Abstract
Neurological disorders such as neurodegenerative diseases or traumatic brain injury are associated with cognitive, motor and behavioural changes that influence the quality of life of the patients. Although different therapeutic strategies have been developed and tried until now to decrease the neurological decline, no treatment has been found to cure these pathologies. In the last decades, the implication of the endocannabinoid system in the neurological function has been extensively studied, and the cannabinoids have been tried as a new promising potential treatment. In this study, we aimed to overview the recent available literature regarding in vivo potential of natural and synthetic cannabinoids with underlying mechanisms of action for protecting against cognitive decline and motor impairments. The results of studies on animal models showed that cannabinoids in traumatic brain injury increase neurobehavioral function, working memory performance, and decrease the neurological deficit and ameliorate motor deficit through down-regulation of pro-inflammatory markers, oedema formation and blood–brain barrier permeability, preventing neuronal cell loss and up-regulating the levels of adherence junction proteins. In neurodegenerative diseases, the cannabinoids showed beneficial effects in decreasing the motor disability and disease progression by a complex mechanism targeting more signalling pathways further than classical receptors of the endocannabinoid system. In light of these results, the use of cannabinoids could be beneficial in traumatic brain injuries and multiple sclerosis treatment, especially in those patients who display resistance to conventional treatment.
1. Introduction
Neurological disorders that affect different subsets of neurons such as neurodegenerative diseases (NDs) are associated with several cognitive, motor and behavioural changes that influence the quality of life of patients and the evolution and prognosis of the disease [1,2,3]. Similar changes have also been observed in those suffering from traumatic brain injury (TBI) or haemorrhagic/ischemic stroke [4,5]. Cognitive decline and motor impairments are usually associated with patients affected by neurodegeneration [6]. Cognitive decline is characterized by a decrease in executive function, attention and working memory [7], while in motor decline appear extrapyramidal rigidity, spasticity, motor impairment and gait problems [8]. The cognitive, behavioural and motor impairment is determined by the death of neurons in different regions of the central nervous system (CNS), and unfortunately, it cannot be treated. Even if a lot of progress has been made to understand the mechanism implicated in the pathogenesis of cognitive and motor impairment in neurodegenerative and brain injuries [9,10,11], no treatment has been found to cure these pathologies. Several strategies and therapies have been developed and tried until now to treat some symptoms and to decrease the neurological decline in order to improve the quality of life of patients [12], and many others underwent preclinical testing [13,14].
In the last years, the implication of the endocannabinoid (eCB) system in the neurological function has been extensively studied. The eCB system is formed from cannabinoid receptors, eCBs and enzymes implicated in the synthesis and metabolization of eCBs [15].
There are two cannabinoid receptors identified, cannabinoid receptor type 1 (CB1R) and type 2 (CB2R), that are coupled with G-protein and act through activation of mitogen-activated protein kinase (MAPK), modulating potassium channels and inhibiting voltage-gated calcium channels and adenylyl cyclases [15]. CB1R is localized mainly in the CNS in the cortex, caudate nucleus, globus pallidum, putamen, amygdala, hypothalamus, hippocamp, cerebellum, substantia nigra, and dorsal vagal complex, and CB2R is localized mainly in the periphery in immune cells and hematopoietic systems, such as spleen, leukocytes and tonsils, and mostly under pathological states in peripheral organs, such as testis, liver, muscle and intestine [16]. CB2R was also identified in CNS, mainly localized in microglia, astrocytes localized in the cortex [17], striatum [18], amygdala [19], hippocampus [20], cerebellum [21] and brainstem [22], but this expression is mostly observed in pathological conditions, possibly due to infiltration of peripheral cells or an increased expression on “activated” microglia or astroglia. CB2R is not usually identified in healthy brain cells. CB1R is mainly localized presynaptic, in the GABAergic terminals of peripheral and central neurons, being implicated in neurotransmitter release and psychoactivity by decreasing presynaptic GABA release that leads to a decrease in GABAergic inhibitory control of postsynaptic neurons, producing postsynaptic neurons exhibition [23]. In the periphery, CB1R is localized in testis, liver, uterus, adipose tissue and immune cells [24].
Studies showed that compared with CB1R, CB2R is mainly expressed in CNS after a specific insult such as anxiety, addiction and inflammation, while in resting microglia, no CB2R was detected [22]. CB2R are mainly localized postsynaptically in the CNS, and their activation produces hyperpolarization of membrane potential and inhibition of neuronal function having opposite effects compared with CB1R [25].
Cannabinoids are a group of chemicals that bind to the cannabinoid receptors acting as direct agonists, producing different effects according to the affinity to one or both cannabinoid receptors [26,27]. The cannabinoids are classified into three categories: i)endocannabinoids that are synthesized by mammals, ii)natural cannabinoids (phytocannabinoids) that are isolated from plants and iii) synthetic cannabinoids that are synthesized in the laboratory and have the same or all physiological properties of phytocannabinoids and endocannabinoids [28,29].
