Protective Ventilation Trends and Practice Patterns: A Report from the Multicenter Perioperative Outcomes Group. Anesth. Analg. 2015, 121, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.Y.; et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N. Engl. J. Med. 2013, 369, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Ladha, K.; Vidal Melo, M.F.; McLean, D.J.; Wanderer, J.P.; Grabitz, S.D.; Kurth, T.; Eikermann, M. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: Hospital based registry study. BMJ 2015, 351, h3646. [Google Scholar] [CrossRef] [PubMed]
- Severgnini, P.; Selmo, G.; Lanza, C.; Chiesa, A.; Frigerio, A.; Bacuzzi, A.; Dionigi, G.; Novario, R.; Gregoretti, C.; de Abreu, M.G.; et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013, 118, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Serpa Neto, A.; Hemmes, S.N.; Barbas, C.S.; Beiderlinden, M.; Biehl, M.; Binnekade, J.M.; Canet, J.; Fernandez-Bustamante, A.; Futier, E.; Gajic, O.; et al. Protective versus Conventional Ventilation for Surgery: A Systematic Review and Individual Patient Data Meta-analysis. Anesthesiology 2015, 123, 66–78. [Google Scholar] [CrossRef]
- Hemmes, S.N.; Gama de Abreu, M.; Pelosi, P.; Schultz, M.J. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): A multicentre randomised controlled trial. Lancet (London, UK) 2014, 384, 495–503. [Google Scholar] [CrossRef]
- Bohm, S.H.; Thamm, O.C.; von Sandersleben, A.; Bangert, K.; Langwieler, T.E.; Tusman, G.; Strate, T.G.; Standl, T.G. Alveolar recruitment strategy and high positive end-expiratory pressure levels do not affect hemodynamics in morbidly obese intravascular volume-loaded patients. Anesth. Analg. 2009, 109, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.A.; McCormick, P.J.; Lin, H.M.; Hosseinian, L.; Fischer, G.W. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br. J. Anaesth. 2014, 113, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Sivan, Y.; Deakers, T.W.; Newth, C.J. Effect of positive end-expiratory pressure on respiratory compliance in children with acute respiratory failure. Pediatr. Pulmonol. 1991, 11, 103–107. [Google Scholar] [CrossRef]
- Giffin, F.; Greenough, A. Effect of positive end expiratory pressure and mean airway pressure on respiratory compliance and gas exchange in children with liver disease. Eur. J. Pediatr. 1994, 153, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Paulson, T.E.; Spear, R.M.; Silva, P.D.; Peterson, B.M. High-frequency pressure-control ventilation with high positive end-expiratory pressure in children with acute respiratory distress syndrome. J. Pediatr. 1996, 129, 566–573. [Google Scholar] [CrossRef]
- Tusman, G.; Böhm, S.H.; Tempra, A.; Melkun, F.; García, E.; Turchetto, E.; Mulder, P.G.; Lachmann, B. Effects of recruitment maneuver on atelectasis in anesthetized children. Anesthesiology 2003, 98, 14–22. [Google Scholar] [CrossRef]
