Elevated Plasma Vitamin B12 in Patients with Hepatic Glycogen Storage Diseases
Abstract
:1. Background
2. Patients and Methods
2.1. Patients
2.2. Handling of Blood Samples
2.3. Determination of Total Vitamin B12, Triglycerides and Transaminases in Plasma
2.4. Determination of tHcy and MMA
2.5. Calculation of the Combined Vitamin B12 Index (cB12)
2.6. Estimation of Vitamin B12 Intake
2.7. Statistical Analyses
3. Results
3.1. Characteristics of the Cohort
3.2. Assessment of Vitamin B12 Intake and Correlation between Plasma Vitamin B12 Concentration and Vitamin B12 Intake
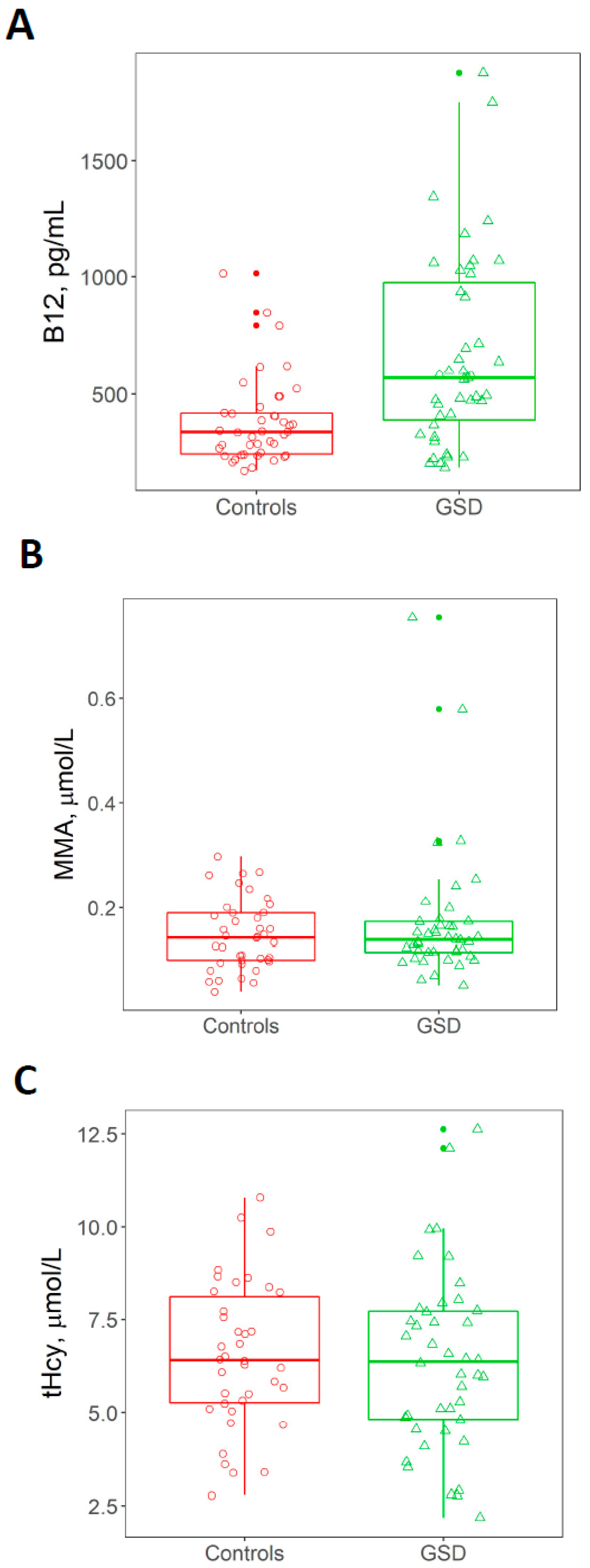
3.3. Plasma Concentration of tHcy, MMA and Vitamin B12
3.4. Combined Vitamin B12 Index, cB12
3.5. Associations between Vitamin B12 Status and Age, Gender and BMI in GSD
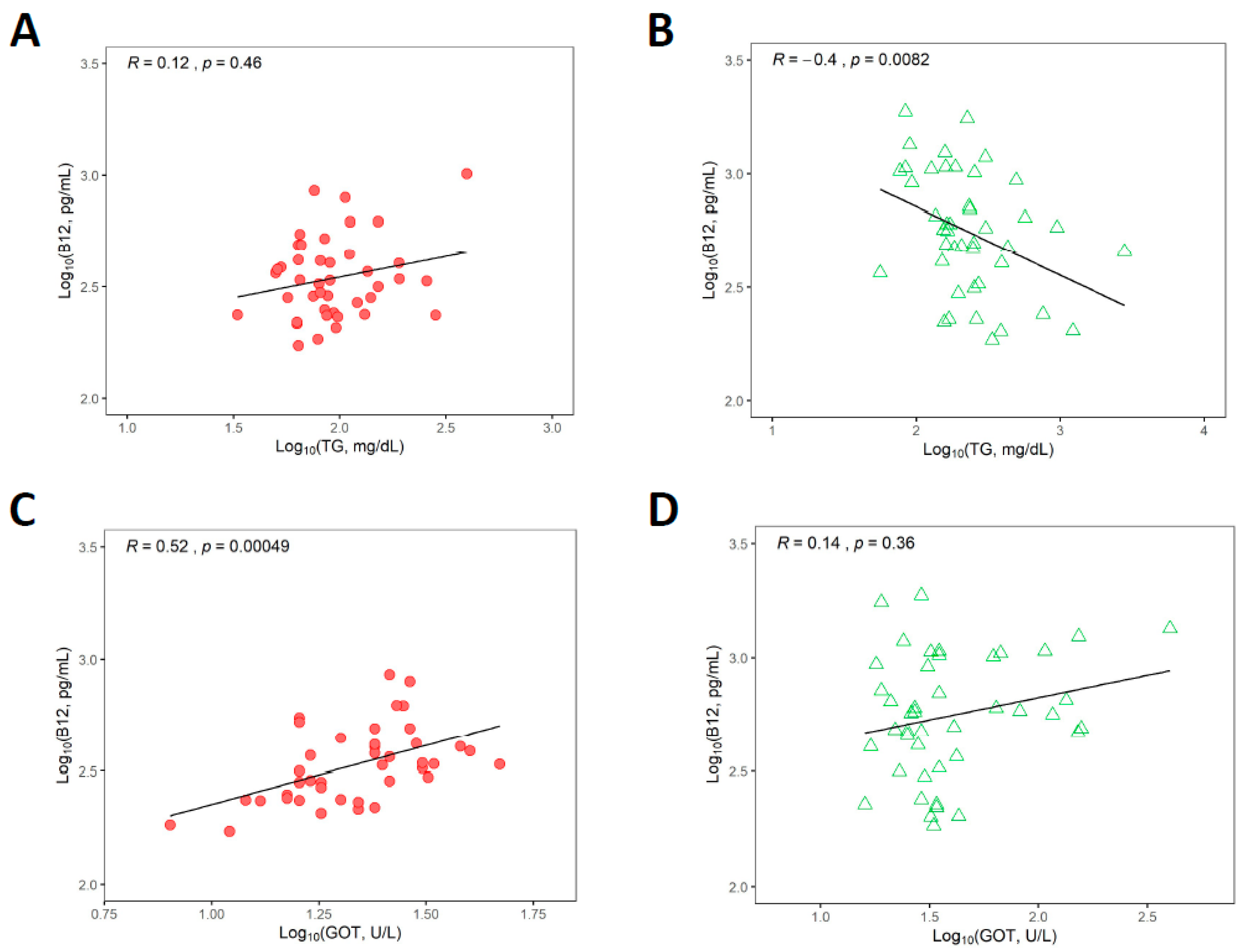
3.6. Associations between Vitamin B12 Status and Triglycerides and Liver Transaminases
3.7. Relationships between Biomarkers upon Merging Healthy Control and GSD Groups as a Continuum
4. Discussion
4.1. Causes and Biological Activity of Elevated Plasma Vitamin B12
4.2. Biomarkers of Vitamin B12 Status in GSD
4.3. Vitamin B12 Status and Liver Disease in GSD
4.4. Behaviour of Biomarkers in a Mathematical Continuum of Healthy Controls and GSD Patients
4.5. Clinical Implications of Elevated Vitamin B12 in Patients with Hepatic GSD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| cB12 | Combined vitamin B12 index |
| Cbl | Cobalamin |
| DGE | German Society of Nutrition |
| GSD | Glycogen storage disease |
| tHcy | Total plasma homocysteine |
| MCM | Methylmalonyl-CoA mutase |
| MMA | Methylmalonic acid |
| MS | Methionine synthase |
References
- Chen, M.A.; Weinstein, D.A. Glycogen storage diseases: Diagnosis, treatment and outcome. Transl. Sci. Rare Dis. 2016, 1, 45–72. [Google Scholar] [CrossRef] [Green Version]
- Martens, J.H.; Barg, H.; Warren, M.J.; Jahn, D. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 2002, 58, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; Lysne, V.; Bjorke-Monsen, A.L.; Behringer, S.; Grunert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci. 2016, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedosov, S.N. Physiological and molecular aspects of cobalamin transport. Sub-Cell. Biochem. 2012, 56, 347–367. [Google Scholar]
- Fedosov, S.N.; Berglund, L.; Fedosova, N.U.; Nexo, E.; Petersen, T.E. Comparative analysis of cobalamin binding kinetics and ligand protection for intrinsic factor, transcobalamin, and haptocorrin. J. Biol. Chem. 2002, 277, 9989–9996. [Google Scholar] [CrossRef] [Green Version]
- Juul, C.B.; Fedosov, S.N.; Nexo, E.; Heegaard, C.W. Kinetic analysis of transcellular passage of the cobalamin-transcobalamin complex in Caco-2 monolayers. Mol. Biol. Cell 2019, 30, 467–477. [Google Scholar] [CrossRef]
- Pons, L.; Guy, M.; Lambert, D.; Hatier, R.; Gueant, J. Transcytosis and coenzymatic conversion of [(57)Co]cobalamin bound to either endogenous transcobalamin II or exogenous intrinsic factor in caco-2 cells. Cell. Physiol. Biochem. 2000, 10, 135–148. [Google Scholar] [CrossRef]
