Pachydrusen in Fellow Eyes Predict Response to Aflibercept Monotherapy in Patients with Polypoidal Choroidal Vasculopathy
Abstract
1. Introduction
2. Methods
2.1. Subjects
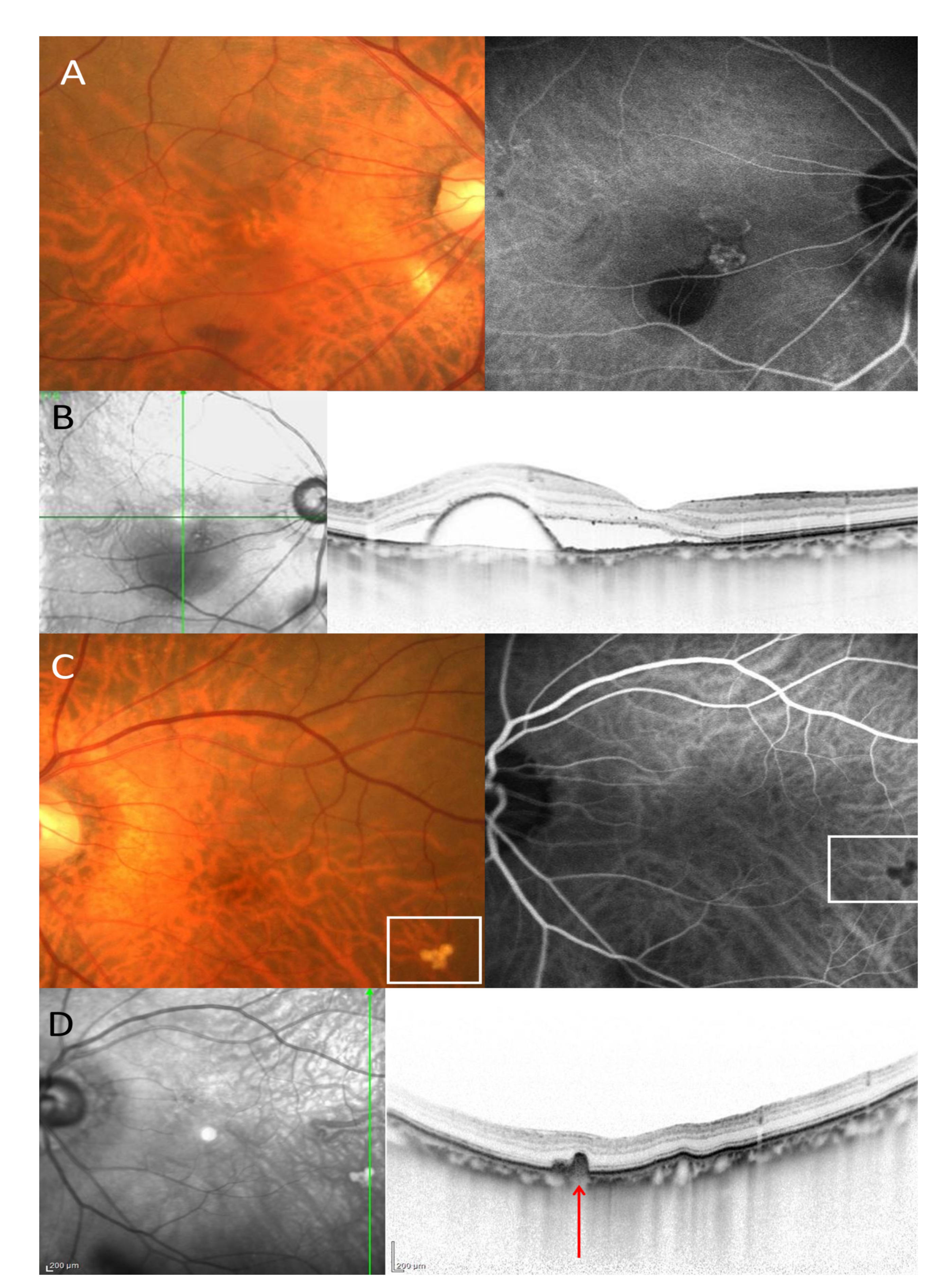
2.2. Group Classification
2.3. Treatment and Follow-Up
2.4. Genotyping
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Dansingani, K.K.; Gal-Or, O.; Sadda, S.R.; A Yannuzzi, L.; Freund, K.B. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): A lesson in the taxonomy of ‘expanded spectra’—A review. Clin. Exp. Ophthalmol. 2017, 46, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Yoneyama, S.; Sugiyama, A.; Tanabe, N.; Kikushima, W.; Mabuchi, F.; Kume, A.; Kubota, T.; Iijima, H. Prevalence and Genetic Characteristics of Geographic Atrophy among Elderly Japanese with Age-Related Macular Degeneration. PLoS ONE 2016, 11, e0149978. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Lee, H.; Bae, K.; Shin, J.Y.; Kim, S.J.; Kim, J.M. Korean Age-related Maculopathy Study (KARMS) Group Investigation of precursor lesions of polypoidal choroidal vasculopathy using contralateral eye findings. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 255, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina 2018, 38, 708–716. [Google Scholar] [CrossRef]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.-Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef]
- Rivera, A.; Fisher, S.A.; Fritsche, L.G.; Keilhauer, C.N.; Lichtner, P.; Meitinger, T.; Weber, B.H.F. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum. Mol. Genet. 2005, 14, 3227–3236. [Google Scholar] [CrossRef]
- Hayashi, H.; Yamashiro, K.; Gotoh, N.; Nakanishi, H.; Nakata, I.; Tsujikawa, A.; Otani, A.; Saito, M.; Iida, T.; Matsuo, K.; et al. CFHandARMS2Variations in Age-Related Macular Degeneration, Polypoidal Choroidal Vasculopathy, and Retinal Angiomatous Proliferation. Investig. Opthalmology Vis. Sci. 2010, 51, 5914–5919. [Google Scholar] [CrossRef]
- Yoneyama, S.; Sakurada, Y.; Mabuchi, F.; Sugiyama, A.; Kubota, T.; Iijima, H. Genetic Variants in the SKIV2L Gene in Exudative Age-related Macular Degeneration in the Japanese Population. Ophthalmic Genet. 2014, 35, 151–155. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Yamashiro, K.; Chen, L.J.; Ahn, J.; Huang, L.; Huang, L.; Cheung, C.M.G.; Miyake, M.; Cackett, P.D.; Yeo, I.Y.; et al. New loci and coding variants confer risk for age-related macular degeneration in East Asians. Nat. Commun. 2015, 6, 6063. [Google Scholar] [CrossRef]