- von Ungern-Sternberg, B.S.; Regli, A.; Schibler, A.; Hammer, J.; Frei, F.J.; Erb, T.O. The impact of positive end-expiratory pressure on functional residual capacity and ventilation homogeneity impairment in anesthetized children exposed to high levels of inspired oxygen. Anesth. Analg. 2007, 104, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Khemani, R.G.; Markovitz, B.P.; Curley, M.A.Q. Characteristics of children intubated and mechanically ventilated in 16 PICUs. Chest 2009, 136, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Pulitanò, S.; Mancino, A.; Pietrini, D.; Piastra, M.; De Rosa, S.; Tosi, F.; De Luca, D.; Conti, G. Effects of positive end expiratory pressure (PEEP) on intracranial and cerebral perfusion pressure in pediatric neurosurgical patients. J. Neurosurg. Anesth. 2013, 25, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Ingaramo, O.A.; Ngo, T.; Khemani, R.G.; Newth, C.J. Impact of positive end-expiratory pressure on cardiac index measured by ultrasound cardiac output monitor*. Pediatr. Crit. Care Med. 2014, 15, 15–20. [Google Scholar] [CrossRef] [PubMed]
- de Jager, P.; Burgerhof, J.G.; van Heerde, M.; Albers, M.J.; Markhorst, D.G.; Kneyber, M.C. Tidal volume and mortality in mechanically ventilated children: A systematic review and meta-analysis of observational studies*. Crit. Care Med. 2014, 42, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Randolph, A.G. Management of acute lung injury and acute respiratory distress syndrome in children. Crit. Care Med. 2009, 37, 2448–2454. [Google Scholar] [CrossRef]
- Cheifetz, I.M. Management of acute lung injury: Sharing data between adults and children. Respir. Care 2011, 56, 1258–1268. [Google Scholar] [CrossRef]
- Kneyber, M.C. Intraoperative mechanical ventilation for the pediatric patient. Best Pract. Res. Clin. Anaesthesiol 2015, 29, 371–379. [Google Scholar] [CrossRef]
- Fernandez-Bustamante, A.; Wood, C.L.; Tran, Z.V.; Moine, P. Intraoperative ventilation: Incidence and risk factors for receiving large tidal volumes during general anesthesia. BMC Anesth. 2011, 11, 22. [Google Scholar] [CrossRef]
- Jaber, S.; Coisel, Y.; Chanques, G.; Futier, E.; Constantin, J.M.; Michelet, P.; Beaussier, M.; Lefrant, J.Y.; Allaouchiche, B.; Capdevila, X.; et al. A multicentre observational study of intra-operative ventilatory management during general anaesthesia: Tidal volumes and relation to body weight. Anaesthesia 2012, 67, 999–1008. [Google Scholar] [CrossRef]
- Lellouche, F.; Dionne, S.; Simard, S.; Bussières, J.; Dagenais, F. High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology 2012, 116, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Hemmes, S.N.; Barbas, C.S.; Beiderlinden, M.; Fernandez-Bustamante, A.; Futier, E.; Gajic, O.; El-Tahan, M.R.; Ghamdi, A.A.; Günay, E.; et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: A meta-analysis of individual patient data. Lancet Respir. Med. 2016, 4, 272–280. [Google Scholar] [CrossRef]
- Güldner, A.; Kiss, T.; Serpa Neto, A.; Hemmes, S.N.; Canet, J.; Spieth, P.M.; Rocco, P.R.; Schultz, M.J.; Pelosi, P.; Gama de Abreu, M. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: A comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 2015, 123, 692–713. [Google Scholar] [CrossRef]
- Helmerhorst, H.J.; Roos-Blom, M.J.; van Westerloo, D.J.; de Jonge, E. Association Between Arterial Hyperoxia and Outcome in Subsets of Critical Illness: A Systematic Review, Meta-Analysis, and Meta-Regression of Cohort Studies. Crit. Care Med. 2015, 43, 1508–1519. [Google Scholar] [CrossRef]
- Damiani, E.; Adrario, E.; Girardis, M.; Romano, R.; Pelaia, P.; Singer, M.; Donati, A. Arterial hyperoxia and mortality in critically ill patients: A systematic review and meta-analysis. Crit. Care 2014, 18, 711. [Google Scholar] [CrossRef]
