Efficacy of Modified Treat-and-Extend Aflibercept Regimen for Macular Edema Due to Branch Retinal Vein Occlusion: 1-Year Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Approval
2.2. Methods and Ophthalmological Examination
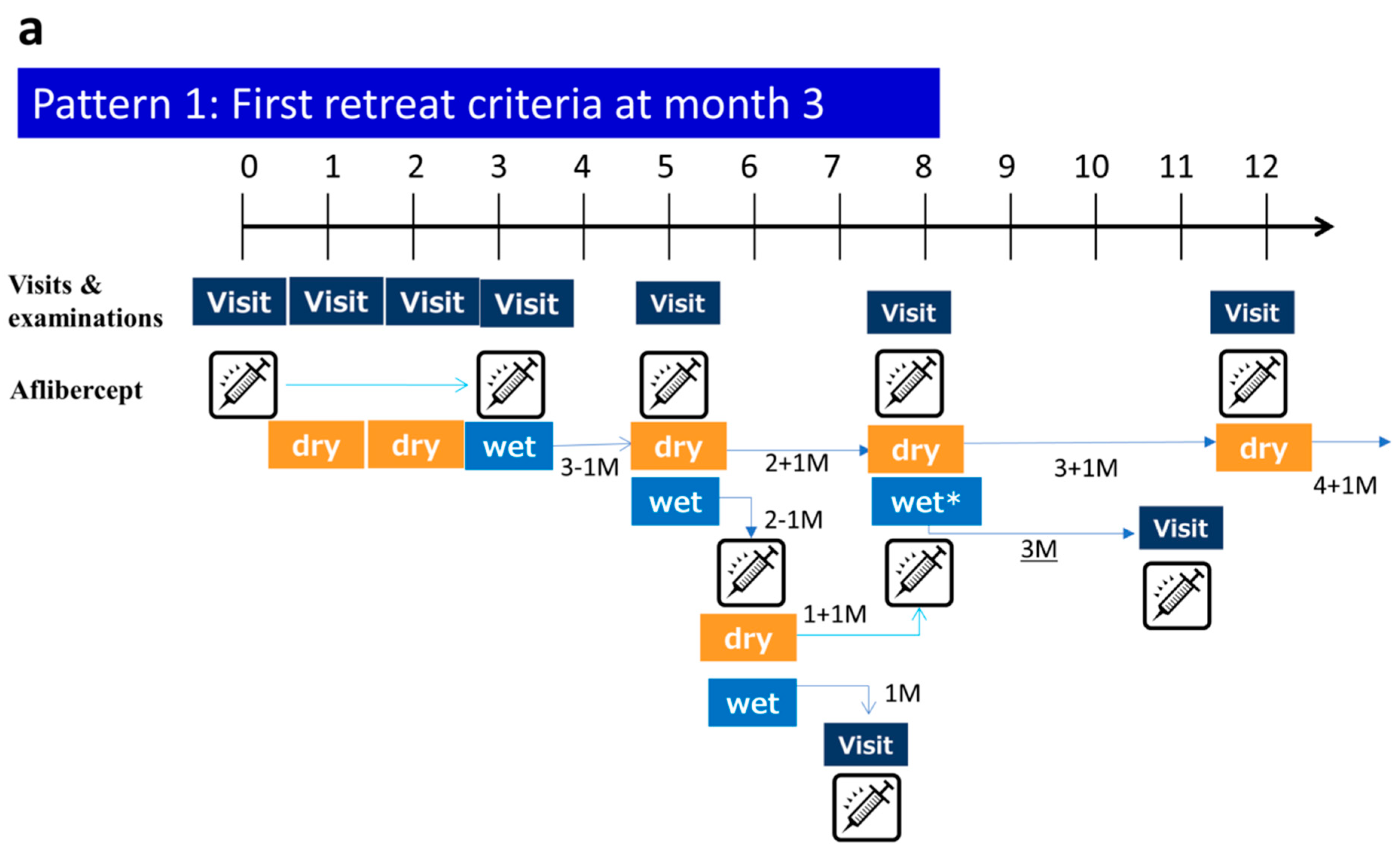
2.3. mTAE Regimen
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. VA Outcomes and CST
3.3. Mean Numbers of IVA Injections and Clinic Visits
3.4. Time to First Recurrence from First Injection
3.5. Comparison between Major BRVO and Macular BRVO
3.6. Safety Outcomes through Month 12
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, R.; Klein, B.E.; Moss, S.E.; Meuer, S.M. The epidemiology of retinal vein occlusion: The Beaver Dam Eye Study. Trans. Am. Ophthalmol. Soc. 2000, 98, 133–141. [Google Scholar] [PubMed]
- Klein, R.; Moss, S.E.; Meuer, S.M.; Klein, B.E.K. The 15-year cumulative incidence of retinal vein occlusion: The Beaver Dam Eye Study. Arch. Ophthalmol. 2008, 126, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.; Paques, M.; Mones, J.; Glacet-Bernard, A. Retinal vein occlusions. Dev. Ophthalmol. 2010, 47, 111–135. [Google Scholar] [PubMed]
- Hayreh, S.S. Ocular vascular occlusive disorders: Natural history of visual outcome. Prog. Retin. Eye Res. 2014, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Wrong, T.Y.; Scott, I.U. Clinical practice. Retinal-vein occlusion. N. Engl. J. Med. 2010, 363, 2135–2144. [Google Scholar]
- Koss, M.J.; Pjister, M.; Rothweiler, F.; Michaelis, M.; Cinatl, J.; Schubert, R.; Koch, F.H. Comparison of cytokine levels from undiluted vitreous of untreated patients with retinal vein occlusion. Acta Ophthalmol. 2012, 90, 98–103. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Heier, J.S.; Feiner, L.; Gray, S.; Saroj, N.; Rundle, A.C.; Murahashi, W.Y.; Rubio, R.G.; BRAVO Investigators. Ranibizumab for macular edema following branch retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology 2010, 117, 1102–1112. [Google Scholar] [CrossRef]
- Clark, W.L.; Boyer, D.S.; Heier, J.S.; Brown, D.M.; Haller, J.A.; Vitti, R.; Kazmi, H.; Berliner, A.J.; Erickson, K.; Chu, K.W.; et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion. 52-week results of the VIBRANT study. Ophthalmology 2016, 123, 330–336. [Google Scholar] [CrossRef]
- Tadayoni, R.; Waldstein, S.M.; Boscia, F.; Gerding, H.; Pearce, I.; Priglinger, S.; Wenzel, A.; Barnes, E.; Gekkieva, M.; Pilz, S.; et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: Six-month results of BRIGHTER. Ophthalmology 2016, 123, 1332–1344. [Google Scholar] [CrossRef]
