Should Lung-Sparing Surgery Be the Standard Procedure for Malignant Pleural Mesothelioma?
Abstract
1. Introduction
- EPP: en bloc resection of the visceral and parietal pleura, lung, ipsilateral hemidiaphragm and pericardium.
- P/D: parietal and visceral pleurectomy.
- Extended P/D: parietal and visceral pleurectomy with resection of diaphragm and pericardium.
2. Methods
2.1. Search Strategy and Article Selection
2.2. Statistical Analyses
3. Results
3.1. Extended PD vs. EPP
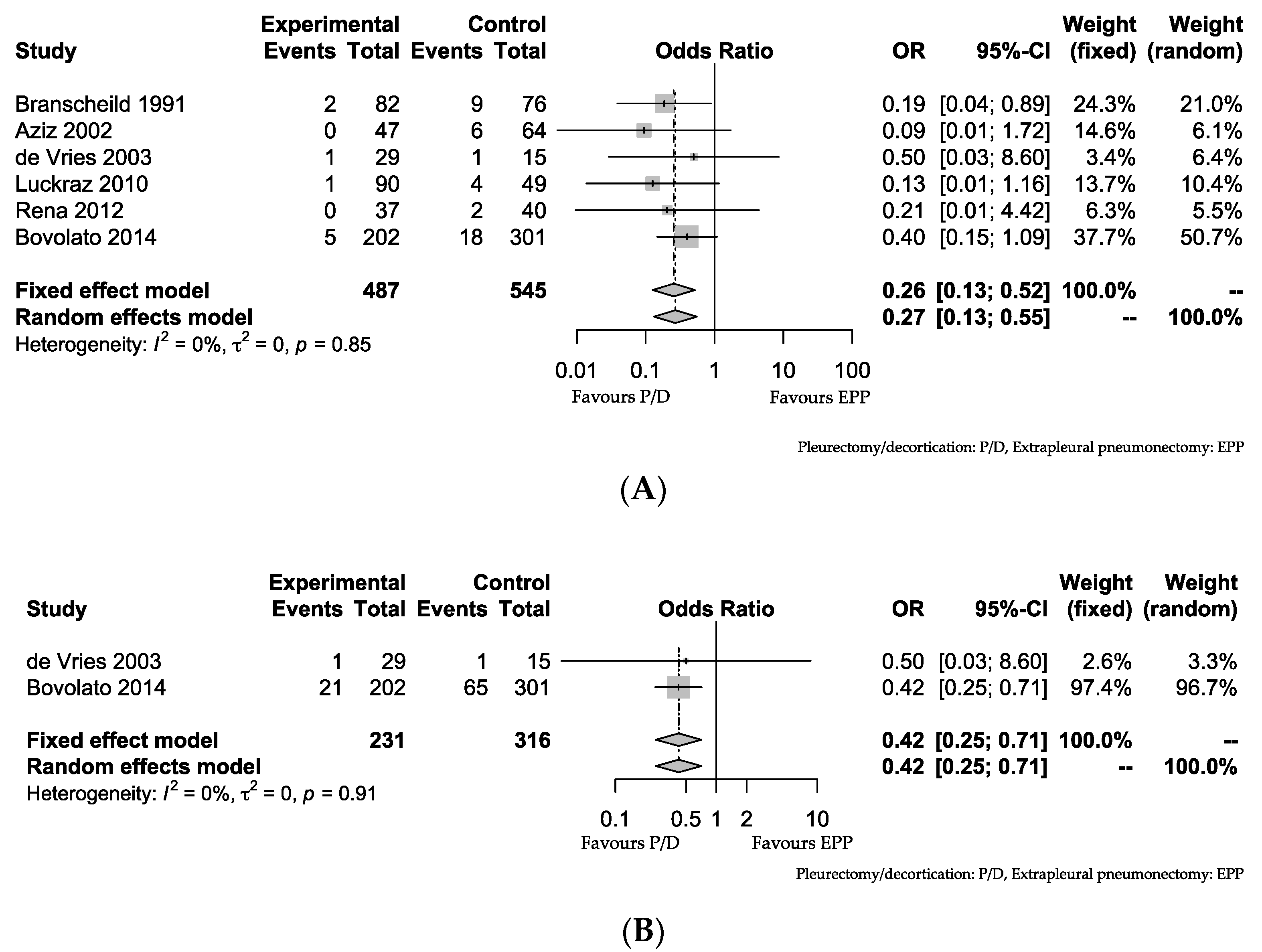
3.2. PD vs. EPP
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gokce, M.; Okur, E.; Baysungur, V.; Ergene, G.; Sevilgen, G.; Halezeroglu, S. Lung decortication for chronic empyema: Effects on pulmonary function and thoracic asymmetry in the late period. Eur. J. Cardiothorac. Surg. 2009, 36, 754–758. [Google Scholar] [CrossRef]
- Rzymann, W.; Skokowski, J.; Romanowicz, G.; Lass, P.; Dziadziuszko, R. Decortication in chronic empyema—Effect on lung function. Eur. J. Cardiothorac. Surg. 2002, 21, 502–507. [Google Scholar] [CrossRef]
- Sarot, I.A. Extrapleural pneumonectomy and pleurectomy in pulmonary tuberculosis. Thorax 1949, 4, 173–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butchart, E.G.; Ashcroft, T.; Barnsley, W.C.; Holden, M.P. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976, 31, 15–24. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, M.; Sugarbaker, D.J. Technique of extrapleural pneumonectomy. Oper. Tech. Thorac. Cardiovasc. Surg. 2010, 15, 282–293. [Google Scholar] [CrossRef][Green Version]
- Friedberg, J.S. State of the art in the technical performance of lung sparing operations for pleural mesothelioma. Semi. Thorac. Cardiothorac. Surg. 2013, 25, 125–143. [Google Scholar] [CrossRef]
- Ichiki, Y.; Takenoyama, M.; Mizukami, M.; So, T.; Sugaya, M.; Yasuda, M.; So, T.; Hanagiri, T.; Sugio, K.; Yasumoto, K. Simultaneous cellular and humoral immune response against mutated p53 in a patient with lung cancer. J. Immunol. 2004, 172, 4844–4850. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, M.; Takenoyama, M.; Shigematsu, Y.; Baba, T.; Fukuyama, T.; Nagata, Y.; Mizukami, M.; So, T.; Ichiki, Y.; Yasuda, M.; et al. Identification of HLA-A24 restricted shared antigen recognized by autologous cytotoxic T lymphocytes from a patient with large cell carcinoma of the lung. Int. J. Cancer 2007, 120, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, M.; Hanagiri, T.; Yasuda, M.; Kuroda, K.; Shigematsu, Y.; Baba, T.; Fukuyama, T.; Nagata, Y.; So, T.; Ichiki, Y.; et al. Antitumor effect of antibody against a SEREX-defined antigen (UOEH-LC-1) on lung cancer xenotransplanted into severe combined immunodeficiency mice. Cancer Res. 2007, 67, 8351–8357. [Google Scholar] [CrossRef][Green Version]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use soft ware ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Mimura, T.; Ohbayashi, C.; Sakuma, T.; Soejima, T.; Tsubota, N. Institutional report-Thoracic general. Radical surgery for malignant pleural mesothelioma: Results and prognosis. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Flore, R.M.; Pass, H.I.; Seshan, V.E.; Dycoco, J.; Zakowski, M.; Carbone, M.; Bains, M.S.; Rusch, V.W. Extrapleural pneumonectomy versus pleurectomy/decrtication in the surgical management of malignant pleural mesothelioma: Results in 663 patients. J. Thorac. Cardiovasc. Surg. 2008, 135, 620–626. [Google Scholar] [CrossRef]