- Rachmale, S.; Li, G.; Wilson, G.; Malinchoc, M.; Gajic, O. Practice of excessive F(IO(2)) and effect on pulmonary outcomes in mechanically ventilated patients with acute lung injury. Respir. Care 2012, 57, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Bar-Yishay, E.; Prais, D.; Klinger, G.; Mei-Zahav, M.; Mussaffi, H.; Steuer, G.; Hananya, S.; Matyashuk, Y.; Gabarra, N.; et al. Encouraging pulmonary outcome for surviving, neurologically intact, extremely premature infants in the postsurfactant era. Chest 2012, 142, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Rowland, R.; Newman, C.G. Pulmonary complications of oxygen therapy. J. Clin. Pathol. 1969, 22, 192–198. [Google Scholar] [CrossRef]
- Cohen, M.M.; Cameron, C.B.; Duncan, P.G. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth. Analg. 1990, 70, 160–167. [Google Scholar] [CrossRef]
- Gollin, G.; Bell, C.; Dubose, R.; Touloukian, R.J.; Seashore, J.H.; Hughes, C.W.; Oh, T.H.; Fleming, J.; O’Connor, T. Predictors of postoperative respiratory complications in premature infants after inguinal herniorrhaphy. J. Pediatric Surg. 1993, 28, 244–247. [Google Scholar] [CrossRef]
- Steward, D.J. Preterm infants are more prone to complications following minor surgery than are term infants. Anesthesiology 1982, 56, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Gregory, G. Life-threatening perioperative apnea in the ex- “premie”. Anesthesiology 1986, 65, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Kurth, C.D.; Spitzer, A.R.; Broennle, A.M.; Downes, J.J. Postoperative apnea in preterm infants. Anesthesiology 1987, 66, 483–488. [Google Scholar] [CrossRef]
- Tiret, L.; Nivoche, Y.; Hatton, F.; Desmonts, J.M.; Vourc’h, G. Complications related to anaesthesia in infants and children. A prospective survey of 40240 anaesthetics. Br. J. Anaesth. 1988, 61, 263–269. [Google Scholar] [CrossRef]
- Aplin, S.; Baines, D.; De Lima, J. Use of the ASA Physical Status Grading System in pediatric practice. Paediatr. Anaesth. 2007, 17, 216–222. [Google Scholar] [CrossRef]
- Abouleish, A.E.; Leib, M.L.; Cohen, N.H. ASA Provides Examples to Each ASA Physical Status Class. ASA Newsl. 2015, 79, 38–49. [Google Scholar]
- Hurwitz, E.E.; Simon, M.; Vinta, S.R.; Zehm, C.F.; Shabot, S.M.; Minhajuddin, A.; Abouleish, A.E. Adding Examples to the ASA-Physical Status Classification Improves Correct Assignment to Patients. Anesthesiology 2017, 126, 614–622. [Google Scholar] [CrossRef]
- Jimenez, N.; Posner, K.L.; Cheney, F.W.; Caplan, R.A.; Lee, L.A.; Domino, K.B. An update on pediatric anesthesia liability: A closed claims analysis. Anesth. Analg. 2007, 104, 147–153. [Google Scholar] [CrossRef]
- Della Torre, V.; Badenes, R.; Corradi, F.; Racca, F.; Lavinio, A.; Matta, B.; Bilotta, F.; Robba, C. Acute respiratory distress syndrome in traumatic brain injury: How do we manage it? J. Thorac. Dis. 2017, 9, 5368–5381. [Google Scholar] [CrossRef]
- Young, N.; Rhodes, J.K.; Mascia, L.; Andrews, P.J. Ventilatory strategies for patients with acute brain injury. Curr. Opin. Crit. Care 2010, 16, 45–52. [Google Scholar] [CrossRef]
- Shweikeh, F.; Foulad, D.; Nuño, M.; Drazin, D.; Adamo, M.A. Differences in surgical outcomes for patients with craniosynostosis in the US: Impact of socioeconomic variables and race. J. Neurosurg. Pediatrics 2016, 17, 27–33. [Google Scholar] [CrossRef]
