Abstract
Little information is available on the functional activity of leukocytes after arthroplasty or the expansion of populations with immune suppressive properties during the acute post-operative period. Synovial fluid and matched pre- and post-surgical blood samples were collected from total hip and knee arthroplasty patients (THA and TKA, respectively) to examine the impact of surgery on peripheral blood leukocyte frequency, bactericidal activity, and inflammatory mediator expression. For spinal surgeries, inflammatory mediator production by peripheral blood mononuclear cells (PBMCs) pre- and post-surgery was examined. An expansion of immune suppressive granulocytic myeloid-derived suppressor cells (G-MDSCs) was observed following arthroplasty, which correlated with significantly increased serum interleukin-10 (IL-10) levels. Analysis of synovial fluid from THA and TKAs revealed reduced granulocyte colony-stimulating factor (G-CSF) and soluble CD40 ligand (sCD40L) and increased interleukin-6 (IL-6), monocyte chemoattractant protein 2 (CCL2) and Fms-like tyrosine kinase 3 ligand (Flt-3L) compared to pre- and post-surgical serum. For the spinal surgery cohort, stimulation of PBMCs isolated post-surgery with bacterial antigens produced significantly less pro-inflammatory (IL-1α, IL-1β, interleukin-1 receptor antagonist (IL-1RA), IL-12p40, growth-related oncogene-α/GRO-α (CXCL1) and 6Ckine (CCL21)) and more anti-inflammatory/tissue repair mediators (IL-10, G-CSF and granulocyte-macrophage colony-stimulating factor (GM-CSF)) compared to PBMCs recovered before surgery. The observed bias towards systemic anti-inflammatory changes without concomitant increases in pro-inflammatory responses may influence susceptibility to infection following orthopaedic surgery in the context of underlying co-morbidities or risk factors.
1. Introduction
Joint arthroplasty is the most common orthopaedic procedure in the United States [1]. Total knee and hip arthroplasties (TKA and THA, respectively) alleviate pain and improve mobility and quality-of-life. Over 1 million procedures are performed in the United States each year [2] and by 2030, it is projected that the demand for primary and revision TKA and THA will total over 4 million cases [3,4]. This increase is driven by an aging population, higher rates of diagnosis and treatment of advanced arthritis, and expansion of surgical treatment to younger, more active patients [2]. Between 1998 and 2008, the annual number of spinal fusions in the United States increased from approximately 174,000 to over 400,000, in part, from improvements in instrumentation and surgical technique [5].
Surgical trauma can lead to alterations in hemodynamic, metabolic, and immune responses during the post-operative period [6]. A local inflammatory response in the surgical wound, typified by polymorphonuclear neutrophil (PMN) and monocyte recruitment, serves to limit tissue damage and remove cell debris to promote the healing process. Several studies have characterized this post-operative inflammatory reaction by the heightened production of several proinflammatory mediators, including interleukin (IL)-1β and tumor necrosis factor α (TNF-α), which can induce the release of other cytokines, such as IL-6 that has been correlated with post-operative complications after arthroplasty [7].
However, unlike reports of heightened pro-inflammatory responses post-surgery, other studies have shown that surgery induces immune suppression. For example, PMN, monocyte, and macrophage phagocytic activity is reduced during the post-operative period, providing a potential window for infection susceptibility [8,9,10]. We have shown that a heterogeneous subset of immature monocytes and granulocytes, called myeloid-derived suppressor cells (MDSCs), are recruited to tissues during prosthetic joint infection (PJI), where they exert anti-inflammatory effects [11,12,13]. However, to our knowledge, no studies have directly evaluated MDSC subsets after acute surgery in the orthopaedic setting, which may represent a potential biomarker to evaluate post-surgical inflammation. Increased levels of anti-inflammatory molecules, including IL-4, IL-10, soluble tumor necrosis factor receptor 1 (sTNFR1), IL-1 receptor antagonist (IL-1Ra), and transforming growth factor β (TGF-β) can also be produced during the post-operative period [14,15], which may further bias the anti-inflammatory attributes of wound-associated leukocytes.
Due to current discrepancies as to whether orthopaedic surgery elicits pro- vs. anti-inflammatory signatures, the objective of this study was to determine if a post-surgical immune signature exists that may explain, in part, why a subset of patients develop post-surgical infectious complications in the context of underlying co-morbidities or risk factors. To this end, we investigated pre- and post-operative changes in peripheral blood leukocyte populations and their activation status as well as inflammatory mediators in matched serum and synovial fluid samples in patients undergoing THA, TKA, and spinal procedures. These distinct orthopaedic procedures were compared to determine whether inflammatory signatures would be conserved, which would suggest that post-surgical inflammatory changes could play a broader role than previously appreciated.