- Fukuda, Y.; Sakurada, Y.; Yoneyama, S.; Kikushima, W.; Sugiyama, A.; Matsubara, M.; Tanabe, N.; Iijima, H. Clinical and genetic characteristics of pachydrusen in patients with exudative age-related macular degeneration. Sci. Rep. 2019, 9, 11906. [Google Scholar] [CrossRef]
- Koh, A.; Lee, W.K.; Chen, L.-J.; Chen, S.-J.; Hashad, Y.; Kim, H.; Lai, T.Y.Y.; Pilz, S.; Ruamviboonsuk, P.; Tokaji, E.; et al. EVEREST STUDY. Retina 2012, 32, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Kikushima, W.; Sakurada, Y.; Sugiyama, A.; Tanabe, N.; Kume, A.; Iijima, H. Comparison of initial treatment between 3-monthly intravitreal aflibercept monotherapy and combined photodynamic therapy with single intravitreal aflibercept for polypoidal choroidal vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 255, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Matsumiya, W.; Keiko, O.; Miki, A.; Nakamura, M.; Honda, S. The 24-month outcomes of intravitreal aflibercept combined with photodynamic therapy for polypoidal choroidal vasculopathy. Jpn. J. Ophthalmol. 2018, 63, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kikushima, W.; Sakurada, Y.; Sugiyama, A.; Yoneyama, S.; Tanabe, N.; Matsubara, M.; Mabuchi, F.; Iijima, H. Comparison of two-year outcomes after photodynamic therapy with ranibizumab or aflibercept for polypoidal choroidal vasculopathy. Sci. Rep. 2017, 7, 16461. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef]
- Yamamoto, A.; Okada, A.A.; Kano, M.; Koizumi, H.; Saito, M.; Maruko, I.; Sekiryu, T.; Iida, T. One-Year Results of Intravitreal Aflibercept for Polypoidal Choroidal Vasculopathy. Ophthalmology 2015, 122, 1866–1872. [Google Scholar] [CrossRef]
- Morimoto, M.; Matsumoto, H.; Mimura, K.; Akiyama, H. Two-year results of a treat-and-extend regimen with aflibercept for polypoidal choroidal vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1891–1897. [Google Scholar] [CrossRef]
- Sakurada, Y.; Kikushima, W.; Sugiyama, A.; Yoneyama, S.; Tanabe, N.; Matsubara, M.; Iijima, H. AREDS simplified severity scale as a predictive factor for response to aflibercept therapy for typical neovascular age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 256, 99–104. [Google Scholar] [CrossRef]
- Wataru, K.; Sugiyama, A.; Yoneyama, S.; Matsubara, M.; Fukuda, Y.; Parikh, R.; Sakurada, Y. Five-year outcomes of photodynamic therapy combined with intravitreal injection of ranibizumab or aflibercept for polypoidal choroidal vasculopathy. PLoS ONE 2020, 15, e0229231. [Google Scholar] [CrossRef]