- Patria, M.F.; Ghislanzoni, S.; Macchini, F.; Lelii, M.; Mori, A.; Leva, E.; Principi, N.; Esposito, S. Respiratory Morbidity in Children with Repaired Congenital Esophageal Atresia with or without Tracheoesophageal Fistula. Int. J. Environ. Res. Public Health 2017, 14, 1136. [Google Scholar] [CrossRef] [PubMed]
- Friedmacher, F.; Kroneis, B.; Huber-Zeyringer, A.; Schober, P.; Till, H.; Sauer, H.; Höllwarth, M.E. Postoperative Complications and Functional Outcome after Esophageal Atresia Repair: Results from Longitudinal Single-Center Follow-Up. J. Gastrointest Surg. 2017, 21, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, P.K.; Rawat, M.; Madappa, R.; Rothstein, D.H.; Lakshminrusimha, S. Congenital Diaphragmatic hernia-a review. Matern. Health Neonatol. Perinatol. 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Corey, K.M.; Hornik, C.P.; Laughon, M.M.; McHutchison, K.; Clark, R.H.; Smith, P.B. Frequency of anomalies and hospital outcomes in infants with gastroschisis and omphalocele. Early Hum. Dev. 2014, 90, 421–424. [Google Scholar] [CrossRef]
- Islam, S. Advances in surgery for abdominal wall defects: Gastroschisis and omphalocele. Clin. Perinatol. 2012, 39, 375–386. [Google Scholar] [CrossRef]
- Bakhsheshian, J.; Jin, D.L.; Chang, K.E.; Strickland, B.A.; Donoho, D.A.; Cen, S.; Mack, W.J.; Attenello, F.; Christian, E.A.; Zada, G. Risk factors associated with the surgical management of craniopharyngiomas in pediatric patients: Analysis of 1961 patients from a national registry database. Neurosurg. Focus 2016, 41, E8. [Google Scholar] [CrossRef]
- Aleksic, V.; Radulovic, D.; Milakovic, B.; Nagulic, M.; Vucovic, D.; Antunovic, V.; Djordjevic, M. A retrospective analysis of anesthesiologic complications in pediatric neurosurgery. Paediatr. Anaesth. 2009, 19, 879–886. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.N.; Wang, Y.S.; Liu, Y.Z.; Hai, Y. Risk factors for pulmonary complications after posterior spinal instrumentation and fusion in the treatment of congenital scoliosis: A case-control study. BMC Musculoskelet Disord 2019, 20, 331. [Google Scholar] [CrossRef]
- Raval, M.V.; Dillon, P.W.; Bruny, J.L.; Ko, C.Y.; Hall, B.L.; Moss, R.L.; Oldham, K.T.; Richards, K.E.; Vinocur, C.D.; Ziegler, M.M. American College of Surgeons National Surgical Quality Improvement Program Pediatric: A phase 1 report. J. Am. Coll Surg. 2011, 212, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nafiu, O.O.; Burke, C.C.; Chimbira, W.T.; Ackwerh, R.; Reynolds, P.I.; Malviya, S. Prevalence of habitual snoring in children and occurrence of peri-operative adverse events. Eur. J. Anaesthesiol 2011, 28, 340–345. [Google Scholar] [CrossRef] [PubMed]
- von Ungern-Sternberg, B.S.; Habre, W.; Erb, T.O.; Heaney, M. Salbutamol premedication in children with a recent respiratory tract infection. Paediatr. Anaesth. 2009, 19, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Scalfaro, P.; Sly, P.D.; Sims, C.; Habre, W. Salbutamol prevents the increase of respiratory resistance caused by tracheal intubation during sevoflurane anesthesia in asthmatic children. Anesth. Analg. 2001, 93, 898–902. [Google Scholar] [CrossRef]