2. Materials and Methods
2.1. Patient Population and Sample Procurement
Informed consent was obtained during the pre-surgical visit of patients undergoing primary THA and TKA or spine surgery. The demographics, surgery type, and diagnosis of subjects for both cohorts are provided in Table 1 and Table 2 (THA/TKA and spine surgery, respectively) with exclusion criteria in Table 3. The subjects reported in this manuscript represent a subset of two larger studies with objectives that were outside the scope of the immune parameters reported here. The spinal subjects were included in a study that enrolled 254 arthroplasty patients without synovial fluid collection (274 consented subjects, which included 33 spinal procedures, with 20 withdrawing from the study prior to surgery or for screen failures). The THA/TKA subjects were from a study that enrolled 114 patients with synovial fluid collection (145 consented subjects with 31 withdrawals for cancelled/rescheduled surgery, failure to obtain blood samples, or screen failure). Subjects who consented but had a negative synovial fluid sample (n = 3) were evaluated for pre and post changes in leukocyte frequency only. Samples for this study were collected from the larger studies at regular intervals without any bias to diagnosis. For all patients, venous blood was collected at the pre-surgical visit and again within 30 min post-surgery, during recovery. Synovial fluid was collected intraoperatively for THA and TKA only and was immediately transferred to a sterile specimen container and held on ice until processing. Both study protocols were approved by the Institutional Review Board of the University of Nebraska Medical Center (Omaha, NE, USA) (#177-14-FB and 792-16-EP).

Table 1.
Hip and knee arthroplasty subjects.

Table 2.
Spinal procedure subjects.

Table 3.
Study exclusion criteria.
2.2. Flow Cytometry
PBMCs from a subset of patients undergoing THA or TKA were analyzed by flow cytometry (n = 19). Within 60 min after each draw, blood was layered over Ficoll-Paque PLUS, leukocytes were collected from the interface, and remaining red blood cells (RBCs) were lysed using RBC Lysis Buffer (BioLegend; San Diego, CA, USA). After lysis, cells were washed, incubated with Human FcR Binding Inhibitor (eBioscience; San Diego, CA, USA), and stained with anti-human CD8a-AlexaFluor488, CD4-PE, CD66b-PE-Dazzle, CD25-APC, CD45-APC-Cy7, CD127-PerCP-Cy5.5, CD14-PE-Cy7, HLA-DR-BV421, CD15-BV510, CD19-BV605, CD33-BV711, and CD16-BV650 (all from BioLegend; San Diego, CA, USA). Dead cells were excluded using a LIVE/DEAD Fixable Blue Dead Cell Stain Kit (Life Technologies; Eugene, OR, USA) and analysis was performed using BD FACS-DIVA software, as previously described with the gating strategy depicted in Supplementary Figure S1 [16].
2.3. Multi-Analyte Microbead Array
Inflammatory mediator expression in samples was quantified using a MILLIPLEX MAP Human Cytokine/Chemokine Multiplex Assay (Cat. #HCYTOMAG-60K; Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Results were analyzed using a Bio-Plex Workstation (Bio-Rad, Hercules, CA, USA).
2.4. Whole Blood Killing Assay
S. aureus USA300 LAC 13c [17] was grown for 12 h in tryptic soy broth (250 rpm, 37 °C), washed twice in phosphate buffered saline (PBS), and diluted to 10⁶ colony forming units (CFU)/mL. A total of 100 µL of bacteria (10⁵ CFU) was mixed with 400 µL of freshly drawn blood in 5 mL fluorescence-activated cell sorting (FACS) tubes. Samples were incubated at 37 °C for 30, 60, and 120 min with constant agitation, whereupon dilutions were plated on trypticase soy agar with 5% sheep blood for enumeration of surviving CFU. In some experiments, blood was serially diluted to evaluate the possible action of interfering molecules (i.e., prozone phenomenon), which was not observed (Supplementary Figure S2).
2.5. Peripheral Blood Mononuclear Cell (PBMC) Stimulation
PBMCs were isolated within 60 min of each blood draw from patients before and after spinal surgery (n = 22) by Ficoll-Paque PLUS as described above, and cultured in a 96-well plate at 2 × 10⁵ cells/well in medium containing autologous serum. PBMCs were incubated for 1 h at 37 °C, 5% CO₂ before stimulation with S. aureus peptidoglycan (PGN; 2, 20, and 200 µg/mL), the synthetic triacylated lipopeptide Pam3CysSerLys4 (Pam3CSK4; 2, 20, and 200 µg/mL), or heat-killed S. aureus USA300 LAC 13c (10⁵, 10⁶, and 10⁷ CFU/mL) for 24 h, whereupon cell-free supernatants were collected and stored at −20 °C until assayed using the MILLIPLEX MAP Human Cytokine/Chemokine Multiplex Assay described above. Any value that was at least 2-fold higher than the unstimulated control was considered to be a positive response and assigned the following ranking: 0, no response at any concentration tested; 1 = positive response at the lowest dose tested (i.e., 2 µg/mL PGN/Pam3CSK4 or 10⁵ CFU heat-killed S. aureus); 2 = positive response at the middle dose tested (i.e., 20 µg/mL PGN/Pam3CSK4 or 10⁶ CFU heat-killed S. aureus); and 3 = positive response at the highest dose tested (i.e., 200 µg/mL PGN/Pam3CSK4 or 10⁷ CFU heat-killed S. aureus).