- Kuroda, Y.; Yamashiro, K.; Miyake, M.; Yoshikawa, M.; Nakanishi, H.; Oishi, A.; Tamura, H.; Ooto, S.; Tsujikawa, A.; Yoshimura, N. Factors Associated with Recurrence of Age-Related Macular Degeneration after Anti-Vascular Endothelial Growth Factor Treatment. Ophthalmology 2015, 122, 2303–2310. [Google Scholar] [CrossRef]
- Kikushima, W.; Sakurada, Y.; Yoneyama, S.; Sugiyama, A.; Tanabe, N.; Kume, A.; Mabuchi, F.; Iijima, H. Incidence and risk factors of retreatment after three-monthly aflibercept therapy for exudative age-related macular degeneration. Sci. Rep. 2017, 7, 44020. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, H.; Cheng, C.-Y.; Wen, F.; Tam, P.O.S.; Zhao, P.; Chen, H.; Li, Z.; Chen, L.; Tai, Z.; et al. A missense variant in FGD6 confers increased risk of polypoidal choroidal vasculopathy. Nat. Genet. 2016, 48, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Kubota, T.; Mabuchi, F.; Imasawa, M.; Tanabe, N.; Iijima, H. Association of LOC387715 A69S With Vitreous Hemorrhage in Polypoidal Choroidal Vasculopathy. Am. J. Ophthalmol. 2008, 145, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Kubota, T.; Imasawa, M.; Tsumura, T.; Mabuchi, F.; Tanabe, N.; Iijima, H. angiographic lesion size associated with loc387715 a69s genotype in subfoveal polypoidal choroidal vasculopathy. Retina 2009, 29, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Kubota, T.; Imasawa, M.; Mabuchi, F.; Tateno, Y.; Tanabe, N.; Iijima, H. Role of Complement Factor H I62V and Age-Related Maculopathy Susceptibility 2 A69S Variants in the Clinical Expression of Polypoidal Choroidal Vasculopathy. Ophthalmology 2011, 118, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, S.; Sakurada, Y.; Kikushima, W.; Sugiyama, A.; Tanabe, N.; Mabuchi, F.; Kubota, T.; Iijima, H. genetic factors associated with choroidal vascular hyperpermeability and subfoveal choroidal thickness in polypoidal choroidal vasculopathy. Retina 2016, 36, 1535–1541. [Google Scholar] [CrossRef]
- Valverde-Megías, A.; Veganzones, S.; Donate-López, J.; Maestro-De-Las-Casas, M.L.; Megías-Fresno, A.; Garcia-Feijoo, J. ARMS2 A69S polymorphism is associated with the number of ranibizumab injections needed for exudative age-related macular degeneration in a pro re nata regimen during 4 years of follow-up. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2091–2098. [Google Scholar] [CrossRef]
