The Acrosomal Status of Density Purified Spermatozoa Differentiates Men from Couples in IVF and ICSI Treatment and Is Associated with Fecundity
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Collection and Processing of Semen Samples
2.3. Measurement of Acrosomal Status
2.4. Statistical Analysis
2.5. Ethical Approval
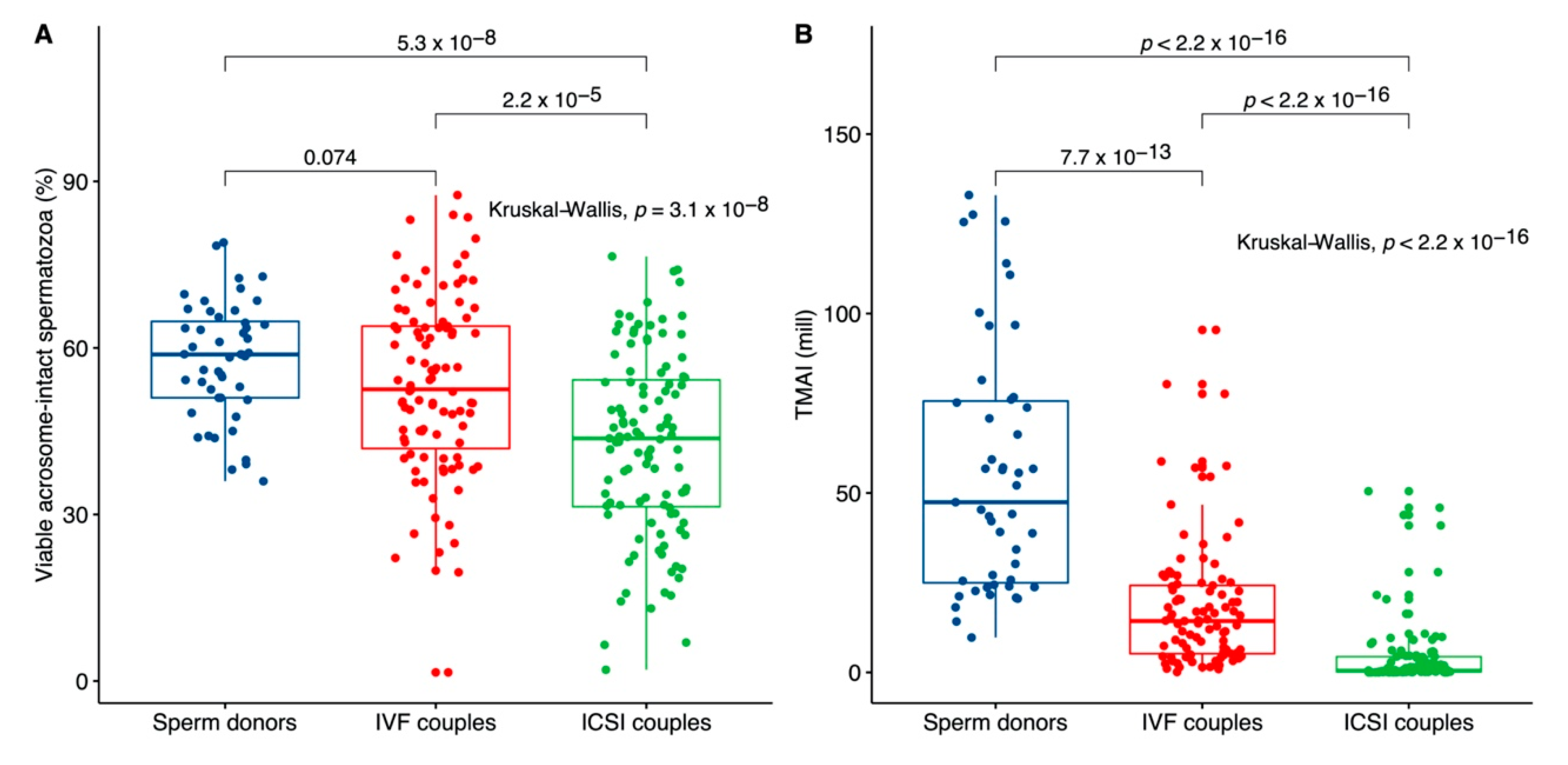
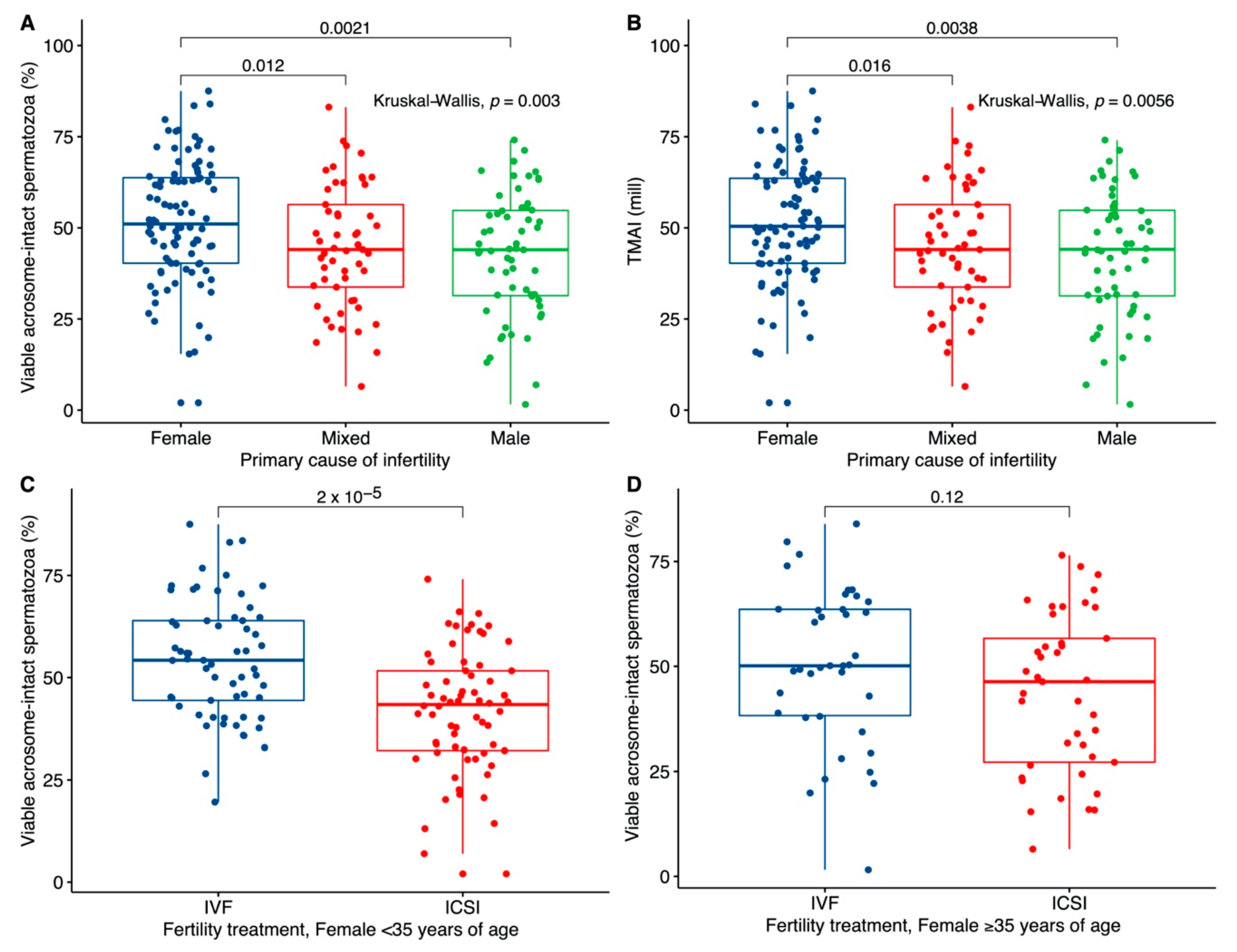
3. Results
3.1. Comparison between Sperm Donors, IVF and ICSI Couples
3.2. Association with the Primary Cause of Infertility
3.3. The Influence of Lifestyle and Intra-Individual Variation
3.4. Association with Fecundity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V.; et al. ART in Europe, 2014: Results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum. Reprod. 2018, 33, 1586–1601. [Google Scholar] [CrossRef]
- García, D.; Massucci, F.A.; Mosca, A.; Ràfols, I.; Rodríguez, A.; Vassena, R. Mapping research in assisted reproduction worldwide. Reprod. Biomed. Online 2020, 40, 71–81. [Google Scholar] [CrossRef]
- Kushnir, V.A.; Barad, D.H.; Albertini, D.F.; Darmon, S.K.; Gleicher, N. Systematic review of worldwide trends in assisted reproductive technology 2004–2013. Reprod. Biol. Endocrinol. 2017, 15, 6. [Google Scholar] [CrossRef]
- Dansk Fertilitetsselskab. Available online: https://fertilitetsselskab.dk/dataark-pr-aar/ (accessed on 7 January 2020).