2.6. Statistics
For the PBMC analysis, a Wilcoxon signed rank test was used to compare the distribution of pre- and post-surgical responses. Significant differences in peripheral blood leukocyte populations pre- and post-arthroplasty were determined by an unpaired two-tailed Student’s t-test, while significant differences in Milliplex data between pre- and post-surgery sera and synovial fluid was determined by a one-way analysis of variance (ANOVA), using GraphPad Prism version 6 (La Jolla, CA, USA). For all analyses, a p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Immune Alterations in the Peripheral Blood and Synovial Fluid of THA and TKA Patients
The first purpose of this study was to determine if orthopaedic procedures caused the expansion of MDSCs that possess immune inhibitory activity and whether this translated into differences in leukocyte effector function. Twenty-six patients were included in this study cohort, with the majority (~92%) requiring THA or TKA for osteoarthritis (OA) (Table 1). The average age was 63.4 years (range of 40–83) with 12 males and 14 females enrolled.
Two MDSC subsets have been described, namely CD33+HLA-DR-CD66b-CD14+ monocytic-MDSCs (M-MDSCs) and CD33+HLA-DR-CD66b+CD14low/- granulocytic-MDSCs (G-MDSCs) [18]. Although the percentages of total CD45+ leukocytes and MDSCs in the blood was not affected by surgery (Figure 1A,B, respectively), the granulocytic fraction of this population (G-MDSCs; CD66b+CD14- of CD33+HLA-DR-) was significantly elevated following surgery for all arthroplasties and THA compared to pre-surgical matched controls (Figure 1C), whereas M-MDSCs were not affected (Figure 1D). PMNs (CD16highCD15+) were significantly increased after surgery in all procedures compared to pre-surgical matched controls, which was driven by THA patients (Figure 1E). Collectively, the observed increases in G-MDSCs and PMNs reflect a transition toward increased granulocytic populations in the blood post-arthroplasty.
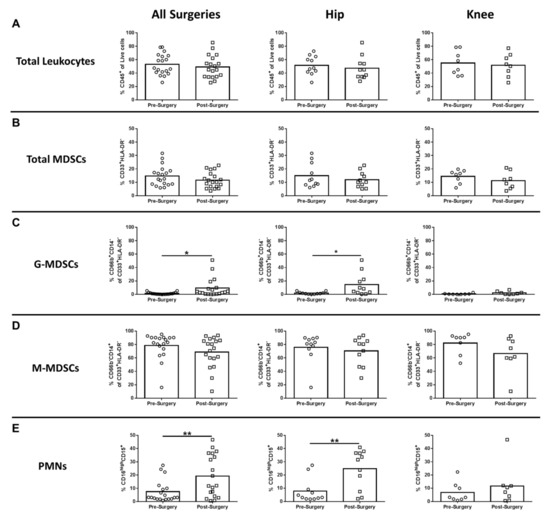
Figure 1.
Hip/knee arthroplasty results in increased granulocytic myeloid-derived suppressor cells (G-MDSCs) and polymorphonuclear neutrophils (PMNs) in the peripheral circulation. Blood samples collected from patients pre- and post-surgery for total hip (THA) (n = 11) and total knee arthroplasties (TKA) (n = 8) were analyzed by flow cytometry. Results were calculated after gating on live CD45+ cells and quantitation of (A) total CD45+ leukocytes, (B) total myeloid-derived suppressor cells (MDSCs) (CD33+HLA-DR-), (C) G-MDSCs (% CD66b+CD14- of the total MDSC population), (D) M-MDSCs (% CD66b-CD14+ of the total MDSC population), and (E) CD16highCD15+ neutrophils (PMNs) is reported (* p < 0.05; ** p < 0.01; unpaired two-tailed Student’s t-test).
In humans, monocytes comprise three subsets based on CD14 and CD16 expression, which include classical, intermediate, and non-classical monocytes [19,20]. Non-classical monocytes are considered the primary inflammatory subtype, whereas classical monocytes are phagocytic with limited inflammatory attributes. Intermediate monocytes comprise only a small percentage of circulating monocytes and are transitional, displaying both phagocytic and inflammatory function [19]. Only CD14-/lowCD16+ non-classical monocytes were significantly increased after surgery compared to pre-surgical matched controls for all procedures, which was driven by TKA (Figure 2C). Both CD14+CD16- classical and CD14+CD16+ intermediate monocytes were unchanged following surgery (Figure 2A,B). With regard to adaptive immunity, CD4+ cells decreased significantly for all surgeries and TKA compared to pre-surgical matched controls (Figure 3A), whereas CD8+ T cells were unaffected (Figure 3B). B cells were also significantly decreased post-surgery for all procedures versus pre-surgical matched controls, which was largely influenced by patients with TKA (Figure 3C).
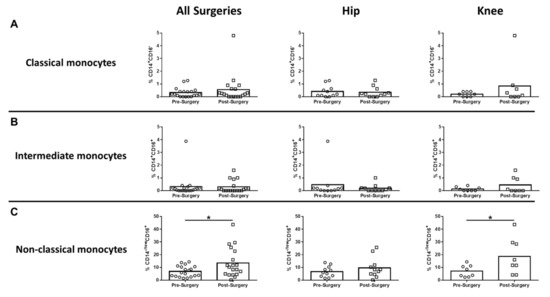
Figure 2.