- Shipper, P.H.; Nichols, F.C.; Thomse, K.M.; Deschamps, C.; Cassivi, S.D.; Allen, M.S.; Pairolero, P.C. Malignant pleural mesothelioma: Surgical management in 285 patients. Ann. Thorac. Surg. 2008, 85, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Lang-Lazdunski, L.; Bille, A.; Lal, R.; Cane, P.; McLean, E.; Landau, D.; Steele, J.; Spicer, J. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J. Thorac. Oncol. 2012, 7, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Nakas, A.; von Meyenfeldt, E.; Lau, K.; Muller, S.; Waller, D. Long-term survival after lung-sparing total pleurectomy for locally advanced (International Mesothelioma Interest Group Stage T3-T4) non-sarcomatoid malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2012, 41, 1031–1036. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ploenes, T.; Osei-Agyemang, T.; Krohn, A.; Waller, C.F.; Duncker-Rohr, V.; Elze, M.; Passlick, B. Changes in lung function after surgery for mesothelioma. Asian Cardiovasc. Thorac. Ann. 2013, 21, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bedirhan, M.A.; Cansever, L.; Demir, A.; Ceyhan, S.; Akin, H.; Ürer, H.N.; Ölçmen, A.; Kocatürk, C.; Dinçer, Í. Which type of should become the preferred procedure for malignant pleural mesothelioma: Extrapleural pneumonectomy or extended pleurectomy? J. Thorac. Dis. 2013, 5, 446–454. [Google Scholar] [PubMed]
- Sharkey, A.J.; Tenconi, S.; Nakas, A.; Waller, D.A. The effects of an intentional transition from extrapleural pneumonectomy to extended pleuectomy/decortication. Eur. J. Cardiothorac. Surg. 2016, 49, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Morenghi, E.; Bottoni, E.; Zucali, P.; Rahal, D.; Morlacchi, A.; Ascolese, A.M.; Rose, F.D.; Navarria, P.; Crepaldi, A.; et al. Comorbidity, postoperative morbidity and survival in patients undergoing radical surgery for malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2016, 50, 1077–1082. [Google Scholar] [CrossRef]
- Branscheid, D.; Krysa, S.; Bauer, E.; Bülzebruk, H.; Schirren, J. Diagnosis and therapeutic strategy in malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 1991, 5, 466–473. [Google Scholar] [CrossRef]
- Aziz, T.; Jilaihawi, A.; Prakash, D. The management of malignant pleural mesothelioma; single centre experience in 10 years. Eur. J. Cardiothorac. Surg. 2002, 22, 298–305. [Google Scholar] [CrossRef]
- Bovolato, P.; Casadio, C.; Bille, A.; Ardissone, F.; Santambrogio, L.; Ratto, G.B.; Garofalo, G.; Bedini, A.V.; Garassino, M.; Porcu, L.; et al. Dose surgery improve survival of patients with malignant pleural mesothelioma? A multicenter retrospective analysis of 1365 consecutive patients. J. Thorac. Oncol. 2014, 9, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Rena, O.; Casadio, C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: A harmful procedure. Lung Cancer 2012, 77, 151–155. [Google Scholar] [CrossRef] [PubMed]