- von Ungern-Sternberg, B.S.; Sommerfield, D.; Slevin, L.; Drake-Brockman, T.F.E.; Zhang, G.; Hall, G.L. Effect of Albuterol Premedication vs Placebo on the Occurrence of Respiratory Adverse Events in Children Undergoing Tonsillectomies: The REACT Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 527–533. [Google Scholar] [CrossRef]
- Patino, M.; Sadhasivam, S.; Mahmoud, M. Obstructive sleep apnoea in children: Perioperative considerations. Br. J. Anaesth. 2013, 111 (Suppl. 1), i83–i95. [Google Scholar] [CrossRef]
- Valley, R.D.; Freid, E.B.; Bailey, A.G.; Kopp, V.J.; Georges, L.S.; Fletcher, J.; Keifer, A. Tracheal extubation of deeply anesthetized pediatric patients: A comparison of desflurane and sevoflurane. Anesth. Analg. 2003, 96, 1320–1324. [Google Scholar] [CrossRef]
- Patel, R.I.; Hannallah, R.S.; Norden, J.; Casey, W.F.; Verghese, S.T. Emergence airway complications in children: A comparison of tracheal extubation in awake and deeply anesthetized patients. Anesth. Analg. 1991, 73, 266–270. [Google Scholar] [CrossRef]
- Šešlija, N.; Janković, I.; Rosić, R.; Milenković, A. The importance of preoperative assessment and children preparation in determination and reduction of anesthetic risks. Anesth. Iugosl. 1996, 21, 155–160. [Google Scholar]
- Dimick, J.B.; Chen, S.L.; Taheri, P.A.; Henderson, W.G.; Khuri, S.F.; Campbell, D.A., Jr. Hospital costs associated with surgical complications: A report from the private-sector National Surgical Quality Improvement Program. J. Am. Coll Surg. 2004, 199, 531–537. [Google Scholar] [CrossRef]
- Johnson, R.G.; Arozullah, A.M.; Neumayer, L.; Henderson, W.G.; Hosokawa, P.; Khuri, S.F. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: Results from the patient safety in surgery study. J. Am. Coll Surg. 2007, 204, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Oofuvong, M.; Ratprasert, S.; Chanchayanon, T. Risk prediction tool for use and predictors of duration of postoperative oxygen therapy in children undergoing non-cardiac surgery: A case-control study. BMC Anesth. 2018, 18, 137. [Google Scholar] [CrossRef]
- Gupta, H.; Gupta, P.K.; Fang, X.; Miller, W.J.; Cemaj, S.; Forse, R.A.; Morrow, L.E. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest 2011, 140, 1207–1215. [Google Scholar] [CrossRef]
- Brueckmann, B.; Villa-Uribe, J.L.; Bateman, B.T.; Grosse-Sundrup, M.; Hess, D.R.; Schlett, C.L.; Eikermann, M. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology 2013, 118, 1276–1285. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Haider, N.; Saran, K.A.; Mathis, M.; Kim, J.; Morris, M.; O’Reilly, M. Life-threatening critical respiratory events: A retrospective study of postoperative patients found unresponsive during analgesic therapy. J. Clin. Anesth 2011, 23, 207–213. [Google Scholar] [CrossRef]
- Taylor, S.; Kirton, O.C.; Staff, I.; Kozol, R.A. Postoperative day one: A high risk period for respiratory events. Am. J. Surg. 2005, 190, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Verghese, S.T.; Hannallah, R.S. Acute pain management in children. J. Pain. Res. 2010, 3, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Chertin, B.; Zeldin, A.; Kocherov, S.; Ioscovich, A.; Ostrovsky, I.A.; Gozal, Y. Use of Caudal Analgesia Supplemented with Low Dose of Morphine in Children Who Undergo Renal Surgery. Curr. Urol. 2016, 9, 132–137. [Google Scholar] [CrossRef][Green Version]