Non-classical monocytes are increased systemically following hip/knee arthroplasty. Blood samples collected from patients pre- and post-surgery for THA (n = 11) and TKA (n = 8) were analyzed by flow cytometry. Results were calculated after gating on live CD45+ cells and removing granulocyte and T cell populations from the analysis. Quantification of (A) CD14+CD16- classical monocytes, (B) CD14+CD16+ intermediate monocytes, and (C) CD14lowCD16+ non-classical monocytes is presented (* p < 0.05; unpaired two-tailed Student’s t-test).
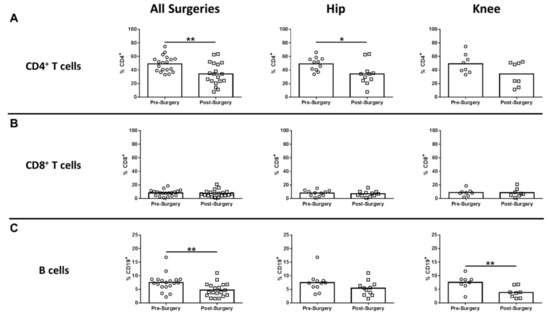
Figure 3.
Hip/knee arthroplasty reduces CD4+ T and B cells in the peripheral circulation. Blood samples collected from patients pre- and post-surgery for THA (n = 11) and TKA (n = 8) were analyzed by flow cytometry. Results were calculated after gating on live CD45+ cells and quantitation of (A) CD4+ T cells, (B) CD8+ T cells, and (C) CD19+ B cells is presented (* p < 0.05; ** p < 0.01; unpaired two-tailed Student’s t-test).
Next, inflammatory mediator expression was quantified in pre- and post-surgical sera as well as synovial fluid in THA/TKA patients to identify novel biomarkers that might serve a prognostic value. Of the mediators examined, only serum IL-10 was significantly increased in all procedures post-surgery, which was driven by THA (Figure 4A). In contrast, several mediators were significantly altered in synovial fluid versus pre- or post-surgical sera. For example, granulocyte-colony stimulating factor (G-CSF) was significantly decreased in the synovial fluid for all procedures compared to pre- and post-surgical sera (Figure 4B). Conversely, both IL-6 and monocyte chemoattractant protein 2 (CCL2) were increased in the synovial fluid of THA and TKA compared with pre- and post-surgical sera (Figure 4C,D, respectively), demonstrating the compartmentalization of inflammatory responses. In addition to IL-6 and CCL2, three additional mediators were differentially expressed in the synovial fluid, namely soluble CD40 ligand (sCD40L), Fms-like tyrosine kinase 3 ligand (Flt-3L), and basic fibroblast growth factor (FGF-2) (Figure 5).
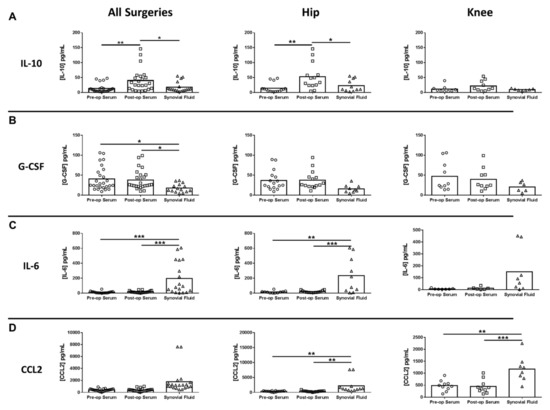
Figure 4.
Cytokine and chemokine expression in serum and synovial fluid of hip/knee arthroplasty patients. Quantitation of (A) IL-10, (B) granulocyte colony-stimulating factor (G-CSF), (C) IL-6, and (D) monocyte chemoattractant protein 2 (CCL2) expression in pre- and post-sera and synovial fluid collected from THA (n = 16) and TKA (n = 10) subjects (* p < 0.05; ** p < 0.01; *** p < 0.001; one-way ANOVA).
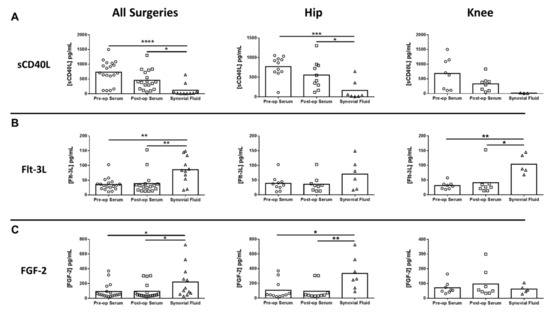
Figure 5.
Differentially expressed mediators in the synovial fluid of hip/knee arthroplasty patients. Quantitation of (A) soluble CD40 ligand (sCD40L), (B) Fms-like tyrosine kinase 3 ligand (Flt-3L), and (C) basic fibroblast growth factor (FGF-2) expression in pre- and post-sera and synovial fluid collected from THA (n = 11) and TKA (n = 8) subjects (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; one-way ANOVA).