- Hannibal, L.; Bolisetty, K.; Axhemi, A.; DiBello, P.M.; Quadros, E.V.; Fedosov, S.; Jacobsen, D.W. Transcellular transport of cobalamin in aortic endothelial cells. FASEB J. 2018, 32, 5506–5519. [Google Scholar] [CrossRef]
- Zulfiqar, A.A.; Sebaux, A.; Drame, M.; Andres, E. Hypervitaminemia B12 and malignant diseases: Report of a cross-sectional study in an acute geriatric unit. Ann. Biol. Clin. 2017, 75, 193–203. [Google Scholar] [CrossRef]
- Ermens, A.A.; Vlasveld, L.T.; Lindemans, J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin. Biochem. 2003, 36, 585–590. [Google Scholar] [CrossRef]
- Arendt, J.F.; Pedersen, L.; Nexo, E.; Sorensen, H.T. Elevated plasma vitamin B12 levels as a marker for cancer: A population-based cohort study. J. Natl. Cancer Inst. 2013, 105, 1799–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanidi, A.; Carreras-Torres, R.; Larose, T.L.; Yuan, J.M.; Stevens, V.L.; Weinstein, S.J.; Albanes, D.; Prentice, R.; Pettinger, M.; Cai, Q.; et al. Is high vitamin B12 status a cause of lung cancer? Int. J. Cancer 2019, 145, 1499–1503. [Google Scholar] [CrossRef] [Green Version]
- Baker, H.; Frank, O.; DeAngelis, B. Plasma vitamin B12 titres as indicators of disease severity and mortality of patients with alcoholic hepatitis. Alcohol. Alcohol. 1987, 22, 1–5. [Google Scholar] [PubMed]
- Baker, H.; Leevy, C.B.; DeAngelis, B.; Frank, O.; Baker, E.R. Cobalamin (vitamin B12) and holotranscobalamin changes in plasma and liver tissue in alcoholics with liver disease. J. Am. Coll. Nutr. 1998, 17, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Osifo, B.O.; Ayoola, A.; Parmentier, Y.; Gerard, P.; Nicolas, J.P. Correlation between serum enzymes and serum unsaturated vitamin B12 binding proteins in primary liver carcinoma. Enzyme 1988, 39, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Mechie, N.C.; Goralzcyk, A.D.; Reinhardt, L.; Mihm, S.; Amanzada, A. Association of serum vitamin B12 levels with stage of liver fibrosis and treatment outcome in patients with chronic hepatitis C virus genotype 1 infection: A retrospective study. BMC Res. Notes 2015, 8, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Guerrero, J.L.; Minovic, I.; Groothof, D.; Gruppen, E.G.; Riphagen, I.J.; Kootstra-Ros, J.; Kobold, A.M.; Hak, E.; Navis, G.; Gansevoort, R.T.; et al. Association of Plasma Concentration of Vitamin B12 With All-Cause Mortality in the General Population in the Netherlands. JAMA Netw. Open 2020, 3, e1919274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzywanski, J.; Mikulski, T.; Pokrywka, A.; Mlynczak, M.; Krysztofiak, H.; Fraczek, B.; Ziemba, A. Vitamin B12 Status and Optimal Range for Hemoglobin Formation in Elite Athletes. Nutrients 2020, 1, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suman, S.G.; Gretarsdottir, J.M. Chemical and Clinical Aspects of Metal-Containing Antidotes for Poisoning by Cyanide. Met. Ions Life Sci. 2019, 19. [Google Scholar] [CrossRef]
