Effect of Vedolizumab on Anemia of Chronic Disease in Patients with Inflammatory Bowel Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Statistical Analysis
3. Results
3.1. Frequency of ACD in IBD
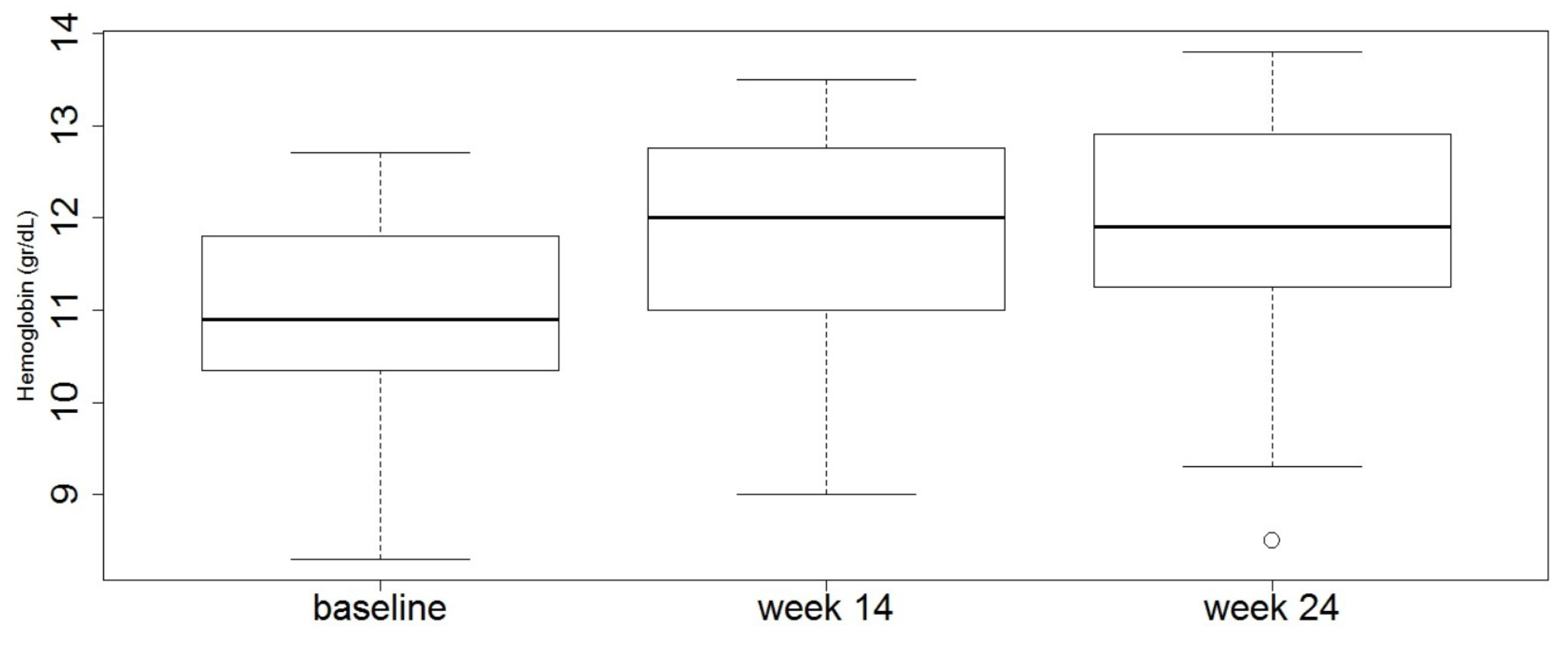
3.2. Effect of Vedolizumab on ACD Course
3.3. Relationship between Clinical Response and ACD Resolution
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ganz, T. Anemia of inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegard, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. J. Crohns Colitis 2015, 99, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef]
- Wang, C.Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Orsini, M.; Chateauvieux, S.; Rhim, J.; Gaigneaux, A.; Cheillan, D.; Christov, C.; Dicato, M.; Morceau, F.; Diederich, M. Sphingolipid-mediated inflammatory signaling leading to autophagy inhibition converts erythropoiesis to myelopoiesis in human hematopoietic stem/progenitor cells. Cell Death Differ. 2019, 26, 1796–1812. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Cooper, A.C. Erythropoietin resistance: The role of inflammation and pro-inflammatory cytokines. Nephrol. Dial. Transplant. 2002, 17, 39–43. [Google Scholar] [CrossRef]
- Gasché, C.; Dejaco, C.; Waldhoer, T.; Tillinger, W.; Reinisch, W.; Fueger, G.F.; Gangl, A.; Lochs, H. Intravenous iron and erythropoietin for anemia associated with Crohn disease. A randomized, controlled trial. Ann. Intern. Med. 1997, 126, 782–787. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Di Sabatino, A.; Albertini, R.; Ardizzone, S.; Biancheri, P.; Bonetti, E.; Cassinotti, A.; Cazzola, P.; Markopoulos, K.; Massari, A.; et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica 2010, 95, 199–205. [Google Scholar] [CrossRef]
- Rubin, D.T.; Mulani, P.; Chao, J.; Pollack, P.F.; Bensimon, A.G.; Yu, A.P.; Ghosh, S. Effect of adalimumab on clinical laboratory parameters in patients with Crohn’s disease: Results from the CHARM trial. Inflamm. Bowel Dis. 2012, 18, 818–825. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Van Assche, G.; Gómez-Ulloa, D.; García-Álvarez, L.; Lara, N.; Black, C.M.; Kachroo, S. Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2017, 15, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fedyk, E.R.; Wyant, T.; Yang, L.-L.; Csizmadia, V.; Burke, K.; Yang, H.; Kadambi, V.J. Exclusive antagonism of the α4β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm. Bowel Dis. 2012, 18, 2107–2119. [Google Scholar] [CrossRef]
- Feagan, B.G.; Greenberg, G.R.; Wild, G.; Fedorak, R.N.; Paré, P.; McDonald, J.W.D.; Dubé, R.; Cohen, A.; Steinhart, H.A.; Landau, S.; et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N. Engl. J. Med. 2005, 352, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Fedorak, R.N.; Paré, P.; McDonald, J.W.; Dubé, R.; Cohen, A.; Steinhart, A.H.; Landau, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef]
- Sands, B.E.; Feagan, B.G.; Rutgeerts, P.; Colombel, J.F.; Sandborn, W.J.; Sy, R.; D’Haens, G.; Ben-Horin, S.; Xu, J.; Rosario, M.; et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment had failed. Gastroenterology 2014, 147, 618–627. [Google Scholar] [CrossRef]