To investigate whether leukocyte function may be altered after surgery, S. aureus killing assays were performed on whole blood collected from THA and TKA patients. Although bacterial killing was more robust in some patients, overall pre- or post-surgical status had no effect on S. aureus bactericidal activity (Figure 6).
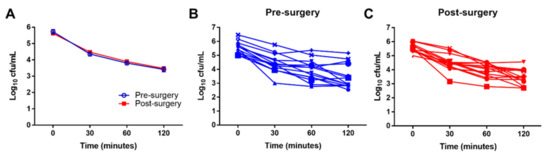
Figure 6.
Surgery does not impair leukocyte bactericidal activity. S. aureus whole blood killing assays were performed from blood collected from patients pre- and post-surgery for THA and TKA (n = 14). Numbers of viable bacteria are reported as Log10 colony forming units (cfu) per mL for (A) the average killing for all patients pre- and post-arthroplasty and stratified into (B) pre-surgery and (C) post-surgery.
3.2. Effect of Spinal Arthroplasty on PBMC Responsiveness
Another purpose of this study was to determine whether orthopaedic surgery altered the activation state of PBMCs. For this analysis, a separate cohort of 22 spinal surgery patients was examined (Table 2), where PBMC responses to S. aureus-relevant stimuli were tested. The average age of subjects was 66.5 years (range of 38–87) with 12 males and 10 females enrolled in the study. Due to variation in the absolute values of mediators between patients, data were categorized based on at least a 2-fold-change in each mediator compared to unstimulated PBMCs from the same patient. Post-surgery, PBMCs had reduced expression of several pro-inflammatory mediators, including IL-12p40, IL-1β, IL-1α, and 6Ckine (CCL21) compared to pre-surgical matched controls (Table 4). In contrast, the anti-inflammatory cytokine IL-10 and growth factors (G-CSF and GM-CSF) were increased post-arthroplasty versus the pre-surgical control group (Table 5). To our knowledge, these findings are the first to report that surgery biases leukocytes towards a functional anti-inflammatory phenotype, in agreement with the post-surgical elevation in serum IL-10 in the THA/TKA cohort.

Table 4.
Mediators decreased in PBMCs following spinal arthroplasty.

Table 5.
Mediators increased in PBMCs following spinal arthroplasty.
4. Discussion
Currently, some discrepancies exist as to whether orthopaedic surgery elicits pro- versus anti-inflammatory changes. Although prior studies have reported cytokine alterations following THA/TKA [21,22,23,24,25], it is paramount to address what effect this post-surgical immune response has on leukocyte function, which has received less attention. We were also interested in determining whether inflammatory signatures would be conserved across distinct orthopaedic procedures, which led to the investigation of both spinal procedures and THA/TKA. Our findings that anti-inflammatory responses are elicited following surgery in both orthopaedic settings suggests that post-surgical inflammatory skewing could play a broader role than previously appreciated. The anti-inflammatory bias reported in this study following orthopaedic surgeries may be a contributing, but not causative, factor for infectious complications, since the low incidence of PJI implies the involvement of other co-morbidities or risk factors [26,27,28,29].
Orthopaedic surgery induced the expansion of G-MDSCs that potently inhibit T cell proliferation [30,31], in agreement with the significant reduction in CD4+ T cells post-arthroplasty compared to matched pre-surgical samples. The expansion of G-MDSCs post-arthroplasty coincided with increased serum G-CSF compared to synovial fluid, a critical growth factor for MDSC and PMN expansion/differentiation [32,33]. In addition, PBMCs exposed to S. aureus-relevant stimuli produced significantly more G-CSF and GM-CSF after spinal procedures, suggesting that surgery primes leukocytes for increased growth factor production. Our findings of PMN expansion and CD4+ T cell contraction after arthroplasty are in agreement with prior studies that reported increased neutrophil-to-lymphocyte ratios in TKA and THA patients during the acute post-surgical period [14,34,35,36,37,38].
IL-10 was increased in both THA/TKA and spine patients, which may enhance patient susceptibility to infectious complications during the post-operative period in combination with other underlying risk factors or co-morbidities. Indeed, our recent study reported that IL-10 levels were significantly increased in patients with PJI compared to aseptic loosening [16] and in a mouse PJI model IL-10 was critical for promoting S. aureus persistence, in part, via inhibition of monocyte/macrophage pro-inflammatory activity that was mediated by MDSCs [11]. Elevated IL-10 levels have also been associated with impaired long-term functional performance following TKA [39] as well as in patients with traumatic bone injuries, which was predictive of sepsis development [40].