1.1. Endocannabinoids
2-arachidonoylglycerol (2-AG) and arachidonoyl ethanolamide (AEA, anandamide) are two of the best-studied endocannabinoids that bind to the CB receptors and produce effects similar to the psychoactive compounds found in cannabis [26]. AEA is a lipid neurotransmitter that has the ability to stimulate several receptors such as presynaptic and postsynaptic CB1R, CB2R, 5-hydroxytryptamine and Transient Receptor Potential Vanilloid 1 (TRPV1) ion channel. 2-AG is synthesized from glycerol and omega-6 fatty acid arachidonic acid and has a higher affinity for CB1R and CB2R when compared to AEA [30].
In recent years, other compounds that bind to the cannabinoid receptors have been identified such as 2-arachidonoyl glyceryl ether (noladin ether, 2-AGE), N-arachidonoyldopamine (NADA) and O-arachidonoylethanolamine (virodhamine) [26]. 2-AGE was the first compound isolated from porcine brain and considered as an ether linked-analogue of 2-AG. It is able to inhibit the intracellular accumulation of AEA and bound to CB1 and weakly to CB2 [31].
Virodhamine is formed from two molecules of arachidonic acid and ethanolamine, which are linked by an ester bond, and was detected in peripheral tissues and brain. It shows to be a partial agonist; more exactly, it acts in vivo as an antagonist at CB1R level and agonist of CB2R level [32]. NADA is an endocannabinoid that binds to and activates TRPV1 but also acts as endogenous TRPM8 antagonist [33].
The concentration of endocannabinoids is regulated by different mechanisms such as degradation that involves reuptake into the presynaptic cell, followed by rapid hydrolysis by fatty acid amide hydrolase (FAAH) of the amide or ester bonds or metabolization through monoacylglycerol lipase (MAGL) only for 2-AG [34].
1.2. Phytocannabinoids
Cannabis sativa is a plant that contains at least 104 cannabinoids identified until now, with different structure and different functional profile [35]. Two of these components form the majority and have been intensely studied in the last years: delta-9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). Other compounds such as cannabinol (CBN), cannabigerolic acid (CBGA), cannabigerol (CBG), cannabidiolic acid (CBDA), cannabichromene (CBC), cannabielsoic acid A, cannabicyclol (CBL), Δ9-tetrahydrocannabinolic acid (Δ9-THCA) and Δ9-tetrahydrocannabivarin (Δ9-THCV) form the minority of phytocannabinoids found in Cannabis sativa plant [35].
Δ9-THC is the most psychoactive molecule found in the cannabis plant. It is a partial agonist of CB1R and CB2R and shows several physiological properties similar to endocannabinoids [26]. Its psychoactive activity is determined by the activation of CB1R in CNS, which leads to the inhibition of adenyl cyclase and decrease cAMP levels [30].
CBD is the non-psychotropic component isolated from cannabis, especially from fibre-type Cannabis species, and is used to treat several pathologies such as neurological diseases and cancer [26]. The affinity of this compound is low compared to Δ9-THC for the two receptors CB1R and CB2R and is considered as an inverse agonist of the human CB2R receptor [26]. The CBD exerts an inhibitory effect on the metabolism of tetrahydrocannabinol by blocking its conversion by cytochrome P-450 to give a more psychoactive molecule. Besides, it plays a counterbalancing role to regulate the negative effect of Δ9-THC produced by high doses consumption [36].
CBN was the first phytocannabinoid isolated from cannabis and is also the major metabolite of tetrahydrocannabinol. In general, this compound has a high affinity for CB2 receptors, but this affinity remains weak compared to that of tetrahydrocannabinol, and it is considered as a partial agonist of CB1R receptors [30].
CBDA is the non-psychoactive precursor of CBD, found in the fresh cannabis plant [30]. CBDA has the same capacity as CBD to antagonize TRPM8 receptor and to stimulate the transient receptor potential (TRP) cation channels, TRPV1 and TRPA1 with significantly less potency than CBD [37].
CBG is the major phytocannabinoid that has been found in several Cannabis varieties [38] acting as a CB2R antagonist and low CB1R antagonist similar to CBD [30]. It also acts as an agonist of α2-adrenergic and 5-HT1A receptors [30].
CBC is found in high amount in dry cannabis plants. In association with other cannabinoids such as CBD and Δ9-THC, it produces antidepressant effects and also promotes neurogenesis [39]. CBC is also an essential stimulator of transient receptor potential (TRP) ankyrin 1-type (TRPA1) channels [39].