- Forti, G.; Krausz, C. Evaluation and Treatment of the Infertile Couple. J. Clin. Endocrinol. Metab. 1998, 83, 4177–4188. [Google Scholar] [CrossRef]
- Gerkowicz, S.A.; Crawford, S.B.; Hipp, H.S.; Boulet, S.L.; Kissin, D.M.; Kawwass, J.F. Assisted reproductive technology with donor sperm: National trends and perinatal outcomes. Am. J. Obstet. Gynecol. 2018, 218, 421.e1–421.e10. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010; ISBN 9789241547789. [Google Scholar]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm morphology, motility, and concentration in fertile and infertile men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef]
- Miller, D.J. Review: The epic journey of sperm through the female reproductive tract. Animal 2018, 12, s110–s120. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Martins da Silva, S.J. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Yanagimachi, H.; Rogers, B.J. The use of zona-free animal ova as a test-system for the assessment of the fertilizing capacity of human spermatozoa. Biol. Reprod. 1976, 15, 471–476. [Google Scholar] [CrossRef]
- Uhlenbruck, G.; Herrmann, W.P. Agglutination of normal, coated and enzyme-treated human spermatozoa with heterophile agglutinins. Vox Sang. 1972, 23, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.K. Lectins as probes of the spermatozoon surface. Arch. Androl. 1981, 6, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Egeberg Palme, D.L.; Rehfeld, A.; Bang, A.K.; Nikolova, K.A.; Kjærulff, S.; Petersen, M.R.; Jeppesen, J.V.; Glensbjerg, M.; Juul, A.; Skakkebæk, N.E.; et al. Viable acrosome-intact human spermatozoa in the ejaculate as a marker of semen quality and fertility status. Hum. Reprod. 2018, 33, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, A.; Egeberg Palme, D.L.; Almstrup, K.; Juul, A.; Skakkebaek, N.E. Medium-throughput Screening Assays for Assessment of Effects on Ca2+-Signaling and Acrosome Reaction in Human Sperm. J. Vis. Exp. 2019, e59212. [Google Scholar] [CrossRef]
- Zoppino, F.C.M.; Halón, N.D.; Bustos, M.A.; Pavarotti, M.A.; Mayorga, L.S. Recording and sorting live human sperm undergoing acrosome reaction. Fertil. Steril. 2012, 97, 1309–1315. [Google Scholar] [CrossRef]
- Ng, F.L.; Liu, D.Y.; Baker, H.W. Comparison of Percoll, mini-Percoll and swim-up methods for sperm preparation from abnormal semen samples. Hum. Reprod. 1992, 7, 261–266. [Google Scholar] [CrossRef]
- Prakash, P.; Leykin, L.; Chen, Z.; Toth, T.; Sayegh, R.; Schiff, I.; Isaacson, K. Preparation by differential gradient centrifugation is better than swim-up in selecting sperm with normal morphology (strict criteria). Fertil. Steril. 1998, 69, 722–726. [Google Scholar] [CrossRef]
- Krog, M.; Prior, M.; Carlsen, E.; Loft, A.; Forman, J.; Pinborg, A.; Andersen, A.N. Fertilization failure after IVF in 304 couples—A case-control study on predictors and long-term prognosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 184, 32–37. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Khalil, M.R.; Rasmussen, P.E.; Erb, K.; Laursen, S.B.; Rex, S.; Westergaard, L.G. Intrauterine insemination with donor semen. An evaluation of prognostic factors based on a review of 1131 cycles. Acta Obstet. Gynecol. Scand. 2001, 80, 342–348. [Google Scholar] [CrossRef]