Flt-3L, a cytokine that promotes hematopoietic cell proliferation [41], was identified as a potential novel biomarker for OA, since its expression was significantly increased in the synovial fluid of arthroplasty patients. CCL2 levels were also increased in the synovial fluid, in agreement with an earlier report in patients with knee arthroscopy for cartilage tears [42]. IL-6 is a pro-inflammatory cytokine involved in many physiological and pathological processes [43,44], including MDSC expansion and activation [45]. IL-6 levels were increased in the synovial fluid of THA/TKA patients, where approximately 92% of these subjects were diagnosed with OA, in agreement with prior reports [44,46,47]. The overall lack of pro-inflammatory mediators in the serum after surgery in our study differs from earlier reports describing elevations in IL-6, TNF-α, and IL-1β following THA or TKA [21,22,23,24,25]. The reasons for this discrepancy remain unknown, but could be explained by the exclusion criteria for our study, since consented subjects had fewer underlying conditions that may potentiate pro-inflammatory reactions after surgery. We acknowledge the limitations of our study, including the cohort size and lack of serial sampling. However, prior studies have reported that many inflammatory changes after THA/TKA manifest during the acute post-surgical period [22,34,36], similar to the interval examined here.
This report advances the field by revealing that arthroplasty induces changes in leukocyte activation status. This is reflected by an overall decrease in pro-inflammatory mediator expression by PBMCs post-surgery concomitant with increased anti-inflammatory (IL-10) and growth factor production, molecules that are typically associated with wound healing. The finding that anti-inflammatory responses are elicited following diverse orthopaedic procedures (i.e., spinal surgery and THA/TKA) suggests that post-surgical inflammatory skewing could play a broader role than previously appreciated. Future longitudinal studies should be performed to examine the potential of peripheral blood G-MDSCs as a biomarker for the risk of developing PJI or monitoring the extent of post-surgical inflammation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/7/2123/s1, Figure S1: Gating strategy to quantitate leukocyte populations in whole blood. Single cells were gated from the total events using FSC-A vs. FSC-H, followed by exclusion of dead cells. Live, CD45+ leukocytes were separated into granulocytes (CD16highCD15+) and non- granulocytes. Non-granulocyte populations were identified using a series of gates to avoid duplicate counting. First, MDSCs were identified as CD33+HLA-DR- cells, followed by CD4+ and CD8+ T cell populations out of the non-MDSC population. CD19 was used to identify B cells within the non-T cell population and finally, the abundance of monocyte populations was determined using CD14 and CD16 expression, Figure S2: S. aureus whole blood killing assay is not influenced by prozone phenomenon. Matched pre-/post-arthroplasty blood samples were collected from a patient and serially diluted to determine whether inhibitory factors influenced S. aureus killing. Results are representative of findings from two individual patients.
Author Contributions
Conceptualization, C.W.H., N.M., A.S.A., and T.K.; methodology, C.E.H., K.J.Y., R.F., J.O., D.M.S., E.R.L., M.J.A., R.A., D.V., C.W.H., B.S.K., C.A.C., and K.L.G.; investigation, C.E.H., K.J.Y., J.O., and D.V.; writing—original draft, C.E.H.; writing—review and editing, C.E.H., K.J.Y., R.F., J.O., D.M.S., E.R.L., M.J.A., R.A., C.W.H., B.S.K., C.A.C., K.L.G., N.M., A.S.A., and T.K.; funding acquisition, A.S.A.; resources, C.W.H., B.S.K., C.A.C., and K.L.G.; supervision, T.K. All authors have reviewed and approved the manuscript.
Funding
This work was supported by Pfizer Vaccine Research and Development. The University of Nebraska Flow Cytometry Research Facility receives partial support from the National Cancer Institute by the Fred & Pamela Buffett Cancer Center Support Grant (P30CA036727).
Acknowledgments
The authors thank the patients for their participation in these studies. The authors also thank Drs. Mark Rupp, Paul Fey, and Angela Hewlett for their contributions during the initial discussions of this project and Dillon Ellis for assistance with patient consents. We thank Pfizer colleagues, C. Hal Jones for his help with study design and conduct, and Alejandra Gurtman for thoughtful comments and review of the manuscript.
Conflicts of Interest
N.M. and A.S.A. are current employees of Pfizer and may own stock in the company. The other authors have no commercial or other associations to report that might pose a conflict of interest for this study.