Δ9-THCV is an n-propyl analogue of Δ9-THC that have similar pharmacological effects and molecular targets. This compound is a partial agonist of CB2R and GPR55 and also has the capacity to activate 5HT1A receptors and different TRP channels [38]. Other phytocannabinoids found in Cannabis sativa plant have been isolated and identified, but their activity and molecular targets have not been revealed so far.
1.3. Synthetic Cannabinoids
This class of compounds is the most diverse group concerning the chemical structure and biological activities. These are classified into seven structural groups: aminoalkylindoles (JWH-018, JWH-073 and AM-2201), tetrahydrocannabinols (Δ9-THC, HU 210), naphthylmethylindoles (JWH-185), cyclohexylphenols (CP47,497), naphthoylpyrroles (JWH-030), phenylacetylindoles (JWH-250, RCS-4), and naphthylmethylindenes (JWH-176)[40]. The synthetic cannabinoids have full agonist activity with a higher affinity to CB1R and CB2R receptors and higher dose-dependent efficacy compared with Δ9-THC [41].
In this study, we aimed to review the recent available literature regarding the in vivo potential of cannabinoids in protecting against cognitive decline and motor impairments that appear in acute brain injury and chronic brain injury. There are several chronic brain injuries produced especially by neurodegeneration, so in order to overview the changes that can appear in the modulation of cognitive decline and motor impairment between acute and chronic injuries, we chose as a model for chronic brain injury multiple sclerosis (MS). MS is defined as a chronic inflammatory demyelinating disease of the CNS associated with several degrees of degeneration and loss of axons [42]. Having also an autoimmune component, MS can affect both young and old people [42].
2. Methodology
Multiple searches on Pub Med were performed for the articles published between January 2009 and December 2019 using as keywords: “cannabinoids” or “cannabis”, or “brain injury” or “multiple sclerosis”. After analysing the results, only the relevant studies were included.
Inclusion criteria for the articles were in vivo studies on animal models of TBI or MS that analyse cognitive and motor impairment, written in English.
Exclusion criteria for the articles were articles written in languages other than English, studies published only as abstracts, reviews and meta-analysis, in vitro studies, human case reports, human clinical trials. For the review articles, their references were checked in order to identify significant ones.
3. Results
After an initial check, we identified 769 studies, 286 for TBI and 483 for MS. After we excluded the articles written in other languages than English, 733 articles remained to be analysed, 280 for TBI and 453 for MS. The reviews, commentaries and editorials were excluded, and 449 articles remained to be checked for relevance. After abstract analysis, 115 articles, 49 for TBI and 66 for MS were included and reviewed by reading the full text. Finally, 47 studies, 18 for TBI and 29 for MS were included in the analysis. The selection procedure is detailed in the PRISMA flow chart (Figure 1).
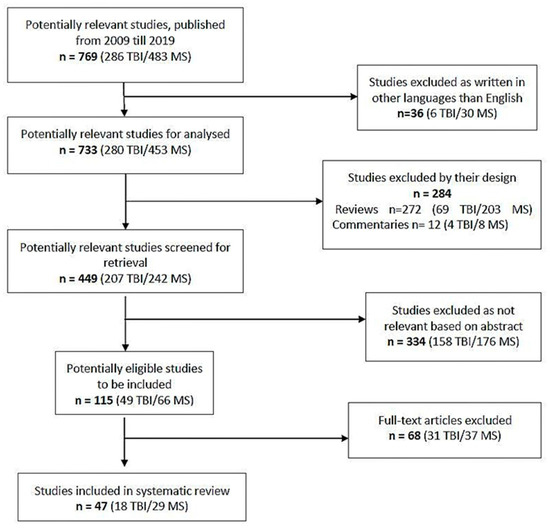
Figure 1.
PRISMA flow chart of the study adapted after [43].
3.1. Cannabinoids Effects in Cognitive and Motor Impairment in Traumatic Brain Injury (TBI)
In Table 1 are presented in vivo studies published between 2009 and 2019 that evaluate the cannabinoids protective effects in TBI models (Table 1).

Table 1.
Preclinical studies that show a correlation between cannabinoid treatment and cognitive and motor improvement in TBI animal models.
We identify 13 studies that evaluate the protective effects of synthetic cannabinoids active as CB2R agonists as HU-910 and HU-914 [44], JWH133 [45,46,54], GP1a [47], SMM-189 [1,49,51], ACEA [52], 0-1966 [57,60], KN38-7271 [59], ACEA [52] in TBI animal models, 2 studies that evaluated the protective effect of natural cannabinoid CBD [48,58] in TBI animal models, 1 study that evaluated the beneficial effect of exogenous administration of the AraS endocannabinoid in TBI animal models [56] and 2 studies that evaluated the protective effect of increasing the endocannabinoid levels in TBI animal models by inhibiting the enzymes that metabolize the endocannabinoids as FAAH [53] and ABHD6 [55].
