Management of Bleeding Events Associated with Antiplatelet Therapy: Evidence, Uncertainties and Pitfalls
Abstract
1. Introduction
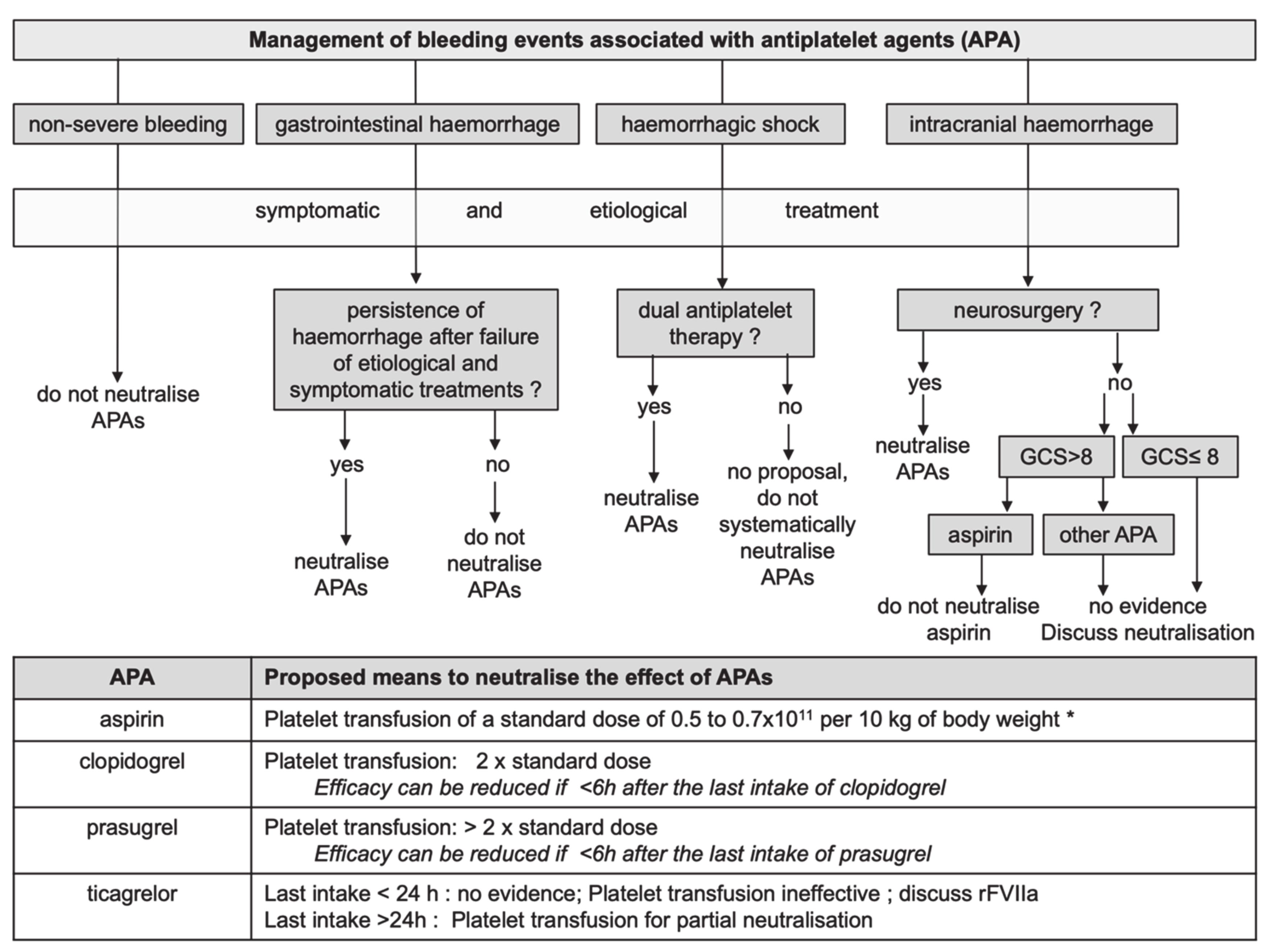
2. Means to Neutralise APAs
3. Management of Bleeding Associated with APAs
3.1. Haemorrhagic Shock
3.2. Intracranial Haemorrhages (ICH)
3.3. Gastrointestinal Haemorrhage
3.4. Non-Severe Haemorrhages
4. Place of Platelet Function Testing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Godier, A.; Garrigue, D.; Lasne, D.; Fontana, P.; Bonhomme, F.; Collet, J.; De Maistre, E.; Ickx, B.; Gruel, Y.; Mazighi, M.; et al. Management of antiplatelet therapy for non elective invasive procedures of bleeding complications: proposals from the French working group on perioperative haemostasis (GIHP), in collaboration with the French Society of Anaesthesia and Intensive Care Medicine (SFAR). Anaesth. Crit. Care Pain Med. 2019, 38, 289–302. [Google Scholar] [CrossRef]
- Bonhomme, F.; Bonvini, R.; Reny, J.-L.; Poncet, A.; Fontana, P. Impact of non-inhibited platelet supplementation on platelet reactivity in patients treated with prasugrel or ticagrelor for an acute coronary syndrome: Anex vivostudy. Platelets 2015, 26, 324–330. [Google Scholar] [CrossRef]
- Martin, A.-C.; Berndt, C.; Calmette, L.; Philip, I.; Decouture, B.; Gaussem, P.; Gouin-Thibault, I.; Samama, C.-M.; Bachelot-Loza, C.; Godier, A. The effectiveness of platelet supplementation for the reversal of ticagrelor-induced inhibition of platelet aggregation. Eur. J. Anaesthesiol. 2016, 33, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Godier, A.; Taylor, G.; Gaussem, P. Inefficacy of Platelet Transfusion to Reverse Ticagrelor. N. Engl. J. Med. 2015, 372, 196–197. [Google Scholar] [CrossRef]
- Zafar, M.U.; Smith, D.A.; Baber, U.; Sartori, S.; Chen, K.; Lam, D.W.; Linares-Koloffon, C.A.; Rey-Mendoza, J.; Britez, G.J.; Escolar, G.; et al. Impact of Timing on the Functional Recovery Achieved With Platelet Supplementation After Treatment With Ticagrelor. Circ. Cardiovasc. Interv. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Bertling, A.; Fender, A.C.; Schüngel, L.; Rumpf, M.; Mergemeier, K.; Geißler, G.; Sibrowski, W.; Kelsch, R.; Waltenberger, J.; Jakubowski, J.A.; et al. Reversibility of platelet P2Y12 inhibition by platelet supplementation: Ex vivo and in vitro comparisons of prasugrel, clopidogrel and ticagrelor. J. Thromb. Haemost. 2018, 16, 1089–1098. [Google Scholar] [CrossRef]
- Kruger, P.C.; Hirsh, J.; Bhagirath, V.C.; Xu, K.; Dale, B.; De Vries, T.A.C.; Ginsberg, J.S.; Eikelboom, J.; Chan, N.C. In Vitro Reversal of the Anti-Aggregant Effect of Ticagrelor Using Untreated Platelets. Thromb. Haemost. 2018, 118, 1895–1901. [Google Scholar] [CrossRef]