- Engel, T.; Ungar, B.; Yung, D.E.; Ben-Horin, S.; Eliakim, R.; Kopylov, U. Vedolizumab in IBD-lessons from real-world experience; a systematic review and pooled analysis. J. Crohns Colitis 2018, 12, 245–257. [Google Scholar] [CrossRef]
- Chaparro, M.; Garre, A.; Ricart, E.; Iborra, M.; Mesonero, F.; Vera, I.; Riestra, S.; García-Sánchez, V.; Luisa De Castro, M.; Martin-Cardona, A.; et al. Short and long-term effectiveness and safety of vedolizumab in inflammatory bowel disease: Results from the ENEIDA registry. Aliment. Pharmacol. Ther. 2018, 48, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s Disease 2016: Part 1: Diagnosis and medical management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Mohammed Vashist, N.; Samaan, M.; Mosli, M.H.; Parker, C.E.; MacDonald, J.K.; Nelson, S.A.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Jairath, V. Endoscopic scoring indices for evaluation of disease activity in ulcerative Colitis. Cochrane Database Syst. Rev. 2018, 16, CD011450. [Google Scholar] [CrossRef] [PubMed]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Parker, C.E.; MacDonald, J.K.; Nelson, S.A.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Jairath, V. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Pakoz, Z.B.; Çekiç, C.; Arabul, M.; Sarıtaş Yuksel, E.; Ipek, S.; Vatansever, S.; Unsal, B. An evaluation of the correlation between hepcidin serum levels and disease activity in inflammatory Bowel disease. Gastroenterol. Res. Pract. 2015, 2015, 810942. [Google Scholar] [CrossRef]
- Mecklenburg, I.; Reznik, D.; Fasler-Kan, E.; Drewe, J.; Beglinger, C.; Hruz, P.; Swiss IBD Cohort Study Group. Serum hepcidin concentrations correlate with ferritin in patients with inflammatory Bowel disease. J. Crohns Colitis 2014, 8, 1392–1397. [Google Scholar] [CrossRef]
- Masson, C. Rheumatoid anemia. Jt. Bone Spine 2011, 78, 131–137. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Bokemeyer, B.; Drabik, A.; Stallmach, A.; Schreiber, S.; Vedolizumab Germany Consortium. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice—A nationwide consecutive German cohort study. Aliment. Pharmacol. Ther. 2016, 43, 1090–1102. [Google Scholar] [CrossRef]
- Eriksson, C.; Marsal, J.; Bergemalm, D.; Vigren, L.; Björk, J.; Eberhardson, M.; Karling, P.; Söderman, C.; SWIBREG Vedolizumab Study Group; Myrelid, P.; et al. Long-term effectiveness of vedolizumab in inflammatory bowel disease: A national study based on the Swedish National Quality Registry for Inflammatory Bowel Disease (SWIBREG). Scand. J. Gastroenterol. 2017, 52, 722–729. [Google Scholar] [CrossRef]
- Amiot, A.; Grimaud, J.C.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2016, 14, 1593–1601. [Google Scholar] [CrossRef]