In vivo animal studies showed that CB2R receptor is the main pharmacological target of natural or synthetic cannabinoids in protection against cognitive and motor impairment after TBI [1,44,45,46,47,48,49,51,54,55,57,58,59,60]. The inhibition of CB1R by SR141716 failed to show any beneficial effect on cognitive and motor impairment after TBI [50].
The main mechanisms through which cannabinoids improve cognitive and motor impairment after TBI are associated with decreasing the pro-inflammatory markers [1,44,46,47,48,52,53,57,58], decreasing oedema formation [44,45,47,54,55,58,60], decreasing BBB permeability [44,45,46,54,55,57], preventing neuronal cell loss [44,49,51,55] and increasing the levels of adherens jonction proteins [45,46,54].
3.2. Cannabinoids Effects in Cognitive and Motor Impairment in Multiple Sclerosis (MS)
In Table 2 are presented in vivo studies published between 2009 and 2019 that evaluate the cannabinoids protective effects in MS models (Table 2).

Table 2.
Preclinical studies that show a correlation between cannabinoid treatment and cognitive and motor improvement in MS animal models.
We identified 5 studies that evaluated the efficacy of the combination of Δ9-THC+CBD in decreasing clinical symptoms of EAE [61,62,63,64,65]; 6 studies that evaluated the efficacy of CB2R agonists in decreasing clinical symptoms of EAE [66,67,68,69,70,71]; 2 studies that evaluated the efficacy of exogenous administration of endocannabinoids or endocannabinoids-derivatives in MS [72,73]; 1 study that evaluated the efficacy of CB1R agonists in decreasing clinical symptoms of EAE [74]; 10 studies that evaluated the efficacy of CB1R, CB2R agonists in decreasing clinical symptoms of EAE [62,75,76,77,78,79,80,81,82,83] and 5 studies that evaluated the protective effect of increasing the endocannabinoid levels in EAE animal models by inhibiting the enzymes that metabolize the endocannabinoids as ABHD6 [84,85] and MAGL [86,87,88].
In vivo animal studies, the pharmacological target of natural and synthetic cannabinoids in decreasing clinical symptoms and progression of EAE are diverse and differ according to the murine model used.
Except for CB1R and CB2R receptors, the stimulation of PPAR-α and GPR55 receptors by cannabinoids is also associated with beneficial effects in EAE models [64,70,71,72,78,81,82,83].
The mechanisms through which cannabinoids decrease motor disability in MS murine models are diverse: decreasing the neuroinflammation [61,62,64,66,67,70,72,73,74,75,76,77,78,80,81,82,85], increasing anti-inflammatory cytokines [66], decreasing cell proliferation [66], increasing remyelination and axonal protection [68,86], decreasing demyelination [70,78,82,85], decreasing microglia activation [63,64,69,70,71,74,79,81,85,88].
4. Discussion
4.1. Cannabinoids Effects in Cognitive and Motor Impairment in Traumatic Brain Injury (TBI)
TBIs is often caused by traumatism and can lead to death. Various treatments such as the use of corticosteroids, barbiturates, hyperventilation, mannitol, hypothermia and cerebrospinal fluid drainage were developed for reducing the damage induced by brain injuries and improving the quality of life of these patients. However, until now, no specific therapy is available for patients who are refractory to conventional treatments, as the mechanisms involved in these pathologies are different and not yet deciphered [90].
Several critical investigations showed that the mentioned treatments exhibit different secondary damages, and therefore, their effects are not significant in the improvement of the treatment of brain injuries. In this way, several pieces of research indicated that novel approaches should be established based on the target, specifically damage generated by injuries [91]. The secondary damage is essentially due to the excitotoxicity induced high concentrations of glutamate and activation of its receptor. This produces an accumulation of intracellular calcium levels, which activate numerous destructive pathways such as reactive oxygen intermediates, caspases and calpains [92]. Another factor that seems to contribute to the management of brain injury is the endothelin. Indeed, the function of endothelin is well understood, which is to be involved in the cerebral circulation. By intermediate of the endothelin-A receptor, endothelin induces vasoconstriction to reduce blood flow and contributes therefore to the genesis of haemorrhagic stroke and ischemia [90].
Various in vivo studies reported that some synthetic, endogenous and natural cannabinoids exhibit effectiveness on several complications caused by brain injuries (Table 1).
The treatment with synthetic CB2R agonists immediately after injury showed promising results in recovery of the neurobehavioral deficits and sensorimotor impairment mainly due to the modulation of inflammatory markers [1,44,45,46,47,57], decrease of oedema formation [44,45,47,54,60], influence in the permeability of BBB [44,45,46,54,57], increase in adherence junction proteins [45,46,54] and prevention of neuronal loss [44,49,51].