- Behringer, S.; Wingert, V.; Oria, V.; Schumann, A.; Grunert, S.; Cieslar-Pobuda, A.; Kolker, S.; Lederer, A.K.; Jacobsen, D.W.; Staerk, J.; et al. Targeted Metabolic Profiling of Methionine Cycle Metabolites and Redox Thiol Pools in Mammalian Plasma, Cells and Urine. Metabolites 2019, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- HBlom, J.; van Rooij, A.; Hogeveen, M. A simple high-throughput method for the determination of plasma methylmalonic acid by liquid chromatography-tandem mass spectrometry. Clin. Chem. Lab. Med. 2007, 45, 645–650. [Google Scholar]
- Fedosov, S.N.; Brito, A.; Miller, J.W.; Green, R.; Allen, L.H. Combined indicator of vitamin B12 status: Modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin. Chem. Lab. Med. 2015, 53, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N. Biochemical markers of vitamin B12 deficiency combined in one diagnostic parameter: The age-dependence and association with cognitive function and blood hemoglobin. Clin. Chim. Acta 2013, 422, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Heseker, H.P.D. Die Nährwerttabelle 2019/2020, 6. Auflage, Aktualisierte 6. Auflage 2019; Umschau Zeitschriftenverlag: Wiesbaden, Germany, 2019. [Google Scholar]
- Strohle, A.; Richter, M.; Gonzalez-Gross, M.; Neuhauser-Berthold, M.; Wagner, K.H.; Leschik-Bonnet, E.; Egert, S. The Revised D-A-CH-Reference Values for the Intake of Vitamin B12: Prevention of Deficiency and Beyond. Mol. Nutr. Food Res. 2019, 63, e1801178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, K.L.; Rich, S.; Rosenberg, I.; Jacques, P.; Dallal, G.; Wilson, P.W.; Selhub, J. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring study. Am. J. Clin. Nutr. 2000, 71, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.K.; Hannibal, L.; Hettich, M.; Behringer, S.; Spiekerkoetter, U.; Steinborn, C.; Grundemann, C.; Zimmermann-Klemd, A.M.; Muller, A.; Simmet, T.; et al. Vitamin B12 Status Upon Short-Term Intervention with a Vegan Diet-A Randomized Controlled Trial in Healthy Participants. Nutrients 2019, 1, 2815. [Google Scholar] [CrossRef] [Green Version]
- Palacios, G.; Sola, R.; Barrios, L.; Pietrzik, K.; Castillo, M.J.; Gonzalez-Gross, M. Algorithm for the early diagnosis of vitamin B12 deficiency in elderly people. Nutr. Hosp. 2013, 28, 1447–1452. [Google Scholar] [PubMed]
- Remacha, A.F.; Sarda, M.P.; Canals, C.; Queralto, J.M.; Zapico, E.; Remacha, J.; Carrascosa, C. Role of serum holotranscobalamin (holoTC) in the diagnosis of patients with low serum cobalamin. Comparison with methylmalonic acid and homocysteine. Ann. Hematol. 2014, 93, 565–569. [Google Scholar] [CrossRef]
- Rossi, E.; Beilby, J.P.; McQuillan, B.M.; Hung, J. Biological variability and reference intervals for total plasma homocysteine. Ann. Clin. Biochem. 1999, 36, 56–61. [Google Scholar] [CrossRef]
- Lin, J.S.; Shen, M.C.; Cheng, W.C.; Tsay, W.; Wang, Y.C.; Lin, B.B.; Hung, M.H. Age, sex and vitamin status affect plasma level of homocysteine, but hyperhomocysteinaemia is possibly not an important risk factor for venous thrombophilia in Taiwanese Chinese. Br. J. Haematol. 2002, 117, 159–163. [Google Scholar] [CrossRef]