- Yamashiro, K.; Mori, K.; Honda, S.; Kano, M.; Yanagi, Y.; Obana, A.; Sakurada, Y.; Sato, T.; Nagai, Y.; Hikichi, T.; et al. A prospective multicenter study on genome wide associations to ranibizumab treatment outcome for age-related macular degeneration. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Sugiyama, A.; Sakurada, Y.; Honda, S.; Miki, A.; Matsumiya, W.; Yoneyama, S.; Kikushima, W.; Iijima, H. Retreatment of Exudative Age-Related Macular Degeneration after Loading 3-Monthly Intravitreal Ranibizumab. Ophthalmologica 2018, 239, 52–59. [Google Scholar] [CrossRef]
- Yoneyama, S.; Sakurada, Y.; Kikushima, W.; Sugiyama, A.; Matsubara, M.; Fukuda, Y.; Tanabe, N.; Parikh, R.; Mabuchi, F.; Kashiwagi, K.; et al. Genetic factors associated with response to as-needed aflibercept therapy for typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Terao, N.; Koizumi, H.; Kojima, K.; Yamagishi, T.; Yamamoto, Y.; Yoshii, K.; Kitazawa, K.; Hiraga, A.; Toda, M.; Kinoshita, S.; et al. Distinct Aqueous Humour Cytokine Profiles of Patients with Pachychoroid Neovasculopathy and Neovascular Age-related Macular Degeneration. Sci. Rep. 2018, 8, 10520. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Mahroo, O.A.; Khan, R.S.; Mohamed, M.D.; McKibbin, M.; Bird, A.; Michaelides, M.; Tufail, A.; Moore, A.T. Differentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog. Retin. Eye Res. 2016, 53, 70–106. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.-Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R. Age-Related Eye Disease Study (AREDS) Research Group A Simplified Severity Scale for Age-Related Macular Degeneration. Arch. Ophthalmol. 2005, 123, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Sugiyama, A.; Kikushima, W.; Yoneyama, S.; Tanabe, N.; Matsubara, M.; Iijima, H. Pseudodrusen pattern and development of late age-related macular degeneration in the fellow eye of the unilateral case. Jpn. J. Ophthalmol. 2019, 63, 374–381. [Google Scholar] [CrossRef]
- Sakurada, Y.; Parikh, R.; Gal-Or, O.; Balaratnasingam, C.; Leong, B.C.; Tanaka, K.; Cherepanoff, S.; Spaide, R.F.; Freund, K.B.; Yannuzzi, L.A. CUTICULAR DRUSEN. Retina 2020, 40, 257–265. [Google Scholar] [CrossRef]





| Group 1: Pachydrusen (n = 16) | Group 2: No Drusen (n = 45) | Group 3: Soft Drusen (n = 35) | Group 4: PCV/Scarring (n = 14) | p-value | |
|---|---|---|---|---|---|
| Mean age(year) | 68.4 | 69.7 | 75.0 | 77.9 | 3.4×10-4☨ |
| p-value (vs Pachydrusen) | NA | 0.55☨☨ | 3.1×10-3☨☨ | 2.5×10-3☨☨ | |
| Male (%) | 12 (75%) | 37 (73.3%) | 27 (77.14%) | 10 (71.43%) | 0.97☨☨☨ |
| p-value (vs Pachydrusen) | NA | 0.90☨☨☨ | 0.87☨☨☨ | 0.83☨☨☨ | |
| BCVA log MAR | 0.26 ± 0.32 | 0.39 ± 0.38 | 0.35 ± 0.29 | 0.31 ± 0.35 | 0.45☨ |