- Miwa, Y.; Muraoka, Y.; Osaka, R.; Ooto, S.; Murakami, T.; Suzuma, K.; Takahashi, A.; Iida, Y.; Yoshimura, N.; Tsujikawa, A. Ranibizumab for macular edema after branch retinal vein occlusion. Retina 2017, 37, 702–709. [Google Scholar] [CrossRef]
- Kawamura, M.; Hirano, Y.; Yoshida, M.; Mizutani, T.; Sugitani, K.; Yasukawa, T.; Ogura, Y. Six-month results of intravitreal ranibizumab for macular edema after branch retinal vein occlusion in a single-center prospective study: Visual outcomes and microaneurysm formation. Clin. Ophthalmol. 2018, 12, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Spade, R. Ranibizumab according to need: A treatment for age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 679–680. [Google Scholar] [CrossRef]
- Toalster, N.; Russell, M.; Ng, P. A 12-month prospective trial of inject and extend regimen for ranibizumab treatment of age-related macular degeneration. Retina 2013, 33, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Croft, D.E.; Brown, D.M.; Wang, R.; Payne, J.; Clark, L.; Abdelfattah, N.S.; Sadda, S.R.; TREX-AMD Study Group. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 2015, 12, 2514–2522. [Google Scholar] [CrossRef]
- Berg, K.; Hadzalic, E.; Gjertsen, I.; Forsaa, V.; Berger, L.H.; Kinge, B.; Henschien, H.; Fossen, K.; Markovic, S.; Pedersen, T.R.; et al. Ranibizumab or Bevacizumab for Neovascular age-related macular degeneration according to the Lucentis compared to Avastin study treat-and-extend protocol: Two-year results. Ophthalmology 2016, 123, 51–59. [Google Scholar] [CrossRef]
- Barthelmes, D.; Nguyen, V.; Daien, V.; Campain, A.; Walton, R.; Guymer, R.; Morlet, N.; Hunyor, A.P.; Essex, R.W.; Arnold, J.J.; et al. Two year outcomes of “Treat and Extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina 2017. epub ahead of print. [Google Scholar] [CrossRef]
- Freund, K.B.; Korobelnik, J.F.; Devenyi, R.; Framme, C.; Galic, J.; Herbert, E.; Hoerauf, H.; Lanzetta, P.; Michels, S.; Mitchell, P.; et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases. A literature review and consensus recommendations. Retina 2015, 35, 1489–1506. [Google Scholar] [CrossRef]
- Rush, R.B.; Simunovic, M.P.; Aragon, A.V.; Ysasaga, J.E. Treat-and-extend intravitreal bevacizumab for branch retinal vein occlusion. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 212–216. [Google Scholar] [CrossRef]
- Hosogi, M.; Morizane, Y.; Shiode, Y.; Kimura, S.; Hosokawa, M.; Doi, S.; Toshima, S.; Takahashi, K.; Fujiwara, A.; SHiraga, F. Results of a treat-and-extend regimen of intravitreal ranibizumab injection for macular edema due to branch retinal vein occlusion. Acta Med. Okayama 2018, 72, 39–45. [Google Scholar] [PubMed]