- Gonzalez-Gross, M.; Benser, J.; Breidenassel, C.; Albers, U.; Huybrechts, I.; Valtuena, J.; Spinneker, A.; Segoviano, M.; Widhalm, K.; Molnar, D.; et al. Gender and age influence blood folate, vitamin B12, vitamin B6, and homocysteine levels in European adolescents: The Helena Study. Nutr. Res. 2012, 32, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Risch, M.; Meier, D.W.; Sakem, B.; Escobar, P.M.; Risch, C.; Nydegger, U.; Risch, L. Vitamin B12 and folate levels in healthy Swiss senior citizens: A prospective study evaluating reference intervals and decision limits. BMC Geriatr. 2015, 15, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adaikalakoteswari, A.; Jayashri, R.; Sukumar, N.; Venkataraman, H.; Pradeepa, R.; Gokulakrishnan, K.; Anjana, R.M.; McTernan, P.G.; Tripathi, G.; Patel, V.; et al. Vitamin B12 deficiency is associated with adverse lipid profile in Europeans and Indians with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adaikalakoteswari, A.; Vatish, M.; Lawson, A.; Wood, C.; Sivakumar, K.; McTernan, P.G.; Webster, C.; Anderson, N.; Yajnik, C.S.; Tripathi, G.; et al. Low maternal vitamin B12 status is associated with lower cord blood HDL cholesterol in white Caucasians living in the UK. Nutrients 2015, 7, 2401–2414. [Google Scholar] [CrossRef]
- Khaire, A.; Rathod, R.; Kale, A.; Joshi, S. Vitamin B12 and omega-3 fatty acids together regulate lipid metabolism in Wistar rats. Prostaglandins Leukot. Essent. Fatty Acids 2015, 99, 7–17. [Google Scholar] [CrossRef]
- Khaire, A.; Rathod, R.; Kale, A.; Joshi, S. Vitamin B12 Deficiency Across Three Generations Adversely Influences Long-chain Polyunsaturated Fatty Acid Status and Cardiometabolic Markers in Rats. Arch. Med. Res. 2016, 47, 427–435. [Google Scholar] [CrossRef]
- Moen, G.H.; Qvigstad, E.; Birkeland, K.I.; Evans, D.M.; Sommer, C. Are serum concentrations of vitamin B-12 causally related to cardiometabolic risk factors and disease? A Mendelian randomization study. Am. J. Clin. Nutr. 2018, 108, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Hauser, E.; Seidl, R.; Freilinger, M.; Male, C.; Herkner, K. Hematologic manifestations and impaired liver synthetic function during valproate monotherapy. Brain Dev. 1996, 18, 105–109. [Google Scholar] [CrossRef]
- Serraj, K.; Mecili, M.; Housni, I.; Andres, E. Hypervitaminemia B12 (high level of cobalamin): Physiopathology, role and interest in clinical practice. Presse Med. 2011, 40, 1120–1127. [Google Scholar] [CrossRef]
- Sugihara, T.; Koda, M.; Okamoto, T.; Miyoshi, K.; Matono, T.; Oyama, K.; Hosho, K.; Okano, J.I.; Isomoto, H.; Murawaki, Y. Falsely Elevated Serum Vitamin B12 Levels Were Associated with the Severity and Prognosis of Chronic Viral Liver Disease. Yonago Acta Med. 2017, 60, 31–39. [Google Scholar]
- Argan, O.; Ural, D.; Karauzum, K.; Bozyel, S.; Aktas, M.; Karauzum, I.Y.; Kozdag, G.; Agir, A.A. Elevated levels of vitamin B12 in chronic stable heart failure: A marker for subclinical liver damage and impaired prognosis. Ther. Clin. Risk Manag. 