- Hansson, E.C.; Malm, C.J.; Hesse, C.; Hornestam, B.; Dellborg, M.; Rexius, H.; Jeppsson, A. Platelet function recovery after ticagrelor withdrawal in patients awaiting urgent coronary surgery. Eur. J. Cardio Thoracic Surg. 2016, 51, 633–637. [Google Scholar] [CrossRef]
- O’Connor, S.A.; Amour, J.; Mercadier, A.; Martin, R.; Kerneis, M.; Abtan, J.; Brugier, D.; Silvain, J.; Barthélémy, O.; Leprince, P.; et al. Efficacy of Ex Vivo Autologous and In Vivo Platelet Transfusion in the Reversal of P2Y 12 Inhibition by Clopidogrel, Prasugrel, and Ticagrelor. Circ. Cardiovasc. Interv. 2015, 8, e002786. [Google Scholar] [CrossRef]
- Teng, R.; Mitchell, P.D.; Butler, K. The effect of desmopressin on bleeding time and platelet aggregation in healthy volunteers administered ticagrelor. J. Clin. Pharm. Ther. 2014, 39, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Pehrsson, S.; Hansson, K.; Nelander, K.; Nylander, S. Boosting the coagulation restores haemostasis in ticagrelor-treated mice. Blood Coagul. Fibrinolysis 2016, 27, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Hindy-François, C.; Bachelot-Loza, C.; Le Bonniec, B.; Grelac, F.; Dizier, B.; Godier, A.; Emmerich, J.; Gaussem, P.; Samama, C.-M. Recombinant activated factor VII does not reduce bleeding in rabbits treated with aspirin and clopidogrel. Thromb. Haemost. 2010, 104, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, F.; Lecompte, T.; Samama, C.M.; Godier, A.; Fontana, P. Evaluation of recombinant factor VIIa, tranexamic acid and desmopressin to reduce prasugrel-related bleeding. Eur. J. Anaesthesiol. 2018, 35, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Angheloiu, A.A.; Gugiu, G.B.; Ruse, C.; Pandey, R.; Dasari, R.R.; Whatling, C. Ticagrelor Removal from Human Blood. JACC. Basic Transl. Sci. 2017, 2, 135–145. [Google Scholar] [CrossRef]
- Buchanan, A.; Newton, P.; Pehrsson, S.; Inghardt, T.; Antonsson, T.; Svensson, P.; Sjögren, T.; Öster, L.; Janefeldt, A.; Sandinge, A.-S.; et al. Structural and functional characterization of a specific antidote for ticagrelor. Blood 2015, 125, 3484–3490. [Google Scholar] [CrossRef]
- Pehrsson, S.; Johansson, K.J.; Janefeldt, A.; Sandinge, A.-S.; Maqbool, S.; Goodman, J.; Sanchez, J.; Almquist, J.; Gennemark, P.; Nylander, S. Hemostatic effects of the ticagrelor antidote MEDI2452 in pigs treated with ticagrelor on a background of aspirin. J. Thromb. Haemost. 2017, 15, 1213–1222. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Pollack, C.V.; Weitz, J.I.; Jennings, L.K.; Xu, S.; Arnold, S.E.; Umstead, B.R.; Mays, M.C.; Lee, J.S. Antibody-Based Ticagrelor Reversal Agent in Healthy Volunteers. N. Engl. J. Med. 2019, 380, 1825–1833. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.; Fries, D.; et al. Management of severe perioperative bleeding. Eur. J. Anaesthesiol. 2017, 34, 332–395. [Google Scholar] [CrossRef]