- De Vries, W.J.; Long, M.A. Treatment of mesothelioma in Bloemfontein, South Africa. Eur. J. Cardiothorac. Surg. 2003, 24, 434–440. [Google Scholar] [CrossRef]
- Luckraz, H.; Rahman, M.; Patel, N.; Szafranek, A.; Gibbs, A.R.; Butchart, E.G. Three decades of experience in the surgical multi-modality management of pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2010, 37, 552–556. [Google Scholar] [CrossRef]
- Dunn, E.J.; Hernandez, J.; Bender, H.W.; Bender, H.W.; Prager, R.L. Alterations in pulmonary function following pneumonectomy for bronchogenic carcinoma. Ann. Thorac. Surg. 1982, 34, 176–180. [Google Scholar] [CrossRef]
- Ichiki, Y.; Nagashima, A.; Chikaishi, Y.; Yasuda, M. Pneumonectomy for non-small lung cancer. Surg. Today 2012, 42, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, D.J.; Jaklitsch, M.T.; Bueno, R.; Richards, W.; Lukanich, J.; Mentzer, S.J.; Colson, Y.; Linden, P.; Chang, M.; Capalbo, L.; et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomy. J. Thorac. Cardiovasc. Surg. 2004, 128, 138–146. [Google Scholar] [CrossRef]
- Yan, T.D.; Boyer, M.; Tin, M.M.; Wong, D.; Kennedy, C.; McLean, J.; Bannon, P.G.; McCaughan, B.C. Extrapleural pneumonectomy for malignant pleural mesothelioma: Outcomes of treatment and prognostic factors. Gen. Thorac. Cardiovasc. Surg. 2009, 138, 619–624. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Treasure, T.; Lang-Lazadunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’Brien, M.; Thomas, G.; Senan, S.; et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the mesothelioma and radical surgery (MARS) randomized feasibility study. Lancet Oncol. 2011, 12, 763–772. [Google Scholar] [CrossRef]
- Bölükbas, S.; Eberlein, M.; Schirren, J. Prospective study on function results after lung-sparing radical pleurectomy in the management of malignant pleural mesothelioma. J. Thorac. Oncol. 2012, 7, 900–905. [Google Scholar] [CrossRef]
- Burkholder, D.; Hadi, D.; Kunnavakkam, R.; Kindler, H.; Todd, K.; Celauro, A.D.; Vigneswaran, W.T. Effects of extended pleurectomy and decortication on quality of life and pulmonary function in patients with malignant pleural mesothelioma. Ann. Thorac. Surg. 2015, 99, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Morishita, S.; Hashimoto, M.; Nakamichi, T.; Uchiyama, Y.; Hasegawa, S.; Domen, K. Physical function and health-related quality of life in the convalescent phase in surgically treated patients with malignant pleural mesothelioma. Support. Care Cancer 2019, 27, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Athanassiadi, K. Updated meta-analysis of survival after extrrapleural pneumonectomy versus pleurectomy/decortication in mesothelioma. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tian, D.; Park, J.; Allan, J.; Pataky, K.A.; Yan, T.D. A systematic review and meta-analysis of surgical treatment for malignant pleural mesothelioma. Lung Cancer 2014, 83, 240–245. [Google Scholar] [CrossRef]
- Tailoli, E.; Wolf, A.S.; Flores, R.M. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann. Thorac. Surg. 2015, 99, 472–481. [Google Scholar] [CrossRef]