2018, 14, 1067–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedosov, S.N. Metabolic signs of vitamin B(12) deficiency in humans: Computational model and its implications for diagnostics. Metabolism 2010, 59, 1124–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendt, J.F.; Nexo, E. Cobalamin related parameters and disease patterns in patients with increased serum cobalamin levels. PLoS ONE 2012, 7, e45979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andres, E.; Serraj, K.; Zhu, J.; Vermorken, A.J. The pathophysiology of elevated vitamin B12 in clinical practice. QJM 2013, 106, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieblau, K.S.; Wasserman, L.R.; Herbert, V. Rapid Charcoal Assay for Intrinsic Factor (If), Gastric Juice Unsaturated B12 Binding Capacity, Antibody to If, and Serum Unsaturated B12 Binding Capacity. Blood 1965, 25, 875–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.N.; Qi, Z.D.; Ouyang, Y.W.; Liao, F.L.; Zhang, Y.; Liu, Y. Studies on interaction between Vitamin B12 and human serum albumin. J. Pharm. Biomed. Anal. 2008, 47, 134–139. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Xu, C.; Ji, B. Effect of pH on the interaction of vitamin B12 with bovine serum albumin by spectroscopic approaches. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 83, 598–608. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M. Investigation of the binding affinity in vitamin B12-Bovine serum albumin system using various spectroscopic methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 184, 262–269. [Google Scholar] [CrossRef]
- Dereven’kov, I.A.; Hannibal, L.; Makarov, S.V.; Makarova, A.S.; Molodtsov, P.A.; Koifman, O.I. Characterization of the complex between native and reduced bovine serum albumin with aquacobalamin and evidence of dual tetrapyrrole binding. J. Biol. Inorg. Chem. 2018, 23, 725–738. [Google Scholar] [CrossRef]
- Bowen, R.A.; Drake, S.K.; Vanjani, R.; Huey, E.D.; Grafman, J.; Horne, M.K., 3rd. Markedly increased vitamin B12 concentrations attributable to IgG-IgM-vitamin B12 immune complexes. Clin. Chem. 2006, 52, 2107–2114. [Google Scholar] [CrossRef] [Green Version]
- Remacha, A.F.; Zapico, E.; Sarda, M.P.; Rojas, E.; Simo, M.; Remacha, J.; Homs, R.; Queralto, J.M. Immune complexes and persistent high levels of serum vitamin B12. Int. J. Lab. Hematol. 2014, 36, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.D.; Garcia, M.I.P.; Bauca, J.M.; Mullor, R.V.; Barcelo, A. Persistently increased vitamin B12 concentration due to cobalamin macrocomplexes: A case report and review of the literature. Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vugteveen, I.; Hoeksma, M.; Monsen, A.L.; Fokkema, M.R.; Reijngoud, D.J.; van Rijn, M.; van Spronsen, F.J. Serum vitamin B12 concentrations within reference values do not exclude functional vitamin B12 deficiency in PKU patients of various ages. Mol. Genet. Metab. 2011, 102, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Rocha, J.C.; van Rijn, M.; Ahring, K.; Belanger-Quintana, A.; MacDonald, A.; Dokoupil, K.; Ozel, H.G.; Lammardo, A.M.; Goyens, P.; et al. Micronutrient status in phenylketonuria. Mol. Genet. Metab. 2013, 110, S6–S17. [Google Scholar] [CrossRef]
- Bali, D.S.; Chen, Y.T.; Austin, S.; Goldstein, J.L. Glycogen Storage Disease Type I. In GeneReviews; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1312/ (accessed on 15 June 2020).