- Spahn, D.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef]
- Godier, A.; Roquet, F.; Hamada, S.R. Tranexamic acid: One more step towards its widespread use. Anaesth. Crit. Care Pain Med. 2020, 39, 15–17. [Google Scholar] [CrossRef]
- Mahmoud, K.D.; Sanon, S.; Habermann, E.B.; Lennon, R.J.; Thomsen, K.M.; Wood, D.L.; Zijlstra, F.; Frye, R.L.; Holmes, D.R. Perioperative Cardiovascular Risk of Prior Coronary Stent Implantation Among Patients Undergoing Noncardiac Surgery. J. Am. Coll. Cardiol. 2016, 67, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Baber, U.; Steg, P.G.; Ariti, C.; Weisz, G.; Witzenbichler, B.; Henry, T.D.; Kini, A.S.; Stuckey, T.; Cohen, D.J.; et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013, 382, 1714–1722. [Google Scholar] [CrossRef]
- Ohmori, T.; Kitamura, T.; Onishi, H.; Ishihara, J.; Nojima, T.; Yamamoto, K. Effect of pre-injury anticoagulant and antiplatelet agents on blood loss in elderly patients with severe trauma. Acute Med. Surg. 2015, 3, 114–119. [Google Scholar] [CrossRef]
- Ott, M.M.; Eriksson, E.A.; Vanderkolk, W.; Christianson, D.; Davis, A.; Scholten, D. Antiplatelet and Anticoagulation Therapies Do Not Increase Mortality in the Absence of Traumatic Brain Injury. J. Trauma Inj. Infect. Crit. Care 2010, 68, 560–563. [Google Scholar] [CrossRef]
- Ohmori, T.; Kitamura, T.; Ishihara, J.; Onishi, H.; Nojima, T.; Yamamoto, K.; Tamura, R.; Muranishi, K.; Matsumoto, T.; Tokioka, T. Early predictors for massive transfusion in older adult severe trauma patients. Injury 2017, 48, 1006–1012. [Google Scholar] [CrossRef]
- Jaben, E.A.; Mulay, S.B.; Stubbs, J. Reversing the Effects of Antiplatelet Agents in the Setting of Intracranial Hemorrhage. J. Intensiv. Care Med. 2014, 30, 3–7. [Google Scholar] [CrossRef]
- McNeil, J.J.; Nelson, M.R.; Woods, R.L.; Lockery, J.E.; Wolfe, R.; Reid, C.M.; Kirpach, B.; Shah, R.C.; Ives, D.G.; Storey, E.; et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1519–1528. [Google Scholar] [CrossRef]
- The ASCEND Study Collaborative Group; ASCEND Study Collaborative Group; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1529–1539. [Google Scholar] [CrossRef]
- CAPRIE Steering Committee. A Randomised, Blinded, Trial of Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE). Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef]