- Kopylov, U.; Ron, Y.; Avni-Biron, I.; Koslowsky, B.; Waterman, M.; Daher, S.; Ungar, B.; Yanai, H.; Maharshak, N.; Ben-Bassat, O.; et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease-the Israeli real-world experience. Inflamm. Bowel Dis. 2017, 23, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Vivio, E.E.; Kanuri, N.; Gilbertsen, J.J.; Monroe, K.; Dey, N.; Chen, C.H.; Gutierrez, A.M.; Ciorba, M.A. Vedolizumab effectiveness and safety over the first year of use in an IBD clinical practice. J. Crohns Colitis 2016, 10, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Jiang, X.; Peerani, F.; Narula, N.; Chaudrey, K.; Whitehead, D.; Hudesman, D.; Lukin, D.; Swaminath, A.; et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: Results from the US VICTORY consortium. Am. J. Gastroenterol. 2016, 111, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Peerani, F.; Meserve, J.; Kochhar, G.; Chaudrey, K.; Hartke, J.; Chilukuri, P.; Koliani-Pace, J.; Winters, A.; Katta, L.; et al. Vedolizumab for ulcerative colitis: Treatment outcomes from the VICTORY consortium. Am. J. Gastroenterol. 2018, 113, 1345–1354. [Google Scholar] [CrossRef]
- Vermeire, S.; Loftus, E.V.; Colombel, J.F.; Feagan, B.; Sandborn, W.; Danese, S.; D’Haens, G.; Kaser, A.; Panaccione, R.; Rubin, D.; et al. Long-term effectiveness and safety of vedolizumab in patients with Crohn’s disease: 5-year cumulative exposure of GEMINI 2 completers rolling into the GEMINI open-label extension study. Gastroenterology 2017, 152, S601. [Google Scholar]
- Amiot, A.; Serrero, M.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: A prospective multicenter cohort study. Aliment. Pharmacol. Ther. 2017, 46, 310–321. [Google Scholar] [CrossRef]
- Amiot, A.; Serrero, M.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. Three-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: A prospective multi-center cohort study. Aliment. Pharmacol. Therap. 2019, 50, 40–53. [Google Scholar] [CrossRef]
- Stallmach, A.; Langbein, C.; Atreya, R.; Bruns, T.; Dignass, A.; Ende, K.; Hampe, J.; Hartmann, F.; Neurath, M.F.; Maul, J.; et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory Bowel disease—A prospective multicenter observational study. Aliment. Pharmacol. Ther. 2016, 44, 1199–1212. [Google Scholar] [CrossRef]
- Danese, S.; Sandborn, W.J.; Colombel, J.F.; Vermeire, S.; Glover, S.C.; Rimola, J.; Siegelman, J.; Jones, S.; Bornstein, J.D.; Feagan, B.G. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology 2019, 157, 1007–1018. [Google Scholar] [CrossRef]
- Löwenberg, M.; Vermeire, S.; Mostafavi, N.; Hoentjen, F.; Franchimont, D.; Bossuyt, P.; Hindryckx, P.; Rispens, T.; de Vries, A.; van der Woude, C.J.; et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn’s disease. Gastroenterology 2019, 157, 997–1006. [Google Scholar]
- Scarozza, P.; Marafini, I.; Laudisi, F.; Troncone, E.; Schmitt, H.; Lenti, M.V.; Costa, S.; Rocchetti, I.; De Cristofaro, E.; Salvatori, S.; et al. Extent of mucosal inflammation in ulcerative colitis influences the clinical remission induced by vedolizumab. J. Clin. Med. 2020, 9, 385. [Google Scholar] [CrossRef]
- Lönnkvist, M.H.; Befrits, R.; Lundberg, J.O.; Lundahl, J.; Fagerberg, U.L.; Hjortswang, H.; van Hage, M.; Hellström, P.M. Infliximab in clinical routine: Experience with Crohn’s disease and biomarkers of inflammation over 5 years. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Assa, A.; Hartman, C.; Weiss, B.; Broide, E.; Rosenbach, Y.; Zevit, N.; Bujanover, Y.; Shamir, R. Long-term outcome of tumor necrosis factor alpha antagonist’s treatment in pediatric Crohn’s disease. J. Crohns Colitis 2013, 77, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Ramos-Rivers, C.; Regueiro, M.; Koutroumpakis, E.; Click, B.; Schwartz, M.; Swoger, J.; Baidoo, L.; Hashash, J.G.; Barrie, A.; et al. The influence of anti-tumor necrosis factor agents on hemoglobin levels of patients with inflammatory Bowel disease. Inflamm. Bowel Dis. 2015, 21, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