- van Lier, F.; van der Geest, P.J.; Hoeks, S.E.; van Gestel, Y.R.; Hol, J.W.; Sin, D.D.; Stolker, R.J.; Poldermans, D. Epidural analgesia is associated with improved health outcomes of surgical patients with chronic obstructive pulmonary disease. Anesthesiology 2011, 115, 315–321. [Google Scholar] [CrossRef]
- Chidambaran, V.; Tewari, A.; Mahmoud, M. Anesthetic and pharmacologic considerations in perioperative care of obese children. J. Clin. Anesth. 2018, 45, 39–50. [Google Scholar] [CrossRef]
- Mahmoud, M.; Mason, K.P. Dexmedetomidine: Review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br. J. Anaesth. 2015, 115, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, L.E.; Murphy, P.B.; Hart, N. Respiratory management of the obese patient undergoing surgery. J. Thorac. Dis. 2015, 7, 943–952. [Google Scholar] [CrossRef]
- Branson, R.D. The scientific basis for postoperative respiratory care. Respir. Care 2013, 58, 1974–1984. [Google Scholar] [CrossRef]
- Cuello-Garcia, C.A.; Mai, S.H.C.; Simpson, R.; Al-Harbi, S.; Choong, K. Early Mobilization in Critically Ill Children: A Systematic Review. J. Pediatr. 2018, 203, 25–33.e26. [Google Scholar] [CrossRef]
- Choong, K.; Awladthani, S.; Khawaji, A.; Clark, H.; Borhan, A.; Cheng, J.; Laskey, S.; Neu, C.; Sarti, A.; Thabane, L.; et al. Early Exercise in Critically Ill Youth and Children, a Preliminary Evaluation: The wEECYCLE Pilot Trial. Pediatr. Crit. Care Med. 2017, 18, e546–e554. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, M.R.; Rosenkranz, P.; McCabe, K.; Rosen, J.E.; McAneny, D. I COUGH: Reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013, 148, 740–745. [Google Scholar] [CrossRef] [PubMed]
| STUDY | YEAR | DESIGN | PRC(s) Described | Sample Size | PRC c Incidence n (%) | Surgical Specialty |
|---|---|---|---|---|---|---|
| Habre et al. [10]. (APRICOT a Study) | 2017 | Prospective, Multi-Center Cohort | PRCs c
| 31127 | Severe Critical Events 1637 (5.3) PRCs c 976 (3.1)
**Most of the Severe Critical Events were PRCs c 59.6% | Multi-Specialty, Elective Surgery Only, Ambulatory Surgery Only |
| Schleelein et al. [11] | 2016 | Retrospective, Case-Control | PRCs c
| 55070 | Anesthesia Now! 213 (0.4) PRCs c: 143 (0.26)
**10.8% of the Anesthesia Now! Events were Cardiac Events. | Multi-specialty, Elective and Emergency |
| Subramanyam et al. [6] | 2016 | Prospectively Collected Data, Retrospective Cohort Analysis, Single Center | PRCs c Intraoperative
| 19059 | 520 (2.8) Intraoperative
| Multi-Specialty, Elective Surgery Only, Ambulatory Surgery Only |
| de Graaff et al. [12] | 2014 | Retrospective Single-Center Cohort | 4 out of 20-Item Complication List were PRCs c:
| 35190 | 1195 Critical Incidents (3.4) 564 (46.5) Respiratory Complications
| Multi-Specialty, Elective, and Emergency |
| Kurth et al. [13] (Wake-Up Safe) | 2013 | Prospective, Multi-Center Cohort | Respiratory Events | 736365 | Serious Adverse Events 740 (0.1) Respiratory Events: 254 (0.03) *Most of the SAE’s d (34%) were Respiratory Events (PRCs c) | Multi-Specialty, Elective and Emergency |
| Von Ungern-Sternberg et al. [14] | 2010 | Prospective, Single-Center Cohort | PRCs c
| 9297 | 1392 (15)
| Otolaryngology, Elective and Emergency |