Short term treatment with CBD in mice model of BCCAO has been shown to prevent motor and cognitive impairment through a complex mechanism related with stimulation of synthesis in the hippocampus of the levels of BDNF and MAP-2 proteins and stimulation of neurogenesis. All these are associated with a decrease in neuroinflammation and neuronal loss in the hippocampus [48,58].
2-AG and AEA are endocannabinoids found in high amount in the CNS and overexpressed after TBI. 2-AG is highly metabolized by MAGL and in less amount by ABHD6. AEA is metabolized by FAAH [93]. Studies showed that the inhibition of monoacylglycerol lipase determined a high increase of 2-AG brain level associated with CBR desensitization and tolerance. In contrast, ABHD6 inhibition produces an increase in 2-AG levels in a range associated with no side effects [94]. The use of WWL70, an inhibitor of ABHD6, showed protective effects in TBI by decreasing the lesion volume associated with 2-AG effects as CB1R agonist [55]. Regarding the neuroprotective effects of 2-AG, these have been associated with activation of both CB1R and CB2R and increasing the phosphorylation of ERK1/2 and AKT kinase implicated in cell survival [55]. The same effects were also observed for PF3845, an inhibitor of FAAH that is associated with an increase of AEA level in the brain [53].
The activation of both CB receptors plays a protective role in cognitive and motor impairments observed in TBI by decreasing the oedema formation, improving neurological score and decreasing BBB disruption (Figure 2). Regarding the effect of CB receptors in microglia activation, CB2R activation has a protective role [95]. CB2R is associated with modulation of activated microglia [96]. There are two states of activated microglia, M1 state in which an increase production of reactive oxygen species (ROS) and inflammatory markers is observed and M2 state which is an anti-inflammatory state associated with healing processes [97]. Lopez-Rodriguez et al. [96] analysed the implication of CB receptors in minocycline protective effects on TBI and showed that the activation of CB1R is less associated with protective effects on TBI compared with CB2R. This effect is related to the fact that microglial cell activation leads to up-regulation of CB2R expression, while CB1R is kept at low levels.
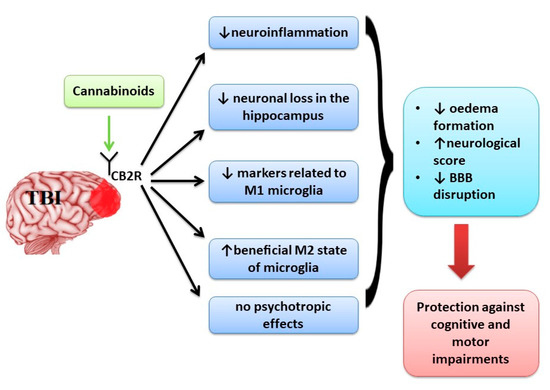
Figure 2.
The main effects of activation of CB2R in TBI.
The activation of CB2R was associated with a decrease of markers related to M1 microglia [1,57,60] and an increase in the beneficial M2 state of microglia [49,51] promoting neuroprotective effects without psychotropic effects determined by CB1R activation (Figure 2).
Targeting CB2R can be a promising therapeutic approach for TBI as the receptor is localized primarily in the microglia and less in neurons, and their expression is increased after a lesion that determines microglia activation.
4.2. Cannabinoids Effects in Cognitive and Motor Impairment in Multiple Sclerosis (MS)
Multiple sclerosis (MS) is a chronic autoimmune inflammatory disorder affecting the central nervous system (CNS) [98,99]. Multiple sclerosis is the most common inflammatory neurological disease in young adults [100]. The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) quantified the global burden of MS concluding that this disease is not common but is a potentially severe cause of neurological disability throughout adult life with substantially increased prevalence in many regions since 1990 [101]. Globally, it has been estimated that two million people suffer from this disease, with over 75% of them being women [102]. The exact aetiology of MS is still rather unknown, and research has suggested the involvement of various genetic and environmental factors, probably modulated by their complex interactions [103]. It is a widely held view that MS is a T cell-mediated autoimmune disorder. Namely, when autoreactive T-lymphocytes cross the blood–brain barrier (BBB) and enter CNS, local inflammation occurs, and subsequently, this results in demyelination, gliotic scarring and axonal damage [104,105]. Depending on the localization of the CNS lesions, MS can present various signs and symptoms such as pain, spasticity, muscle spasms, headache, numbness, fatigue and depression [98].
In vivo studies showed that a large variety of synthetic, endogenous and natural cannabinoids have beneficial effects in improving clinical outcome and evolution of MS by targeting the endocannabinoid system. Cannabinoid-based treatment that acts both on classical targets of the endocannabinoid system as CB1R/CB2R but also on PPAR-γ and GPR5 receptors that modulate neuroinflammation can be beneficial in MS on a case by case basis [64,70,71,72,78,82,83].