- Howard, J.M.; Azen, C.; Jacobsen, D.W.; Green, R.; Carmel, R. Dietary intake of cobalamin in elderly people who have abnormal serum cobalamin, methylmalonic acid and homocysteine levels. Eur. J. Clin. Nutr. 1998, 52, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Van Asselt, D.Z.; de Groot, L.C.; van Staveren, W.A.; Blom, H.J.; Wevers, R.A.; Biemond, I.; Hoefnagels, W.H. Role of cobalamin intake and atrophic gastritis in mild cobalamin deficiency in older Dutch subjects. Am. J. Clin. Nutr. 1998, 68, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Carmel, R. Current concepts in cobalamin deficiency. Annu. Rev. Med. 2000, 51, 357–375. [Google Scholar] [CrossRef]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M.; British, H. Committee for Standards in, Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef]
- Solomon, L.R. Functional cobalamin (vitamin B12deficiency: Role of advanced age and disorders associated with increased oxidative stress. Eur. J. Clin. Nutr. 2015, 69, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Beegle, R.D.; Brown, L.M.; Weinstein, D.A. Regression of hepatocellular adenomas with strict dietary therapy in patients with glycogen storage disease type I. JIMD Rep. 2015, 18, 23–32. [Google Scholar]
- Kumar, K.A.; Lalitha, A.; Pavithra, D.; Padmavathi, I.J.; Ganeshan, M.; Rao, K.R.; Venu, L.; Balakrishna, N.; Shanker, N.H.; Reddy, S.U.; et al. Maternal dietary folate and/or vitamin B12 restrictions alter body composition (adiposity) and lipid metabolism in Wistar rat offspring. J. Nutr. Biochem. 2013, 24, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, V.S.; Ghosh, S.; Sati, S.; Ghose, S.; Kaur, L.; Kumar, K.A.; Shamsudheen, K.V.; Patowary, A.; Singh, M.; Jyothi, V.; et al. Maternal vitamin B12 deficiency in rats alters DNA methylation in metabolically important genes in their offspring. Mol. Cell. Biochem. 2020, 468, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Dalmia, A.; Dib, M.J.; Maude, H.; Harrington, D.J.; Sobczynska-Malefora, A.; Andrew, T.; Ahmadi, K.R. A genetic epidemiological study in British adults and older adults shows a high heritability of the combined indicator of vitamin B12 status (cB12) and connects B12 status with utilization of mitochondrial substrates and energy metabolism. J. Nutr. Biochem. 2019, 70, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Paesold-Burda, P.; Baumgartner, M.R.; Santer, R.; Bosshard, N.U.; Steinmann, B. Elevated serum biotinidase activity in hepatic glycogen storage disorders--a convenient biomarker. J. Inherit. Metab. Dis. 2007, 30, 896–902. [Google Scholar] [CrossRef] [PubMed]