- Amiri, M.F.I. Analysis of prognostic factors for successful outcome in patients undergoning intrauterine insemination. Acta Med. Iran. 2007, 101–106. [Google Scholar]
- Zhang, A.; Ma, X.; Zhang, L.; Zhang, X.; Wang, W. Pregnancy and offspring outcomes after artificial insemination with donor sperm: A retrospective analysis of 1805 treatment cycles performed in Northwest China. Medicine 2019, 98, e14975. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, A.; Egeberg, D.L.; Almstrup, K.; Petersen, J.H.; Dissing, S.; Skakkebæk, N.E. EDC IMPACT: Chemical UV filters can affect human sperm function in a progesterone-like manner. Endocr. Connect. 2018, 7, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, C.; Müller, A.; Egeberg, D.L.; Alvarez, L.; Brenker, C.; Rehfeld, A.; Frederiksen, H.; Wäschle, B.; Kaupp, U.B.; Balbach, M.; et al. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 2014, 15, 758–765. [Google Scholar] [CrossRef]
- Petersen, C.G.; Mauri, A.L.; Vagnini, L.D.; Renzi, A.; Petersen, B.; Mattila, M.; Comar, V.; Ricci, J.; Dieamant, F.; Oliveira, J.B.A.; et al. The effects of male age on sperm DNA damage: An evaluation of 2,178 semen samples. JBRA Assist. Reprod. 2018, 22, 323–330. [Google Scholar]
- Albani, E.; Castellano, S.; Gurrieri, B.; Arruzzolo, L.; Negri, L.; Borroni, E.M.; Levi-Setti, P.E. Male age: Negative impact on sperm DNA fragmentation. Aging 2019, 11, 2749–2761. [Google Scholar] [CrossRef]
- Olesen, I.A.; Joensen, U.N.; Petersen, J.H.; Almstrup, K.; Rajpert-De Meyts, E.; Carlsen, E.; McLachlan, R.; Juul, A.; Jørgensen, N. Decrease in semen quality and Leydig cell function in infertile men: A longitudinal study. Hum. Reprod. 2018, 33, 1963–1974. [Google Scholar] [CrossRef]
- Dunphy, B.C.; Kay, R.; Barratt, C.L.; Cooke, I.D. Female age, the length of involuntary infertility prior to investigation and fertility outcome. Hum. Reprod. 1989, 4, 527–530. [Google Scholar] [CrossRef]
- Navot, D.; Bergh, P.A.; Williams, M.A.; Garrisi, G.J.; Guzman, I.; Sandler, B.; Grunfeld, L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet 1991, 337, 1375–1377. [Google Scholar] [CrossRef]
- Simon, L.; Liu, L.; Murphy, K.; Ge, S.; Hotaling, J.; Aston, K.I.; Emery, B.; Carrell, D.T. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum. Reprod. 2014, 29, 904–917. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Jin, H.; Guo, Y.; Sun, Y. The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl. Androl. Urol. 2019, 8, 356–365. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Baldi, E. Sperm DNA Fragmentation: Mechanisms of Origin. Adv. Exp. Med. Biol. 2019, 1166, 75–85. [Google Scholar]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef]
- Shi, X.; Chan, C.P.S.; Waters, T.; Chi, L.; Chan, D.Y.L.; Li, T.-C. Lifestyle and demographic factors associated with human semen quality and sperm function. Syst. Biol. Reprod. Med. 2018, 64, 358–367. [Google Scholar] [CrossRef]