- Garratt, K.N.; Weaver, W.D.; Jenkins, R.G.; Pow, T.K.; Mauri, L.; Kereiakes, D.J.; Winters, K.J.; Christen, T.; Allocco, D.J.; Lee, D.P. Prasugrel Plus Aspirin Beyond 12 Months Is Associated With Improved Outcomes After Taxus Liberté Paclitaxel-Eluting Coronary Stent Placement. Circulation 2015, 131, 62–73. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Inohara, T.; Xian, Y.; Liang, L.; Matsouaka, R.A.; Saver, J.L.; Smith, E.E.; Schwamm, L.H.; Reeves, M.J.; Hernandez, A.F.; Bhatt, D.L.; et al. Association of Intracerebral Hemorrhage Among Patients Taking Non-Vitamin K Antagonist vs Vitamin K Antagonist Oral Anticoagulants With In-Hospital Mortality. JAMA 2018, 319, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.I.; Siddiqui, F.M.; Goldstein, J.N.; Cox, M.; Xian, Y.; Matsouaka, R.; Heidenreich, P.A.; Peterson, E.D.; Bhatt, D.L.; Fonarow, G.C.; et al. Association Between Previous Use of Antiplatelet Therapy and Intracerebral Hemorrhage Outcomes. Stroke 2017, 48, 1810–1817. [Google Scholar] [CrossRef]
- Kobayashi, L.; Barmparas, G.; Bosarge, P.; Brown, C.V.; Bukur, M.; Carrick, M.M.; Catalano, R.D.; Holly-Nicolas, J.; Inaba, K.; Kaminski, S.; et al. Novel oral anticoagulants and trauma. J. Trauma Acute Care Surg. 2017, 82, 827–835. [Google Scholar] [CrossRef]
- Naidech, A.M.; Liebling, S.M.; Rosenberg, N.F.; Lindholm, P.F.; Bernstein, R.A.; Batjer, H.H.; Alberts, M.J.; Kwaan, H.C. Early platelet transfusion improves platelet activity and may improve outcomes after intracerebral hemorrhage. Neurocritical Care 2012, 16, 82–87. [Google Scholar] [CrossRef]
- Baschin, M.; Selleng, S.; Zeden, J.-P.; Westphal, A.; Kohlmann, T.; Schroeder, H.W.; Greinacher, A.; Thiele, T. Platelet transfusion to reverse antiplatelet therapy before decompressive surgery in patients with intracranial haemorrhage. Vox Sang. 2017, 112, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.B.; David, T.K.P. Is Platelet Transfusion Effective in Patients Taking Antiplatelet Agents Who Suffer an Intracranial Hemorrhage? J. Emerg. Med. 2015, 49, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Baharoglu, M.I.; Cordonnier, C.; Salman, R.A.-S.; De Gans, K.; Koopman, M.M.; Brand, A.; Majoie, C.B.; Beenen, L.F.; Marquering, H.A.; Vermeulen, M.; et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): A randomised, open-label, phase 3 trial. Lancet 2016, 387, 2605–2613. [Google Scholar] [CrossRef]
- Arnone, G.D.; Kumar, P.; Wonais, M.C.; Esfahani, D.R.; Campbell-Lee, S.A.; Charbel, F.T.; Amin-Hanjani, S.; Alaraj, A.; Seicean, A.; Mehta, A.I. Impact of Platelet Transfusion on Intracerebral Hemorrhage in Patients on Antiplatelet Therapy–An Analysis Based on Intracerebral Hemorrhage Score. World Neurosurg. 2018, 111, e895–e904. [Google Scholar] [CrossRef]
- Li, X.; Sun, Z.; Zhao, W.; Zhang, J.; Chen, J.; Li, Y.; Ye, Y.; Zhao, J.; Yang, X.; Xiang, Y.; et al. Effect of acetylsalicylic acid usage and platelet transfusion on postoperative hemorrhage and activities of daily living in patients with acute intracerebral hemorrhage. J. Neurosurg. 2013, 118, 94–103. [Google Scholar] [CrossRef]
- Kelly, K.; Dumont, L.J. Frozen platelets. Transfus. Apher. Sci. 2019, 58, 23–29. [Google Scholar] [CrossRef]