- Hikichi, T.; Higuchi, M.; Matsushita, T.; Kosaka, S.; Matsushita, R.; Takami, K.; Ohtsuka, H.; Kitamei, H.; Shioya, S. Two-year outcomes of intravitreal bevacizumab therapy for macular edema secondary to branch retinal vein occlusion. Br. J. Ophthalmol. 2014, 98, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B. Fundus changes in branch retinal vein occlusion. Retina 2015, 35, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Guichard, M.M.; Xavier, A.R.; Türksever, C.; Pruente, C.; Hatz, K. Spectral-domain optical coherence tomography-driven treat-and-extend and pro re nata regimen in patients with macular edema due to retinal vein occlusion: 24-month evaluation and outcome predictors. Ophthalmic Res. 2018, 60, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Sophie, R.; Pearlman, J.; Brown, D.M.; Boyer, D.S.; Heier, J.S.; Marcus, D.M.; Feiner, L.; Patel, A.; RETAIN Study Groupe. Long-term outcomes inpatients with retinal vein occlusion treated with ranibizumab. The RETAIN study. Ophthalmology 2014, 121, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Pichi, F.; Elbarky, A.M.; Elhamaky, T.R. Outcome of “Treat and Monitor” regimen of aflibercept and ranibizumab in macular edema secondary to non-ishchemic branch retinal vein occlusion. Int. Ophtalmol. 2019, 39, 145–153. [Google Scholar] [CrossRef]
- Ohnaka, M.; Nagai, Y.; Sho, K.; Miki, K.; Kimura, M.; Chihara, T.; Takahashi, K. A modified treat-and-extend regimen of aflibercept for treatment-naïve patients with neovascular age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 657–664. [Google Scholar] [CrossRef]
- Lim, J.W. Intravitreal bevacizumab and cytokine levels in major and macular branch retinal vein occlusion. Ophthalmologica 2011, 225, 150–154. [Google Scholar] [CrossRef]
- Noma, H.; Mimura, T.; Yasuda, K.; Nakagawa, H.; Motohashi, R.; Kotake, O.; Shimura, M. Intravitreal ranibizumab and aqueous humor factors/cytokines in major and macular branch retinal vein occlusion. Ophthalmologica 2016, 235, 203–207. [Google Scholar] [CrossRef]
- Costagliola, C.; Agnifili, L.; Arcidiacona, B.; Duse, S.; Fasanella, V.; Mastropasqua, R.; Verolio, M.; Semeraro, F. Systemic thromboembolic adverse events treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration. Expert Opin. Biol. Ther. 2012, 12, 1299–1313. [Google Scholar] [CrossRef]
- Semeraro, F.; Morescalchi, F.; Duse, S.; Gambicorti, E.; Romano, M.R.; Costagliola, C. Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration: An overview. Expert Opin. Drug Saf. 2014, 13, 785–802. [Google Scholar] [CrossRef]