| Bhananker et al. [15] (POCA b Study) | 2007 | Prospective, Multi-Center Cohort | Perioperative Cardiac Arrests between 1998–2004 PRCs c
| 193 | PRCs c 53 (27)
| Multi-specialty, Elective and Emergency |
| Mamie et al. [16] | 2004 | Prospective, single Center Cohort | PRCs c
| 757 | PRCs c 211 (27.9)
| Multi-Specialty, Elective Surgery Only |
| Murat et al. [17] | 2004 | Prospective, Single Center Cohort | PRCsc
| 23043 | *Most of the intraoperative adverse events (53%) were respiratory events (PRCsc)
| Multi-specialty, Elective and Emergency surgery |
| Budic et al. [18] | 2004 | Prospective, Single Center Cohort | *Adverse Respiratory Events were Defined as any Episode of Perioperative Airway Obstruction (e.g., Laryngospasm), Oxygen Desaturation Less than 90% (for ≥ 10 s), Breath Holding (≥ 15 s), Severe Coughing, and Any Requirement for Unanticipated Endotracheal Intubation. PRCs c
| 682 | PRCs c 39 (5.71)
| Multi-Specialty, Elective and Emergency Surgery |
| Tay et al. [19] | 2001 | Prospective, Single Center Cohort | PRCs c
| 10000 | 297 Damaging Events Described *Most of the Damaging Events (77%) were Respiratory Events (PRCs c) PRCsc 230 (77.44)
| Multi-Specialty, Elective and Emergency Surgery |
| PATIENT FACTORS | PROCEDURE FACTORS |
|---|---|
| Modifiable | Modifiable |
Lung Disease:
Smoking/Passive Smoking/Vaping [10,29,31,32,33,34,35,36,37,38,39,40,41,42,43] Obesity [6,11,44,45,46,47,48,49,50,51,52,53,54,55,56] Laboratory/Clinical Test Findings [57,58,59] | Pediatric Anesthesiologist versus General [10,16,19,73,74,75] GA f Versus Regional [10,14,60,76,77] Duration of Procedure (Long versus Short ) [10,11,78,79,80] Re-Operation [11] Complexity of Procedure (Simple versus Complex) [11] Location (OR i versus Remote Location) [6,11] Long-Acting NMBDs h [7,81] Sugammadex [81,82,83,84,85,86,87] Supraglottic Airway Versus Endotracheal Airway [14,18,88] Open Versus Laparoscopic Abdominal Surgery [89,90,91,92] Intraoperative Blood Product Transfusion [76,93,94,95,96] Mechanical Ventilation Strategy [7,24,25,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129] |
| Non-Modifiable | Non-Modifiable |
| Age (Preemies, Infants, Young Children) [6,11,14,16,17,18,19,21,73,130,131,132,133,134] ASA a III or higher [7,10,12,17,19,130,135,136,137,138,139] Difficult Airway (NEAR4Kids g) [74] Head Injury/TBI l [140,141] Craniofacial Anomalies [142] | Type of Surgery |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egbuta, C.; Mason, K.P. Recognizing Risks and Optimizing Perioperative Care to Reduce Respiratory Complications in the Pediatric Patient. J. Clin. Med. 2020, 9, 1942. https://doi.org/10.3390/jcm9061942
Egbuta C, Mason KP. Recognizing Risks and Optimizing Perioperative Care to Reduce Respiratory Complications in the Pediatric Patient. Journal of Clinical Medicine. 2020; 9(6):1942. https://doi.org/10.3390/jcm9061942
Chicago/Turabian StyleEgbuta, Chinyere, and Keira P. Mason. 2020. "Recognizing Risks and Optimizing Perioperative Care to Reduce Respiratory Complications in the Pediatric Patient" Journal of Clinical Medicine 9, no. 6: 1942. https://doi.org/10.3390/jcm9061942
APA StyleEgbuta, C., & Mason, K. P. (2020). Recognizing Risks and Optimizing Perioperative Care to Reduce Respiratory Complications in the Pediatric Patient. Journal of Clinical Medicine, 9(6), 1942. https://doi.org/10.3390/jcm9061942