A recent in vivo study investigating the effects of Δ9-THC+CBD in EAE model provided further evidence that this combination suppresses neuroinflammation [61]. It was suggested that neuroinflammation could be mediated through regulation of miRNA-mediated signalling pathways based on the results obtained in brain-infiltrating cells [61]. Indeed, the involvement of miRNAs in the pathology of MS has been recently reviewed, and modulators of miRNA expression or function were suggested as disease-modifying therapeutics [106]. In a study utilizing EAE-induced mice, treatment with CBD ameliorated the severity of the clinical signs, suppressed microglial activation and T-cell recruitment in the spinal cord of mice, presumably not acting through known CB1 and CB2 receptors [81].
Moreover, neuroprotective effects against glutamate excitotoxicity, one of the major determinants of neurodegeneration in MS [107], were shown for CBD through CB receptor-independent mechanisms [108]. Other studies in rats with induced EAE disease performed to explore the anti-inflammatory properties of CBD revealed attenuation of EAE disease by evidenced by significantly reduced clinical scores of paralysis, decreased in IL-7 and IFN levels, and reduction in T cell infiltration in CNS were shown [62,77,89]. Interestingly, however, the possible role of immunosuppressive myeloid-derived suppressor cells (MDSCs) in observed CBD-induced amelioration was suggested, since CBD treatment led to a profound increase of these cells in mice. Anti-inflammatory effects of CBD were also shown in the viral model TMEV of MS. This is associated with adenosine A2A receptors participation in some of these effects [79].
BCP is a selective CB2R agonist of natural origin with anti-inflammatory and antioxidant effects that showed beneficial effects on decreasing neuroinflammation in MS models that lead to the decrease of disease progression [66,67]. The administration of the antidepressant imipramine (IMP) in a mouse model of MS improves clinical outcome and pathological score through modulation of inflammatory cytokine levels. In combination with BCP, IMP exerts synergistic effects [66].
PEA is an endogenous compound with cannabinoid-like effects, without psychoactive effects. It acts mainly as G-protein-coupled receptor 55 (GPR55) agonist and by activation of PPAR-α [72,78]. After treatment with PEALut, obtained from PEA and luteolin, the clinical symptoms and motor disabilities decrease in a murine model of EAE by drug modulation of neuroinflammation [72]. Another study that analysed the efficacy of CBD and PEA alone and in combination showed that both CBD and PEA have protective effects on a mice model of MS, but in combination, their effects are antagonistic, explained mainly by opposite effects on GPR55 receptor [78]. This study suggests the possible implication of the GPR55 receptor in the beneficial effects of cannabinoids in MS.
Inhibition of ABHD6 enzyme that leads to increase of 2-AG in the CNS can have promising results in the treatment of MS. KT182 compound, an inhibitor of ABHD6, succeeded in decreasing the motor disability but failed to induce neuro-anti-inflammatory effects [84]. On the other side, WWL70 compound, another inhibitor of ABHD6, proved more promising results by increasing 2-AG levels and improving clinical manifestation of MS, mainly by increasing 2-AG levels in microglia/macrophage and acting on CB2R, leading to decreased microglia activation and decreased production of inflammatory markers and adhesion molecules [85].
CBG is a phytocannabinoid that attracted the attention of researchers due to the lack of psychotropic effects. VCE-003 is a quinone derivative of CBG that has been shown to act mainly as CB2R agonist associated with regulation of microglial neurotoxicity and as PPARγ receptor agonist implicated in decreasing neuroinflammation [70,71]. In vivo studies showed that this compound has promising effects in decreasing progression and motor impairment in MS murine models [70,71].
The study conducted to investigate the efficacy of different extracts of Cannabis sativa revealed that Δ9-THC, CBD and cannabinoid free extracts are all active in CREAE-induced mice at different phases of the disease, likely by distinct mechanisms [65]. These findings are also supported by in vivo studies that investigated the efficacy of Sativex-like combination of phytocannabinoids and each component alone conducted in two models of MS, one on mice infected with Theiler’s murine encephalomyelitis virus (TMEV), inducing demyelinating disease model of MS [64], and one on mice with induced EAE model [63].