- Schwartz, R.M.; Lieberman-Cribbin, W.; Wolf, A.; Flores, R.M.; Taioli, E. Systematic review of quality of life following pleurectomy decortication and extrapleural pneumonectomy for malignant pleural mesothelioma. BMC Cancer 2018, 18, 1188. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Ichiki, Y.; Taira, A.; Shinohara, S.; Kuwata, T.; Hirai, A.; Imanishi, N.; Yoneda, K.; Tsubota, N.; Tanaka, F. Return to work after surgical treatment for malignant pleural mesothelioma: Report of a case. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 897–900. [Google Scholar] [CrossRef]
- Imanishi, N.; Nabe, Y.; Takenaka, M.; Hirai, A.; Ichiki, Y.; Tanaka, F. Extended pleurectomy decortication for thymoma with pleural dissemination. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 814–817. [Google Scholar] [CrossRef]
- Rusch, V.W. The international mesothelioma interest group. A proposed new international TNM staging system for malignant pleural mesothelioma. Chest 1995, 108, 1122–1128. [Google Scholar] [CrossRef]
- Rusch, V.W.; Chansky, K.; Kindler, H.L.; Nowak, A.K.; Pass, H.I.; Rice, D.C.; Shemanski, L.; Galateau-Salle, F.; McCaughan, B.C.; Nakano, T.; et al. The IASLC mesothelioma staging project: Proposals for the M descriptors in the forthcoming (eighth) edition of the TNM classification for mesothelioma. J. Thorac. Oncol. 2016, 11, 2112–2119. [Google Scholar] [PubMed]
- Nowak, A.K.; Chansky, K.; Rice, D.C.; Pass, H.I.; Kindler, H.L.; Shemanski, L.; Bille, A.; Rintoul, R.C.; Batirel, H.F.; Thomas, C.F.; et al. On behalf of the staging and prognostic factors committee, advisory boards and participating institutions. The IASLC mesothelioma staging project: Proposals for revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for pleural mesothelioma. J. Thorac. Oncol. 2016, 11, 2089–2099. [Google Scholar] [PubMed]
- Rusch, V.W.; Venkatraman, E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 1996, 111, 815–826. [Google Scholar] [CrossRef]
- Chailleux, E.; Dobouis, G.; Pioche, D.; de Lajartre, A.Y.; Rembeaux, A.; Germaud, P. Prognostic factors in diffuse malignant mesothelioma. A study of 167 patients. Chest 1998, 93, 159–162. [Google Scholar] [CrossRef]
- Faber, L.P. Pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 1982, 84, 841–845. [Google Scholar]
- Rice, D.; Chansky, K.; Nowak, A.; Pass, H.; Kindler, H.; Shemanski, L.; Opitz, I.; Call, S.; Hasegawa, S.; Kerstine, K.; et al. On behalf of the mesothelioma domain of the IASLC staging and prognostic factors committee, advisory boards and participating institutions. J. Thorac. Oncol. 2016, 11, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.; Brambilla, E.; Muller-Hermelink, H. Pathology and genetics of tumours of the lung, pleura, thymus, and heart. In World Health Organization Classification of Tumours; IARC Press: Lyon, France, 2004. [Google Scholar]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumors of the Lung, Pleura, Thymus and Heart; International Agency for Research on Cancer: Lyon, France, 2015. [Google Scholar]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to the 2015 World Health Organization Classification of tumors of the lung, pleura, thymus, and heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef]