| Group | IVF | ICSI | Sperm Donors | p-Value |
|---|---|---|---|---|
| Samples/men | 99/99 | 107/107 | 199/138 | |
| Fresh purified samples | 99 | 107 | 48 | |
| Frozen-thawed samples | - | - | 199 | |
| Cycle number | 1.0 (1–6) | 2.0 (1–6) | Unknown | 1.3 × 10−5 |
| Infertility cause | ||||
| Male Factor | 10 | 49 | - | |
| Female Factor | 62 | 30 | - | |
| Mixed factor | 26 | 27 | - | |
| Unknown | 1 | 1 | - | |
| Lifestyle factors | ||||
| Uxor age (years) | 33.0 (22–43) | 33.0 (24–43) | Unknown | 0.492 |
| Vir age (years) | 34.0 (23–58) | 35.0 (27–57) | 26.0 (18–41) *** | 0.541 |
| Uxor BMI | 22.5 (17.4–34.4) n = 82 | 22.8 (17.3–33.6) n = 87 | Unknown | 0.552 |
| Uxor alcohol (units per week) | 0.0 (0.0–6.0) n = 75 | 1.0 (0.0–8.0) n = 82 | Unknown | 0.006 |
| Uxor smoking (cigarettes per day) | 0.0 (0.0–10.0) n = 77 | 0.0 (0.0–15.0) n = 82 | Unknown | 0.501 |
| Vir BMI | 23.8 (22.0–29.0) n = 8 | 25.7 (22.2–35.2) n = 18 | 23.8 (18.0–37.9) * | 0.317 |
| Vir alcohol (units per week) | 1.0 (0.0–14.0) n = 33 | 4.5 (0.0–21.0) n = 44 | Unknown | 0.006 |
| Vir smoking (cigarettes per day) | 0.0 (0.0–15.0) n = 37 | 0.0 (0.0–17.0) n = 45 | Unknown | 0.041 |
| Semen samples | ||||
| Ejaculate volume (mL) | 3.0 (0.5–7.5) | 3.0 (0.5–7.0) | 3.5 (0.7–10.2) *** | 0.500 |
| Spermatozoa concentration (mill/mL) | 67.0 (10.0–250.0) | 15.0 (0.1–118.0) n = 106 | 104.9 (30.6–539.1) *** n = 154 | <2.2 × 10−16 |
| Progressive motile spermatozoa (mill/mL) | 40.0 (4.0–150.0) n = 98 | 5.0 (0.0–50.0) | 56.8 (11.3–305.6) *** n = 154 | <2.2 × 10−16 |
| Percentage viable acrosome-intact after processing (%) | 52.5 (1.6–87.5) | 43.7 (2.0–76.5) | 58.8 (36.0–79.0) *** n = 48 | 2.2 × 10−5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norup, P.B.; Egeberg Palme, D.L.; Petersen, M.R.; Main, K.M.; Almstrup, K. The Acrosomal Status of Density Purified Spermatozoa Differentiates Men from Couples in IVF and ICSI Treatment and Is Associated with Fecundity. J. Clin. Med. 2020, 9, 2327. https://doi.org/10.3390/jcm9082327
Norup PB, Egeberg Palme DL, Petersen MR, Main KM, Almstrup K. The Acrosomal Status of Density Purified Spermatozoa Differentiates Men from Couples in IVF and ICSI Treatment and Is Associated with Fecundity. Journal of Clinical Medicine. 2020; 9(8):2327. https://doi.org/10.3390/jcm9082327
Chicago/Turabian StyleNorup, Pernille Badsberg, Dorte L. Egeberg Palme, Morten R. Petersen, Katharina M. Main, and Kristian Almstrup. 2020. "The Acrosomal Status of Density Purified Spermatozoa Differentiates Men from Couples in IVF and ICSI Treatment and Is Associated with Fecundity" Journal of Clinical Medicine 9, no. 8: 2327. https://doi.org/10.3390/jcm9082327
APA StyleNorup, P. B., Egeberg Palme, D. L., Petersen, M. R., Main, K. M., & Almstrup, K. (2020). The Acrosomal Status of Density Purified Spermatozoa Differentiates Men from Couples in IVF and ICSI Treatment and Is Associated with Fecundity. Journal of Clinical Medicine, 9(8), 2327. https://doi.org/10.3390/jcm9082327