The therapeutic potential of a Sativex-like combination to slow MS progression was supported by the data [63,64]. The results obtained in the group treated with single substances showed that CBD acts similarly to Sativex when alleviating motor deterioration acting through PPARγ receptors while other component, Δ9-THC, acts primarily through CB1R and CB2R and produces somewhat weaker effects in the model with mice infected with TMEV [64]. It is interesting that CBD alone has no effect on the other model of MS with the mice-induced EAE, while the Δ9-THC-BDS acts similarly to Sativex when alleviating motor deterioration acting through CB1R and decreasing cell aggregates produced by microglial activation [63]. The differences in the efficacy of cannabinoids between the MS murine models of mice infected with TMEV and mice induced EAE can be explained by the differences observed in the CBR in these models. CB1R is less expressed in the brain of EAE rats [109], while CB2R is up-expressed in mice infected with TMEV [110].
Interestingly, some studies showed the up-expression of CB1R and CB2R in the glial cells of patients suffering from MS, showing the implication of CBR in the pathogenesis of MS disease [111]. Modulation of CB2R in MS is mainly associated with anti-inflammatory effects.
The recent studies showed that using a broad-spectrum cannabinoid combination can have beneficial effects in MS treatment due to the implication of many targeting receptors, further than the classical receptors of the endocannabinoid system (Figure 3).
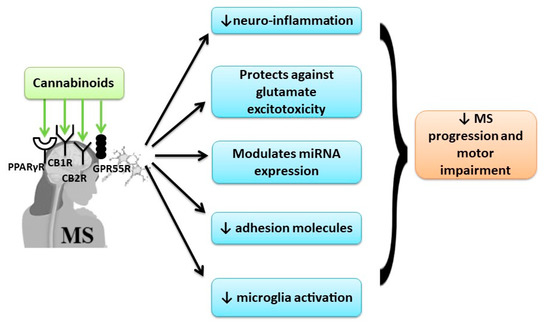
Figure 3.
The beneficial effect of cannabinoids in MS by multi-receptor modulation.
4.3. Limitations and Strength of the Study
The strength of this study is that it comprehensively summarizes the evidence from a large number of meta-analyses covering the impact of cannabinoids in protecting against cognitive decline and motor impairments that occur in acute brain injury and chronic brain injury.
A critical point of this review can be considered that the data were extracted and analysed from a large number of reviews and not from individual studies, although the meta-analyses provide the highest level of scientific evidence. No clinical trials were included, as the main purpose of this comprehensive review was to highlight the molecular mechanisms of action of cannabinoids in brain receptors and this is best done in animal models.
Even the research methodology can be considered a last limiting aspect; although it was systematic, this paper cannot be considered as a systematic review, because the quality of the studies themselves was not analysed in detail.
5. Conclusions
Based on benefits from cannabinoids use observed in animal studies, further clinical studies should be employed to proof the beneficial effect of cannabinoids’ treatment in TBI and MS, especially in those patients who are displaying resistance to conventional treatment.
The use of cannabinoids in TBI increases neurobehavioral function, working memory performance and decreases the neurological deficit and ameliorates motor deficit through down-regulation of pro-inflammatory markers, oedema formation and BBB permeability, preventing neuronal cell loss and up-regulating the levels of adherence junction proteins.
In murine models of MS, cannabinoids showed beneficial effects on improving clinical outcome, decreasing motor impairment and delaying the progression of the disease through down-regulation of neuroinflammation, up-regulation of anti-inflammatory cytokines, decreasing cell proliferation and decreasing demyelination and microglia activation.
In TBI treatment, targeting CB2R is a promising therapeutic approach, as the receptor is localized primarily in the microglia and less in neurons, and their expression is increased after a lesion that determines microglia activation. In contrast, in neurodegenerative diseases like MS, the cannabinoids showed beneficial effects in decreasing the motor disability and disease progression by a complex mechanism targeting more signalling pathways further that classical receptors of the endocannabinoid system.