| Patients with ACD (35/75) | Patients without ACD (40/75) | p Value | |
|---|---|---|---|
| Male gender | 15 (43%) | 21 (52%) | p = 0.695 |
| Age < 65 years | 31 (88%) | 31 (77%) | p = 0.206 |
| Crohn′s disease | 11 (31%) | 14 (35%) | p = 0.743 |
| Ulcerative colitis | 24 (69%) | 26 (65%) | p = 0.743 |
| Current smokers | 3 (8%) | 6 (15%) | p = 0.392 |
| Previous anti-TNF | 26 (74%) | 25 (62%) | p = 0.275 |
| Concomitant steroids | 18 (51%) | 23 (57%) | p = 0.598 |
| Concomitant immunosuppressors | 1 (3%) | 5 (12%) | p = 0.124 |
| Concomitant immuno-inflammatory disorders § | 5 (14%) | 4 (10%) | p = 0.324 |
| Severe clinical activity | 3 (8%) | 1 (2%) | p = 0.243 |
| Moderate clinical activity | 23 (66%) | 29 (73%) | p = 0.524 |
| Mild clinical activity | 9 (26%) | 10 (25%) | p = 0.943 |
| Severe endoscopic activity * | 21 (66%) | 23 (66%) | p = 0.993 |
| Moderate endoscopic activity | 3 (9%) | 9 (26%) | p = 0.081 |
| Mild endoscopic activity | 8 (25%) | 3 (8%) | p = 0.069 |
| Hemoglobin (median, IQR) (gr/dL) | 10.9 (10.35–12.7) | 13.8 (13.2–15.1) | p = 0.0002 |
| Ferritin value (median, IQR) (µg/L) | 86 (45–143) | 103 (84.7–189) | p = 0.019 |
| Transferrin saturation (median, IQR) (%) | 16 (13.2–20) | 22 (21.6–37.1) | p = 0.002 |
| CRP > 5 mg/L | 23 (66%) | 20 (50%) | p = 0.169 |
| CRP value (median, IQR) (mg/L) | 10 (3.75–56.3) | 6.8 (2.85–52) | p = 0.849 |
| IBD clinical response to Vedolizumab at week 14 | 22 (63%) | 22 (55%) | p = 0.490 |
| IBD clinical response to Vedolizumab at week 24 | 22 (63%) | 21 (52%) | p = 0.365 |
| Variable | Patients with ACD Improvement (13/35) | Patients without ACD Improvement (11/35) | p Value |
|---|---|---|---|
| Male gender | 7 (54%) | 4 (36%) | p = 0.391 |
| Age < 65 years | 10 (77%) | 11 (100%) | p = 0.222 |
| Crohn′s Disease | 7 (54%) | 2 (18%) | p = 0.072 |
| Ulcerative colitis | 6 (46%) | 9 (82%) | p = 0.072 |
| Current smokers | 1 (8%) | 0 | p = 1 |
| Previous anti-TNF | 11 (85%) | 7 (64%) | p = 0.236 |
| Concomitant steroids | 8 (61%) | 5 (45%) | p = 0.430 |
| Concomitant immunosuppressors | 1 (8%) | 0 | p = 1 |
| Hemoglobin (median, IQR) (gr/dL) | 10.9 (8.7–12.7) | 11.4 (8.9–12.3) | p = 0.865 |
| Mild anemia (hemoglobin ≥ 9.5 gr/dL) | 11 (85%) | 10 (91%) | p = 0.642 |
| Moderate anemia (hemoglobin 8–9.5 gr/dL) | 2 (15%) | 1 (9%) | p = 0.642 |
| Severe clinical activity ∫ | 1 (8%) | 2 (18%) | p = 0.438 |
| Moderate clinical activity | 10 (77%) | 5 (45%) | p = 0.112 |
| Mild clinical activity | 2 (15%) | 4 (36%) | p = 0.236 |
| Severe endoscopic activity * | 7 (58%) | 9 (82%) | p = 0.221 |
| Moderate endoscopic activity | 5 (42%) | 1 (9%) | p = 0.075 |
| Mild endoscopic activity | 0 | 1 (9%) | p = 0.478 |
| CRP > 5 mg/L | 10 (77%) | 5 (45%) | p = 0.112 |