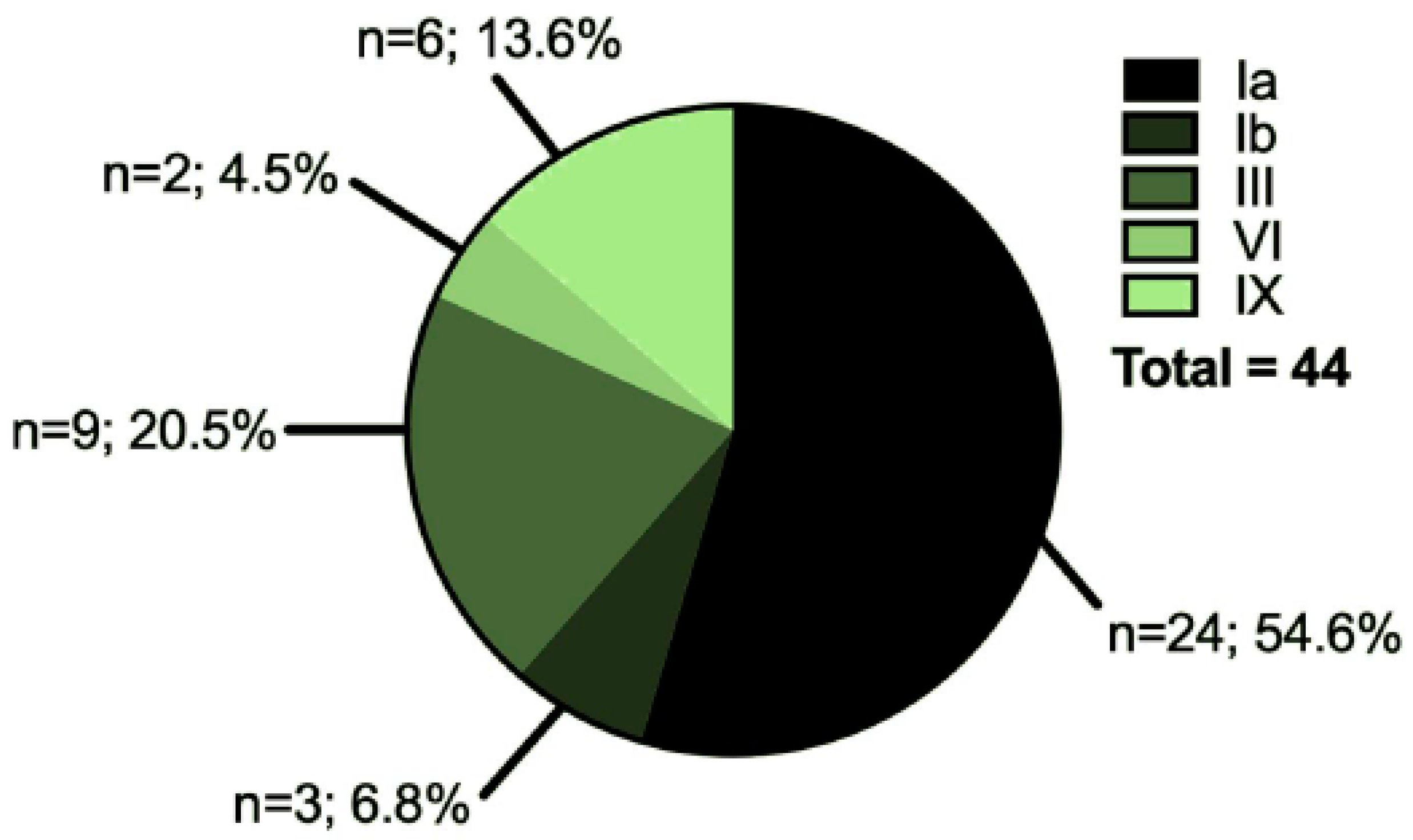



| Healthy Controls | GSD Patients | Healthy Controls vs. GSD Patients | |
|---|---|---|---|
| Biometric parameters and vitamin B12 intake | |||
| Age | 22.67 ± 14.67 (1–62) | 20.77 ± 13.39 (2–59) | NA |
| BMI | 22.22 ± 5.37 (13.7–38.4) | 22.54 ± 4.82 (13.7–35.6) | NA |
| Gender | 19 females, 23 males | 20 females, 24 males | NA |
| Vitamin B12 intake ** (µg/day) | ND | 36.59 ± 177.20 (1.56–1007.47) | NA |
| Biochemical parameters determined in plasma | |||
| Triglycerides (TG) (mg/dL) | 107.64 ± 69.44 (33–397) | 387.32 ± 573.89 (56–2780) | <0.0001 * |
| MMA (µmol/mL) | 0.149 ± 0.07 (0.043–0.2985) | 0.172 ± 0.124 (0.054–0.751) | 0.7132 * |
| tHcy (µmol/L) | 6.53 ± 1.96 (2.77–10.79) | 6.32 ± 2.42 (2.18–12.62) | 0.5452 * |
| Vitamin B12 (pg/mL) | 379 ± 182.93 (172–1015) | 667.28 ± 408.83 (185–1876) | 0.0002 * |
| GOT (ASAT) (IU/L) | 23.02 ± 8.26 (8–47) | 56.86 ± 66.09 (16–401) | <0.0001 * |
| GPT (ALAT) (IU/L) | 20.03 ± 10.89 (<2.5–62) | 66.23 ± 84.94 (10–461) | <0.0001 * |
| Adequate Vitamin B12 Status | Increased Vitamin B12 Status | Decreased Vitamin B12 Status | |
|---|---|---|---|
| Patients (n = 44) | 34 (77.3%) | 8 (18.2%) | 2 (4.6%) |
| Controls (n = 42) | 41 (97.6%) | 1 (2.4%) | 0 (0%) |
| Selected Variables: | Correlation Coefficient | 95% Confidence Interval | p Value |
|---|---|---|---|
| Vitamin B12 and age | −0.11 | −0.41 to 0.21 | 0.475 |
| Vitamin B12 and BMI | −0.11 | −0.42 to 0.22 | 0.516 |
| Vitamin B12 and MMA | 0.12 | −0.20 to 0.43 | 0.442 |
| Vitamin B12 and tHcy | −0.22 | −0.51 to 0.11 | 0.181 |
| Vitamin B12 and GOT (ASAT) | 0.52 | 0.24 to 0.72 | <0.001 |
| Vitamin B12 and GPT (ALAT) | 0.19 | −0.14 to 0.48 | 0.243 |
| Vitamin B12 and triglycerides | 0.12 | −0.20 to 0.41 | 0.46 |
| MMA und tHcy | 0.26 | −0.15 to 0.50 | 0.261 |
| MMA and triglycerides | 0.38 | 0.06 to 0.62 | 0.016 |
| tHcy and triglycerides | 0.21 | −0.13to 0.50 | 0.206 |
| MMA and BMI | 0.17 | −0.17 to 0.47 | 0.319 |
| tHcy and BMI | 0.51 | 0.20 to 0.72 | 0.002 |
| MMA and age | 0.53 | 0.25 to 0.73 | <0.001 |
| tHcy and age | 0.43 | 0.12 to 0.66 | 0.007 |