- Marincowitz, C.; Lecky, F.E.; Townend, W.; Borakati, A.; Fabbri, A.; Sheldon, T. The Risk of Deterioration in GCS13–15 Patients with Traumatic Brain Injury Identified by Computed Tomography Imaging: A Systematic Review and Meta-Analysis. J. Neurotrauma 2018, 35, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Thorn, S.; Güting, H.; Mathes, T.; Schäfer, N.; Maegele, M. The effect of platelet transfusion in patients with traumatic brain injury and concomitant antiplatelet use: A systematic review and meta-analysis. Transfusies 2019, 59, 3536–3544. [Google Scholar] [CrossRef]
- Jehan, F.; Zeeshan, M.; Kulvatunyou, N.; Khan, M.; O’Keeffe, T.; Tang, A.; Gries, L.; Joseph, B. Is There a Need for Platelet Transfusion After Traumatic Brain Injury in Patients on P2Y12 Inhibitors? J. Surg. Res. 2019, 236, 224–229. [Google Scholar] [CrossRef]
- Oakland, K.; Desborough, M.J.; Murphy, M.F.; Schachter, M.; Jairath, V. Rebleeding and Mortality After Lower Gastrointestinal Bleeding in Patients Taking Antiplatelets or Anticoagulants. Clin. Gastroenterol. Hepatol. 2019, 17, 1276–1284.e3. [Google Scholar] [CrossRef]
- Chan, F.K.L.; Ki, E.-L.L.; Wong, G.L.-H.; Ching, J.Y.; Tse, Y.K.; Au, K.W.; Wu, J.C.; Ng, S.C. Risks of Bleeding Recurrence and Cardiovascular Events With Continued Aspirin Use After Lower Gastrointestinal Hemorrhage. Gastroenterology 2016, 151, 271–277. [Google Scholar] [CrossRef]
- Zakko, L.; Rustagi, T.; Douglas, M.; Laine, L. No Benefit From Platelet Transfusion for Gastrointestinal Bleeding in Patients Taking Antiplatelet Agents. Clin. Gastroenterol. Hepatol. 2017, 15, 46–52. [Google Scholar] [CrossRef]
- Acosta, R.D.; Abraham, N.S.; Chandrasekhara, V.; Chathadi, K.V.; Early, D.S.; Eloubeidi, M.A.; Evans, J.A.; Faulx, A.L.; Fisher, D.A.; Fonkalsrud, L.; et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest. Endosc. 2016, 83, 3–16. [Google Scholar] [CrossRef]
- Makris, M.; Van Veen, J.J.; Tait, C.; Mumford, A.D.; Laffan, M.; the British Committee for Standards in Haematology. Guideline on the management of bleeding in patients on antithrombotic agents. Br. J. Haematol. 2012, 160, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Keeling, D.; Tait, R.C.; Watson, H.; the British Committee of Standards for Haematology. Peri-operative management of anticoagulation and antiplatelet therapy. Br. J. Haematol. 2016, 175, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Connelly, C.R.; Yonge, J.; McCully, S.P.; Hart, K.D.; Hilliard, T.C.; Lape, D.E.; Watson, J.J.; Rick, B.; Houser, B.; Deloughery, T.G.; et al. Assessment of three point-of-care platelet function assays in adult trauma patients. J. Surg. Res. 2017, 212, 260–269. [Google Scholar] [CrossRef]