- Borasio, P.; Berruti, A.; Billé, A.; Lausi, P.; Levra, M.G.; Giardino, R.; Ardissone, F. Malignant pleural mesothelioma: Clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur. J. Cardiothorac. Surg. 2008, 33, 307–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edwards, J.G.; Abrams, K.R.; Leverment, J.N.; Spyt, T.J.; Waller, D.A.; O’Byrne, K.J. Prognostic factors for malignant mesothelioma in 142 patients: Validation of CALGB and EORTC prognostic scoring systems. Thorax 2000, 55, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Herdon, J.E.; Green, M.R.; Chahinian, A.P.; Corson, J.M.; Suzuki, Y.; Vogelzang, N.J. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the cancer and leukemia group B. Chest 1998, 113, 723–731. [Google Scholar] [CrossRef]
- Flores, R.M.; Zakowski, M.; Venkatraman, E.; Krug, L.; Rosenzweig, K.; Dycoco, J.; Lee, C.; Yeoh, C.; Bains, M.; Rusch, V. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J. Thorac. Oncol. 2007, 2, 957–965. [Google Scholar] [CrossRef]
- Yan, T.D.; Boyer, M.; Tin, M.M.; Sim, J.; Kennedy, C.; McLean, J.; Bannon, P.G.; McCaughan, B.C. Prognostic features of long-term survivors after surgical management of malignant pleural mesothelioma. Ann. Thorac. Surg. 2009, 87, 1552–1556. [Google Scholar] [CrossRef]
- Brcic, L.; Jakopovic, M.; Brcic, I.; Klaric, V.; Milosevic, M.; Sepac, A.; Samarzija, M.; Seiwerth, S. Reproducibility of histological subtyping of malignant pleural mesothelioma. Virchows Arch. 2014, 465, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Suzuki, K.; Shima, C.S.; Rusch, V.W.; Adusumilli, P.S.; Travis, W.D. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: A clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J. Thorac. Oncol. 2011, 6, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Ichiki, Y.; Nabe, Y.; Tsuda, Y.; Kuwata, T.; Chikaishi, Y.; Hirai, A.; Imanishi, N.; Yoneda, K.; Tanaka, F. Difficulty of treatment for pleural epithelioid hemangioendothelioma: A report of a case. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 190–193. [Google Scholar] [CrossRef]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; van Brummelen, E. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): Preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017, 18, 623–630. [Google Scholar] [CrossRef]
- Ichiki, Y.; Fukuyama, T.; Nakanishi, K. The prospect of combination therapy with immune checkpoint inhibitors and chemotherapy for squamous cell carcinoma of the lung. Transl. Lung Cancer Res. 2020, 9. [Google Scholar] [CrossRef]