| Selected Variables: | Correlation Coefficient | 95% Confidence Interval | p Value |
|---|---|---|---|
| Vitamin B12 and age | −0.17 | −0.45 to 0.15 | 0.275 |
| Vitamin B12 and BMI | −0.24 | −0.51 to 0.07 | 0.114 |
| Vitamin B12 and MMA | −0.43 | −0.65 to −0.14 | 0.004 |
| Vitamin B12 and tHcy | −0.48 | −0.69 to −0.20 | 0.001 |
| Vitamin B12 and GOT (ASAT) | 0.14 | −0.17 to 0.43 | 0.356 |
| Vitamin B12 and GPT (ALAT) | 0.12 | −0.19 to 0.41 | 0.435 |
| Vitamin B12 and triglycerides | −0.40 | −0.63 to −0.10 | 0.008 |
| Vitamin B12 and vitamin B12 intake | 0.24 | −0.13 to 0.56 | 0.193 |
| MMA and tHcy | 0.11 | −0.20 to 0.41 | 0.474 |
| MMA and triglycerides | −0.08 | −0.37 to 0.23 | 0.606 |
| tHcy and triglycerides | 0.39 | 0.09 to 0.63 | 0.01 |
| MMA and BMI | −0.17 | −0.45 to 0.14 | 0.278 |
| tHcy and BMI | 0.47 | 0.19 to 0.68 | 0.001 |
| MMA and age | −0.12 | −0.40 to 0.19 | 0.443 |
| tHcy and age | 0.52 | 0.25 to 0.71 | <0.001 |
| Single Marker | Probability * Controls = Patients | Combined Markers | Probability * Controls = Patients |
|---|---|---|---|
| total B12 | 2 × 10−4 | ½ · log10(B12·TG) | 4 × 10−11 |
| GOT | 4 × 10−6 | ½ · log10(GOT·GPT) | 2 × 10−7 |
| GPT | 2 × 10−7 | ⅓ · log10(GOT·GPT·TG) | 4 × 10−12 |
| TG | 1 × 10−8 | ¼ · log10(B12·GOT·GPT·TG) | 3 × 10−12 |
| ½ · log10(GPT·TG) | 2 × 10−15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinkel, J.; Schmitt, J.; Wurm, M.; Rosenbaum-Fabian, S.; Schwab, K.O.; Jacobsen, D.W.; Spiekerkoetter, U.; Fedosov, S.N.; Hannibal, L.; Grünert, S.C. Elevated Plasma Vitamin B12 in Patients with Hepatic Glycogen Storage Diseases. J. Clin. Med. 2020, 9, 2326. https://doi.org/10.3390/jcm9082326
Hinkel J, Schmitt J, Wurm M, Rosenbaum-Fabian S, Schwab KO, Jacobsen DW, Spiekerkoetter U, Fedosov SN, Hannibal L, Grünert SC. Elevated Plasma Vitamin B12 in Patients with Hepatic Glycogen Storage Diseases. Journal of Clinical Medicine. 2020; 9(8):2326. https://doi.org/10.3390/jcm9082326
Chicago/Turabian StyleHinkel, Julia, Johannes Schmitt, Michael Wurm, Stefanie Rosenbaum-Fabian, Karl Otfried Schwab, Donald W. Jacobsen, Ute Spiekerkoetter, Sergey N. Fedosov, Luciana Hannibal, and Sarah C. Grünert. 2020. "Elevated Plasma Vitamin B12 in Patients with Hepatic Glycogen Storage Diseases" Journal of Clinical Medicine 9, no. 8: 2326. https://doi.org/10.3390/jcm9082326