- Kutcher, M.E.; Redick, B.J.; McCreery, R.C.; Crane, I.M.; Greenberg, M.D.; Cachola, L.M.; Nelson, M.F.; Cohen, M.J. Characterization of platelet dysfunction after trauma. J. Trauma Acute Care Surg. 2012, 73, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wohlauer, M.V.; Moore, E.E.; Thomas, S.; Sauaia, A.; Evans, E.; Harr, J.N.; Silliman, C.C.; Ploplis, V.; Castellino, F.J.; Walsh, M. Early Platelet Dysfunction: An Unrecognized Role in the Acute Coagulopathy of Trauma. J. Am. Coll. Surg. 2012, 214, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Castellino, F.J.; Chapman, M.P.; Donahue, D.L.; Thomas, S.; Moore, E.E.; Wohlauer, M.V.; Fritz, B.; Yount, R.; Ploplis, V.; Davis, P.; et al. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J. Trauma Acute Care Surg. 2014, 76, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.A.; Parry, P.V.; Bauer, J.S.; Zusman, B.E.; Panczykowski, D.M.; Puccio, A.M.; Okonkwo, D.O. Use of Aspirin and P2Y12 Response Assays in Detecting Reversal of Platelet Inhibition with Platelet Transfusion in Patients With Traumatic Brain Injury on Antiplatelet Therapy. Neurosurgery 2016, 80, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Bachelani, A.M.; Bautz, J.T.; Sperry, J.L.; Corcos, A.; Zenati, M.; Billiar, T.R.; Peitzman, A.B.; Marshall, G.T. Assessment of platelet transfusion for reversal of aspirin after traumatic brain injury. Surgery 2011, 150, 836–843. [Google Scholar] [CrossRef]
- Taylor, G.; Osinski, D.; Thevenin, A.; Devys, J.M. Is platelet transfusion efficient to restore platelet reactivity in patients who are responders to aspirin and/or clopidogrel before emergency surgery? J. Trauma Acute Care Surg. 2013, 74, 1367–1369. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godier, A.; Albaladejo, P.; on Perioperative Haemostasis Group, t.F.W.G. Management of Bleeding Events Associated with Antiplatelet Therapy: Evidence, Uncertainties and Pitfalls. J. Clin. Med. 2020, 9, 2318. https://doi.org/10.3390/jcm9072318
Godier A, Albaladejo P, on Perioperative Haemostasis Group tFWG. Management of Bleeding Events Associated with Antiplatelet Therapy: Evidence, Uncertainties and Pitfalls. Journal of Clinical Medicine. 2020; 9(7):2318. https://doi.org/10.3390/jcm9072318
Chicago/Turabian StyleGodier, Anne, Pierre Albaladejo, and the French Working Group on Perioperative Haemostasis (GIHP) Group. 2020. "Management of Bleeding Events Associated with Antiplatelet Therapy: Evidence, Uncertainties and Pitfalls" Journal of Clinical Medicine 9, no. 7: 2318. https://doi.org/10.3390/jcm9072318
APA StyleGodier, A., Albaladejo, P., & on Perioperative Haemostasis Group, t. F. W. G. (2020). Management of Bleeding Events Associated with Antiplatelet Therapy: Evidence, Uncertainties and Pitfalls. Journal of Clinical Medicine, 9(7), 2318. https://doi.org/10.3390/jcm9072318