- Schmid, M.K.; Bachmann, L.M.; Fäs, L.; Kessels, A.G.; Job, O.M.; Thiel, M.A. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age-related macular degeneration: A trade-off analysis. Br. J. Ophthalmol. 2015, 99, 141–146. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Sheu, S.J.; Hu, H.Y.; Chu, D.; Chou, P. Association between retinal vein occlusion and an increased risk of acute myocardial infarction: A nationwide population-based follow-up study. PLoS ONE 2017, 12, e0184016. [Google Scholar] [CrossRef] [PubMed]





| Cases | 50 |
|---|---|
| Age (years; mean (SD)) | 66 (12) |
| Sex (male/female) | 24/26 |
| BCVA (logMAR; mean (SD)) | 0.33 (0.27) |
| CMT (µm; mean (SD)) | 488 (171) |
| Major BRVO (n = 29) | Macular BRVO (n = 17) | p. Value * | |
|---|---|---|---|
| Baseline BCVA (logMAR) | 0.33 (0.26) | 0.34 (0.31) | 0.91 |
| Baseline CRT (µm) | 509 (161) | 477 (191) | 0.55 |
| BCVA at month 12 (logMAR) | 0.071 (0.19) | 0.067 (0.20) | 0.95 |
| CRT at month 12 (µm) | 304 (129) | 282 (71) | 0.54 |
| Mean number of injections | 4.24 | 4.29 | 0.93 |
| Mean number of visits | 6.97 | 6.29 | 0.01 |
| Duration between symptoms and initial therapy (month) | 1.62 | 4.95 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, Y.; Takahashi, H.; Inoda, S.; Sakamoto, S.; Tan, X.; Inoue, Y.; Tominaga, S.; Kawashima, H.; Yanagi, Y. Efficacy of Modified Treat-and-Extend Aflibercept Regimen for Macular Edema Due to Branch Retinal Vein Occlusion: 1-Year Prospective Study. J. Clin. Med. 2020, 9, 2360. https://doi.org/10.3390/jcm9082360
Arai Y, Takahashi H, Inoda S, Sakamoto S, Tan X, Inoue Y, Tominaga S, Kawashima H, Yanagi Y. Efficacy of Modified Treat-and-Extend Aflibercept Regimen for Macular Edema Due to Branch Retinal Vein Occlusion: 1-Year Prospective Study. Journal of Clinical Medicine. 2020; 9(8):2360. https://doi.org/10.3390/jcm9082360
Chicago/Turabian StyleArai, Yusuke, Hidenori Takahashi, Satoru Inoda, Shinichi Sakamoto, Xue Tan, Yuji Inoue, Satoko Tominaga, Hidetoshi Kawashima, and Yasuo Yanagi. 2020. "Efficacy of Modified Treat-and-Extend Aflibercept Regimen for Macular Edema Due to Branch Retinal Vein Occlusion: 1-Year Prospective Study" Journal of Clinical Medicine 9, no. 8: 2360. https://doi.org/10.3390/jcm9082360
APA StyleArai, Y., Takahashi, H., Inoda, S., Sakamoto, S., Tan, X., Inoue, Y., Tominaga, S., Kawashima, H., & Yanagi, Y. (2020). Efficacy of Modified Treat-and-Extend Aflibercept Regimen for Macular Edema Due to Branch Retinal Vein Occlusion: 1-Year Prospective Study. Journal of Clinical Medicine, 9(8), 2360. https://doi.org/10.3390/jcm9082360