| CRP value (median, IQR) (mg/L) | 10.5 (6–28) | 3.9 (1.8–40) | p = 0.033 |
| Iron therapy for anemia | 6 (46%) | 7 (64%) | p = 0.391 |
| IBD clinical response to Vedolizumab at week 14 | 7 (54%) | 5 (45%) | p = 0.682 |
| IBD clinical response to Vedolizumab at week 24 | 7 (54%) | 5 (45%) | p = 0.682 |
| Variable | Patients with ACD Resolution (11/35) | Patients without ACD Improvement (11/35) | p Value |
|---|---|---|---|
| Male gender | 4 (36%) | 4 (36%) | p = 1 |
| Age < 65 years | 10 (91%) | 11 (100%) | p = 1 |
| Crohn′s Disease | 2 (18%) | 2 (18%) | p = 1 |
| Ulcerative colitis | 9 (82%) | 9 (82%) | p = 1 |
| Current smokers | 1 (9%) | 2 (18%) | p = 0.534 |
| Previous anti-TNF | 8 (73%) | 7 (64%) | p = 0.647 |
| Concomitant steroids | 5 (45%) | 5 (45%) | p = 1 |
| Concomitant immunosuppressors | 0 | 0 | p = 1 |
| Hemoglobin (median, IQR) (gr/dL) | 10.9 (8.3–12.5) | 11.4 (8.9–12.3) | p = 0.373 |
| Mild anemia (hemoglobin ≥ 9.5 gr/dL) | 10 (91%) | 10 (91%) | p = 1 |
| Moderate anemia (hemoglobin 8–9.5 gr/dL) | 1 (9%) | 1 (9%) | p = 1 |
| Severe clinical activity ∫ | 0 | 2 (18%) | p = 0.476 |
| Moderate clinical activity | 8 (73%) | 5 (45%) | p = 0.193 |
| Mild clinical activity | 3 (27%) | 4 (36%) | p = 0.647 |
| Severe endoscopic activity * | 5 (56%) | 9 (82%) | p = 0.202 |
| Moderate endoscopic activity | 2 (22%) | 1 (9%) | p = 0.413 |
| Mild endoscopic activity | 2 (22%) | 1 (9%) | p = 0.413 |
| CRP > 5 mg/L | 8 (73%) | 5 (45%) | p = 0.193 |
| CRP value (median, IQR) (mg/L) | 13.3 (5.65–56.3) | 3.9 (1.8–40) | p = 0.138 |
| Iron therapy for anemia | 4 (36%) | 7 (64%) | p = 0.200 |
| IBD clinical response to Vedolizumab at week 14 | 10 (91%) | 5 (45%) | p = 0.022 |
| IBD clinical response to Vedolizumab at week 24 | 10 (91%) | 5 (45%) | p = 0.022 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarozza, P.; De Cristofaro, E.; Scucchi, L.; Rocchetti, I.; Marafini, I.; Neri, B.; Salvatori, S.; Biancone, L.; Calabrese, E.; Monteleone, G. Effect of Vedolizumab on Anemia of Chronic Disease in Patients with Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 2126. https://doi.org/10.3390/jcm9072126
Scarozza P, De Cristofaro E, Scucchi L, Rocchetti I, Marafini I, Neri B, Salvatori S, Biancone L, Calabrese E, Monteleone G. Effect of Vedolizumab on Anemia of Chronic Disease in Patients with Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2020; 9(7):2126. https://doi.org/10.3390/jcm9072126
Chicago/Turabian StyleScarozza, Patrizio, Elena De Cristofaro, Ludovica Scucchi, Irene Rocchetti, Irene Marafini, Benedetto Neri, Silvia Salvatori, Livia Biancone, Emma Calabrese, and Giovanni Monteleone. 2020. "Effect of Vedolizumab on Anemia of Chronic Disease in Patients with Inflammatory Bowel Diseases" Journal of Clinical Medicine 9, no. 7: 2126. https://doi.org/10.3390/jcm9072126
APA StyleScarozza, P., De Cristofaro, E., Scucchi, L., Rocchetti, I., Marafini, I., Neri, B., Salvatori, S., Biancone, L., Calabrese, E., & Monteleone, G. (2020). Effect of Vedolizumab on Anemia of Chronic Disease in Patients with Inflammatory Bowel Diseases. Journal of Clinical Medicine, 9(7), 2126. https://doi.org/10.3390/jcm9072126