References
- Kremers, H.M.; Kremers, W.K.; Berry, D.J.; Lewallen, D.G. Social and Behavioral Factors in Total Knee and Hip Arthroplasty. J. Arthroplast. 2015, 30, 1852–1854. [Google Scholar] [CrossRef] [PubMed]
- Kremers, H.M.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Joint Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Joint Surg. Am. 2018, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Rajaee, S.S.; Bae, H.W.; Kanim, L.E.A.; Delamarter, R.B. Spinal fusion in the United States: Analysis of trends from 1998 to 2008. Spine (Phila. Pa. 1976) 2012, 37, 67–76. [Google Scholar] [CrossRef]
- Lin, E.; Calvano, S.E.; Lowry, S.F. Inflammatory cytokines and cell response in surgery. Surgery 2000, 127, 117–126. [Google Scholar] [CrossRef]
- Randau, T.; Friedrich, M.J.; Wimmer, M.D.; Reichert, B.; Kuberra, D.; Stoffel-Wagner, B.; Limmer, A.; Wirtz, D.C.; Gravius, S. Interleukin-6 in Serum and in Synovial Fluid Enhances the Differentiation between Periprosthetic Joint Infection and Aseptic Loosening. PLoS ONE 2014, 9, e89045. [Google Scholar] [CrossRef]
- Hogan, B.V.; Peter, M.B.; Shenoy, H.G.; Horgan, K.; Hughes, T.A. Surgery induced immunosuppression. Surgery 2011, 9, 38–43. [Google Scholar] [CrossRef]
- Van Dijk, W.C.; Verbrugh, H.A.; Van Rijswijk, R.E.; Vos, A.; Verhoef, J. Neutrophil function, serum opsonic activity, and delayed hypersensitivity in surgical patients. Surgery 1982, 92, 21–29. [Google Scholar]
- Chen, R.-M.; Wu, C.-H.; Chang, H.-C.; Wu, G.-J.; Lin, Y.-L.; Sheu, J.-R.; Chen, T.-L. Propofol Suppresses Macrophage Functions and Modulates Mitochondrial Membrane Potential and Cellular Adenosine Triphosphate Synthesis. Anesthesiology 2003, 98, 1178–1185. [Google Scholar] [CrossRef]
- Heim, C.E.; Vidlak, D.; Kielian, T. Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J. Leukoc. Boil. 2015, 98, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.E.; Vidlak, D.; Scherr, T.D.; Hartman, C.W.; Garvin, K.L.; Kielian, T. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J. Immunol. 2015, 194, 3861–3872. [Google Scholar] [CrossRef]
- Heim, C.E.; Vidlak, D.; Scherr, T.D.; Kozel, J.A.; Holzapfel, M.; Muirhead, D.E.; Kielian, T. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J. Immunol. 2014, 192, 3778–3792. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, P.H.; Ickovics, J.R.; Epel, E.; Nadler, E.; Jokl, P.; Fulkerson, J.P.; Tillie, J.M.; Dhabhar, F.S. Surgical Stress-Induced Immune Cell Redistribution Profiles Predict Short-Term and Long-Term Postsurgical Recovery. J. Bone Joint Surg. Am. 2009, 91, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Stoecklein, V.M.; Osuka, A.; Lederer, J.A. Trauma equals danger--damage control by the immune system. J. Leukoc. Boil. 2012, 92, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.E.; Vidlak, D.; Odvody, J.; Hartman, C.; Garvin, K.L.; Kielian, T. Human prosthetic joint infections are associated with myeloid-derived suppressor cells (MDSCs): Implications for infection persistence. J. Orthop. Res. 2017, 36, 1605–1613. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureusBiofilms Prevent Macrophage Phagocytosis and Attenuate Inflammation In Vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef]
- Mukherjee, R.; Barman, P.K.; Thatoi, P.K.; Tripathy, R.; Das, B.K.; Ravindran, B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015, 5, 13886. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Clementsen, T.; Krohn, C.D.; Reikerås, O. Systemic and local cytokine patterns during total hip surgery. Scand. J. Clin. Lab. Investig. 2006, 66, 535–542. [Google Scholar] [CrossRef]
- Shah, K.; Mohammed, A.; Patil, S.; McFadyen, A.; Meek, R.M.D. Circulating Cytokines after Hip and Knee Arthroplasty: A Preliminary Study. Clin. Orthop. Relat. Res. 2008, 467, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Reikeras, O.; Borgen, P.O.; Reseland, J.E.; Lyngstadaas, S.P. Changes in serum cytokines in response to musculoskeletal surgical trauma. BMC Res. Notes 2014, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Mindrinos, M.N.; Seok, J.; Cuschieri, J.; Cuenca, A.G.; Gao, H.; Hayden, D.L.; Hennessy, L.; Moore, E.E.; Minei, J.P.; et al. A genomic storm in critically injured humans. J. Exp. Med. 2011, 208, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.; Güler, N.; Syed, D.; Hopkinson, W.; McComas, K.N.; Walborn, A.; Hoppensteadt, D.; Fareed, J.; Rondina, M.T.; Hopkins, W. Postoperative Changes in the Systemic Inflammatory Milieu in Older Surgical Patients. Clin. Appl. Thromb. 2017, 24, 583–588. [Google Scholar] [CrossRef]
- Del Pozo, J.; Patel, R. Infection Associated with Prosthetic Joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef]
- Fang, A.; Hu, S.S.; Endres, N.; Bradford, D.S. Risk Factors for Infection After Spinal Surgery. Spine 2005, 30, 1460–1465. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.; Blom, A.W.; Beswick, A.D.; Team, I. Patient-Related Risk Factors for Periprosthetic Joint Infection after Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150866. [Google Scholar] [CrossRef]