| p-value (vs Pachydrusen) | NA | 0.18☨☨ | 0.15☨☨ | 0.75☨☨ | |
| Mean central retinal thickness(µm) | 408 | 373 | 406 | 434 | 0.26☨ |
| p-value (vs Pachydrusen) | NA | 0.56☨☨ | 0.66☨☨ | 0.39☨☨ | |
| Mean subfoveal choroidal thickness(µm) | 325 | 235 | 200 | 260 | 2.2×10-3☨ |
| p-value (vs Pachydrusen) | NA | 6.0×10-3☨☨ | 4.3×10-3☨☨ | 0.21☨☨ | |
| Mean greatest linear dimension(µm) | 3771 | 3669 | 3464 | 4761 | 0.15☨ |
| p-value (vs Pachydrusen) | N/A | 0.81☨☨ | 0.75☨☨ | 0.16☨☨ | |
| ARMS2 A69S T allele frequency | 0.34 | 0.57 | 0.54 | 0.75 | 0.020☨☨☨ |
| p-value (vs Pachydrusen) | NA | 0.038☨☨☨ | 0.047☨☨☨ | 4.0×10-3☨☨☨ | |
| TT | 1 (6.25%) | 16 (35.6%) | 9 (25.7%) | 9 (64.3%) | |
| TG | 9 (56.3%) | 19 (42.2%) | 20 (57.1%) | 3 (21.4%) | |
| GG | 6 (12.5%) | 10 (22.2%) | 6 (17.1%) | 2 (14.3%) | |
| CFH I62V G allele frequency | 0.72 | 0.74 | 0.69 | 0.93 | 0.054☨☨☨ |
| p-value (vs Pachydrusen) | NA | 0.90☨☨☨ | 0.52☨☨☨ | 0.070☨☨☨ | |
| GG | 9 (56.3%) | 25 (55.6%) | 14 (40.0%) | 12 (85.7%) | |
| GA | 5 (31.3%) | 17 (37.8%) | 20 (57.1%) | 2 (14.3%) | |
| AA | 2 (12.5%) | 3 (6.7%) | 1 (2.9%) | 0 (0%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, Y.; Sakurada, Y.; Sugiyama, A.; Yoneyama, S.; Matsubara, M.; Kikushima, W.; Tanabe, N.; Parikh, R.; Kashiwagi, K. Pachydrusen in Fellow Eyes Predict Response to Aflibercept Monotherapy in Patients with Polypoidal Choroidal Vasculopathy. J. Clin. Med. 2020, 9, 2459. https://doi.org/10.3390/jcm9082459
Fukuda Y, Sakurada Y, Sugiyama A, Yoneyama S, Matsubara M, Kikushima W, Tanabe N, Parikh R, Kashiwagi K. Pachydrusen in Fellow Eyes Predict Response to Aflibercept Monotherapy in Patients with Polypoidal Choroidal Vasculopathy. Journal of Clinical Medicine. 2020; 9(8):2459. https://doi.org/10.3390/jcm9082459
Chicago/Turabian StyleFukuda, Yoshiko, Yoichi Sakurada, Atsushi Sugiyama, Seigo Yoneyama, Mio Matsubara, Wataru Kikushima, Naohiko Tanabe, Ravi Parikh, and Kenji Kashiwagi. 2020. "Pachydrusen in Fellow Eyes Predict Response to Aflibercept Monotherapy in Patients with Polypoidal Choroidal Vasculopathy" Journal of Clinical Medicine 9, no. 8: 2459. https://doi.org/10.3390/jcm9082459
APA StyleFukuda, Y., Sakurada, Y., Sugiyama, A., Yoneyama, S., Matsubara, M., Kikushima, W., Tanabe, N., Parikh, R., & Kashiwagi, K. (2020). Pachydrusen in Fellow Eyes Predict Response to Aflibercept Monotherapy in Patients with Polypoidal Choroidal Vasculopathy. Journal of Clinical Medicine, 9(8), 2459. https://doi.org/10.3390/jcm9082459