- Shigematsu, Y.; Hanagiri, T.; Kuroda, K.; Baba, T.; Mizukami, M.; Ichiki, Y.; Yauda, M.; Takenoyama, M.; Sugio, K.; Yasumoto, K. Malignant mesothelioma-associated antigens recognized by tumor-infiltrating B cells and the clinical significance of the antibody titers. Cancer Sci. 2009, 100, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, Y.; Taira, A.; Chikaishi, Y.; Matsumiya, H.; Mori, M.; Kanayama, M.; Nabe, Y.; Shinohara, S.; Kuwata, T.; Takenaka, M.; et al. Prognostic factors of advanced or postoperative recurrent non-small cell lung cancer targeted with immune check point inhibitors. J. Thorac. Dis. 2019, 11, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Righi, L.; Koeppen, H.; Zou, W.; Izzo, S.; Grosso, F.; Libener, R.; Loiacono, M.; Monica, V.; Buttigliero, C.; et al. Molecular and histopathological characterization of the tumor immune microenvironment in advanced stage of malignant pleural mesothelioma. J. Thorac. Oncol. 2017, 13, 124–133. [Google Scholar] [CrossRef]
- Eshleman, J.R.; Markowitz, S.D. Mismatch repair defects in human carcinogenesis. Hum. Mol. Genet. 1996, 5, 1489–1494. [Google Scholar] [CrossRef]
- Ionov, Y.; Peinado, M.A.; Malkhosyan, S.; Shibata, D.; Perucho, M. Ubiquitous somatic mutations in sample repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Swanson, B.J.; Frankel, W.L. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn. Pathol. 2017, 12, 24. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Arulananda, S.; Thapa, B.; Walkiewicz, M.; Zapparoli, G.V.; Williams, D.S.; Dobrovic, A.; John, T. Mismatch repair protein defects and microsatellite instability in malignant pleural mesothelioma. J. Thorac. Oncol. 2018, 13, 1588–1594. [Google Scholar] [CrossRef]
- Hylebos, M.; Van Camp, G.; van Meerbeeck, J.P.; Op de Beeck, K. The genetic landscape of malignant pleural mesothelioma: Results from massively parallel sequencing. J. Thorac. Oncol. 2016, 11, 1615–1626. [Google Scholar] [CrossRef]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016, 48, 407–416. [Google Scholar] [CrossRef] [PubMed]
- De Reynies, A.; Jaurand, M.C.; Renier, A.; Couchy, G.; Hysi, I.; Elarouci, N.; Galateau-Salle, F.; Copin, M.C.; Hofman, P.; Cazes, A.; et al. Molecular classification of malignant pleural mesothelioma: Identification of a poor prognosis subgroup linked to the epithelial-to-mesenchymal transition. Clin. Cancer. Res. 2014, 20, 1323–1334. [Google Scholar] [CrossRef]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Galdney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.D.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol. Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.S.; Simone, C.B.; Culligan, M.J.; Barsky, A.R.; Doucette, A.; McNulty, S.; Hahn, S.M.; Alley, E.; Sterman, D.H.; Glatstein, E.; et al. Extended pleurectomy-decortication-based treatment for advanced stage epithelial mesothelioma yielding a median survival of nearly three years. Ann. Thorac. Surg. 2017, 103, 912–919. [Google Scholar] [CrossRef] [PubMed]



| Advantage | Disadvantage |
|---|---|
| fewer microscopic residual tumor cells | worse safety and QOL * |
| a tendecy toward a shorter operation time | more need for extensive reconstruction |
| less air leakage | less ability to tolerate more aggressive |
| easier performance of adjuvant radiation | chemotherapy |
| better safety and QOL * | more microscopic residual tumor cells |
| less need for extensive reconstruction | a tendecy toward a longer operation time |
| a greater ability to tolerate more aggressive | more air leakage |