- Triantafyllopoulos, G.; Stundner, O.; Memtsoudis, S.; Poultsides, L.A. Patient, Surgery, and Hospital Related Risk Factors for Surgical Site Infections following Total Hip Arthroplasty. Sci. World J. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Gato, M.; Blanco-Luquin, I.; Zudaire, M.; Morentin, X.M.; Pérez-Valderrama, E.; Zabaleta, A.; Kochan, G.; Escors, D.; Fernández-Irigoyen, J.; Santamaría, E. Drafting the proteome landscape of myeloid-derived suppressor cells. Proteomics 2015, 16, 367–378. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Waight, J.D.; Hu, Q.; Miller, A.; Liu, S.; Abrams, S.I. Tumor-Derived G-CSF Facilitates Neoplastic Growth through a Granulocytic Myeloid-Derived Suppressor Cell-Dependent Mechanism. PLoS ONE 2011, 6, e27690. [Google Scholar] [CrossRef]
- Chakraborty, A.; Tweardyabc, D.J. Stat3 and G-CSF-Induced Myeloid Differentiation. Leuk. Lymphoma 1998, 30, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Wasko, M.K.; Struminski, M.; Bobecka, K.; Kowalczewski, J. Neutrophil-to-lymphocyte ratio shows faster changing kinetics than C-reactive protein after total hip and knee arthroplasty. J. Orthop. Transl. 2017, 10, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Gaudillière, B.; Fragiadakis, G.K.; Bruggner, R.V.; Nicolau, M.; Finck, R.; Tingle, M.; Silva, J.; Ganio, E.A.; Yeh, C.G.; Maloney, W.J.; et al. Clinical recovery from surgery correlates with single-cell immune signatures. Sci. Transl. Med. 2014, 6, 255ra131. [Google Scholar] [CrossRef]
- Katoh, N.; Nishino, J.; Nishimura, K.; Kawabata, C.; Hotta, Y.; Matsui, T.; Nakamura, S.; Matsushita, T. Normal sequential changes in neutrophil CD64 expression after total joint arthroplasty. J. Orthop. Sci. 2013, 18, 949–954. [Google Scholar] [CrossRef][Green Version]
- Yombi, J.C.; Schwab, P.E.; Thienpont, E. Neutrophil-to-lymphocyte ratio (NLR) distribution shows a better kinetic pattern than C-reactive protein distribution for the follow-up of early inflammation after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2015, 24, 3287–3292. [Google Scholar] [CrossRef]
- Bossche, W.V.D.; Rykov, K.; Teodosio, C.; Have, B.L.T.; Knobben, B.A.; Sietsma, M.S.; Josiassen, K.; Versteeg, S.D.B.-; Orfao, A.; Van Dongen, J.J.; et al. Flow cytometric assessment of leukocyte kinetics for the monitoring of tissue damage. Clin. Immunol. 2018, 197, 224–230. [Google Scholar] [CrossRef]
- Langkilde, A.; Jakobsen, T.L.; Bandholm, T.; Eugen-Olsen, J.; Blauenfeldt, T.; Petersen, J.; Andersen, O. Inflammation and post-operative recovery in patients undergoing total knee arthroplasty-secondary analysis of a randomized controlled trial. Osteoarthr. Cartil. 2017, 25, 1265–1273. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Smith, R.M.; Perry, S.L.; Windsor, A.J.; Dickson, R.A.; Bellamy, M.C. Immediate IL-10 expression following major orthopaedic trauma: Relationship to anti-inflammatory response and subsequent development of sepsis. Intensiv. Care Med. 2000, 26, 1076–1081. [Google Scholar] [CrossRef]
- Lyman, S.D.; Jacobsen, S.E.W. c-kit Ligand and Flt3 Ligand: Stem/Progenitor Cell Factors With Overlapping Yet Distinct Activities. Blood 1998, 91, 1101–1134. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar, V.G.; Cuéllar, J.M.; Kirsch, T.; Strauss, E.J.; Information, P.E.K.F.C. Correlation of Synovial Fluid Biomarkers With Cartilage Pathology and Associated Outcomes in Knee Arthroscopy. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 475–485. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. Immunotherapeutic implication of IL-6 blockade. Immunotherapy 2012, 4, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, A.; Beekhuizen, M.; Rutgers, M.; Van Osch, G.J.; Bekkers, J.; Bot, A.G.; Geurts, B.; Dhert, W.; Saris, D.; Creemers, L.B. Interleukin-6 is elevated in synovial fluid of patients with focal cartilage defects and stimulates cartilage matrix production in an in vitro regeneration model. Arthritis Res. Ther. 2012, 14, R262. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shen, W.; Zhang, Y.; Liu, M.; Zhang, L.; Liu, Q.; Lu, H.H.; Bo, J. Accumulation of myeloid-derived suppressor cells (MDSCs) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget 2017, 8, 38378–38388. [Google Scholar] [CrossRef]
- Levinger, I.; Levinger, P.; Trenerry, M.K.; Feller, J.A.; Bartlett, J.R.; Bergman, N.; McKenna, M.J.; Cameron-Smith, D. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis Rheum. 2011, 63, 1343–1348. [Google Scholar] [CrossRef]
- Latourte, A.; Cherifi; C.; Maillet, J.; Ea, H.-K.; Bouaziz, W.; Funck-Brentano, T.; Cohen-Solal, M.; Hay, E.; Richette, P. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 2017, 76, 748–755. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).