| chemotherapy preservation of immune reaction from the lung | more difficult performance of adjuvant radiation |
| Study | Extended P/D MST (Month) | Number of Patients Undergoing Extended P/D | EPP MST (Month) | Number of Patients Undergoing EPP |
|---|---|---|---|---|
| Okada 2008 [12] | 17 | 34 | 13 | 31 |
| Schipper 2008 [14] | 17.2 | 10 | 16 | 73 |
| Lang-Lazdunski 2012 [15] | 23 * | 54 | 12.8 * | 22 |
| Nakas 2012 [16] | 13.4 | 67 | 14.7 | 98 |
| Bedirhan 2013 [18] | 27 | 20 | 17 | 31 |
| Sharkey 2016 [19] | 12.3 | 220 | 12.9 | 133 |
| Infrante 2016 [20] | 30 | 47 | 19 | 91 |
| Study | P/D MST (Month) | Number of Patients Undergoing P/D | EPP MST (Month) | Number of Patients Undergoing EPP |
|---|---|---|---|---|
| Branscheid 1991 [21] | 10.5 | 82 | 9.5 | 76 |
| Aziz 2002 [22] | 14 | 47 | 13 | 64 |
| de Vries 2003 [25] | 9 | 29 | 12 | 15 |
| Luckraz 2010 [26] | 10.1 | 90 | 10.3 | 49 |
| Rena 2012 [24] | 25 | 37 | 20 | 40 |
| Bedirhan 2013 [18] | 15 | 20 | 17 | 31 |
| Bovolato 2014 [23] | 20.5 | 202 | 18.8 | 301 |
| Infrante 2016 [20] | 13 | 14 | 19 | 91 |
| Disease | Surgical Procedure | Number of Pateints | Preoperative Status | Postoperative Status | Referrence |
|---|---|---|---|---|---|
| Chronic empyema | P/D | 50 | FEV1.0%: 61.4 ± 11.6, FVC%: 60.8 ± 11.8 | FEV1.0%: 78.9 ± 13.3 *, FVC%: 77.4 ± 14.1 * | [1] |
| Chronic empyema | P/D | 26 | FEV1.0%: 50.0 ± 15.5, VC%: 62.3 ± 13.8 | FEV1.0%: 69.2 ± 12.7 *, VC%: 79.8 ± 12.9 * | [2] |
| MPM | P/D | 16 | FEV1.0%: 60.2 ± 10.3, FVC%: 54.7 ± 9.9 | FEV1.0%: 73.6 ± 11.4 *, FVC%: 68.9 ± 9.1 * | [31] |
| MPM | extended P/D | 36 | PS:0 FEV1.0%: 84.1 ± 16.9, FVC%: 80.2 ± 4.12 PS:1 FEV1.0%: 65.6 ± 14.9, FVC%: 61.7 ± 14.0 | PS:0 FEV1.0%: 72.4 ± 17.3 *, FVC%: 67.1 ± 3.7 * PS:1,2 FEV1.0%: 72.8 ± 17.5, FVC%: 64.8 ± 14.4 | [32] |
| MPM | P/D | 16 | FEV1.0: 2.65 ± 0.65 L, FVC: 3.53 ± 0.91 L | FEV1.0: 2.00 ± 0.44 L *, FVC: 2.51 ± 0.65 L * | [33] |
| MPM | P/D | 23 | median FEV1.0%: 87.1%, median FVC%: 86.5% | median FEV1.0%: 70%, median FVC%: 68.2% | [17] |
| MPM | EPP | 25 | median FEV1.0%: 78.0%, median FVC%: 76.8% | median FEV1.0%: 49.3 *, median VC%: 48.0% * | [17] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichiki, Y.; Goto, H.; Fukuyama, T.; Nakanishi, K. Should Lung-Sparing Surgery Be the Standard Procedure for Malignant Pleural Mesothelioma? J. Clin. Med. 2020, 9, 2153. https://doi.org/10.3390/jcm9072153
Ichiki Y, Goto H, Fukuyama T, Nakanishi K. Should Lung-Sparing Surgery Be the Standard Procedure for Malignant Pleural Mesothelioma? Journal of Clinical Medicine. 2020; 9(7):2153. https://doi.org/10.3390/jcm9072153
Chicago/Turabian StyleIchiki, Yoshinobu, Hidenori Goto, Takashi Fukuyama, and Kozo Nakanishi. 2020. "Should Lung-Sparing Surgery Be the Standard Procedure for Malignant Pleural Mesothelioma?" Journal of Clinical Medicine 9, no. 7: 2153. https://doi.org/10.3390/jcm9072153
APA StyleIchiki, Y., Goto, H., Fukuyama, T., & Nakanishi, K. (2020). Should Lung-Sparing Surgery Be the Standard Procedure for Malignant Pleural Mesothelioma? Journal of Clinical Medicine, 9(7), 2153. https://doi.org/10.3390/jcm9072153