Author Contributions
Conceptualization, D.C., A.M.B. and A.O.D.; validation investigation M.M., A.B. (Aleksandra Buha), C.C. and C.S.; resources, D.C. and A.O.D.; data curation M.M., A.O.D., A.B. (Abdelhakim Bouyahya), N.E.O., N.E.M. and A.B. (Aleksandra Buha); review and editing A.M.B., A.O.D. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
All authors wrote and contributed to the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reiner, A.; Heldt, S.A.; Presley, C.S.; Guley, N.H.; Elberger, A.J.; Deng, Y.; D’Surney, L.; Rogers, J.T.; Ferrell, J.; Bu, W.; et al. Motor, visual and emotional deficits in mice after closed-head mild traumatic brain injury are alleviated by the novel CB2 inverse agonist SMM-189. Int. J. Mol. Sci. 2014, 16, 758–787. [Google Scholar] [CrossRef] [PubMed]
- Hallikainen, I.; Hongisto, K.; Välimäki, T.; Hänninen, T.; Martikainen, J.; Koivisto, A.M. The Progression of Neuropsychiatric Symptoms in Alzheimer’s Disease during a Five-Year Follow-Up: Kuopio ALSOVA Study. J. Alzheimer’s Dis. Jad 2018, 61, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.R.; Piguet, O. Progress and Challenges in Frontotemporal Dementia Research: A 20-Year Review. J. Alzheimer’s Dis. Jad 2018, 62, 1467–1480. [Google Scholar] [CrossRef]
- Douven, E.; Aalten, P.; Staals, J.; Schievink, S.H.J.; van Oostenbrugge, R.J.; Verhey, F.R.J.; Köhler, S. Co-occurrence of depressive symptoms and executive dysfunction after stroke: Associations with brain pathology and prognosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Kokiko-Cochran, O.N.; Godbout, J.P. The Inflammatory Continuum of Traumatic Brain Injury and Alzheimer’s Disease. Front. Immunol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Verghese, J.; Beauchet, O.; Hausdorff, J.M. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 2012, 60, 2127–2136. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Bergman, H.; Phillips, N.A.; Wong, C.H.; Sourial, N.; Chertkow, H. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 2009, 9, 41. [Google Scholar] [CrossRef]
- Ricciardi, L.; Bloem, B.R.; Snijders, A.H.; Daniele, A.; Quaranta, D.; Bentivoglio, A.R.; Fasano, A. Freezing of gait in Parkinson’s disease: The paradoxical interplay between gait and cognition. Parkinsonism Relat. Disord. 2014, 20, 824–829. [Google Scholar] [CrossRef]
- Aloizou, A.M.; Siokas, V.; Vogiatzi, C.; Peristeri, E.; Docea, A.O.; Petrakis, D.; Provatas, A.; Folia, V.; Chalkia, C.; Vinceti, M.; et al. Pesticides, cognitive functions and dementia: A review. Toxicol. Lett. 2020, 326, 31–51. [Google Scholar] [CrossRef]
- Sergievich, A.A.; Khoroshikh, P.P.; Artemenko, A.F.; Zakharenko, A.M.; Chaika, V.V.; Kodintsev, V.V.; Stroeva, O.A.; Lenda, E.G.; Tsatsakis, A.; Burykina, T.I.; et al. Behavioral impacts of a mixture of six pesticides on rats. Sci. Total Environ. 2020, 727, 138491. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Docea, A.O.; Calina, D.; Tsarouhas, K.; Zamfira, L.M.; Mitrut, R.; Sharifi-Rad, J.; Kovatsi, L.; Siokas, V.; Dardiotis, E.; et al. A Mechanistic and Pathophysiological Approach for Stroke Associated with Drugs of Abuse. J. Clin. Med. 2019, 8, 1295. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, W. The Treatment Strategies for Neurodegenerative Diseases by Integrative Medicine. Integr. Med. Int. 2014, 1, 223–225. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Tumer, T.B.; Moreira, A.C.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Marina Salgado Castillo, C.; Martorell, M.; et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LE Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Barroso-Chinea, P.; Rico, A.J.; Conte-Perales, L.; Callén, L.; Roda, E.; Gómez-Bautista, V.; López, I.P.; Lluis, C.; Labandeira-García, J.L.; et al. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J. Psychopharmacol. (Oxf. Engl.) 2011, 25, 97–104. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience 2015, 311, 253–267. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; García-Bueno, B.; Zoppi, S.; Leza, J.C.; Manzanares, J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br. J. Pharmacol. 2012, 165, 951–964. [Google Scholar] [CrossRef]
- Stempel, A.V.; Stumpf, A.; Zhang, H.Y.; Özdoğan, T.; Pannasch, U.; Theis, A.K.; Otte, D.M.; Wojtalla, A.; Rácz, I.; Ponomarenko, A.; et al. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 2016, 90, 795–809. [Google Scholar] [CrossRef]
- Viscomi, M.T.; Oddi, S.; Latini, L.; Pasquariello, N.; Florenzano, F.; Bernardi, G.; Molinari, M.; Maccarrone, M. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 4564–4570. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Griffin-Thomas, L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.J.; García-Merino, A. Neuroprotective agents: Cannabinoids. Clin. Immunol. (Orlando Fla.) 2012, 142, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W.; Newton, C.; Larsen, K.; Lu, L.; Perkins, I.; Nong, L.; Friedman, H. The cannabinoid system and immune modulation. J. Leukoc. Biol. 2003, 74, 486–496. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Gao, M.; Liu, Q.R.; Bi, G.H.; Li, X.; Yang, H.J.; Gardner, E.L.; Wu, J.; Xi, Z.X. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E5007–E5015. [Google Scholar] [CrossRef]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Mihai, L.-G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB₁ and CB₂. Pharm. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Maurya, N.; Velmurugan, B.K. Therapeutic applications of cannabinoids. Chem. Biol. Interact. 2018, 293, 77–88. [Google Scholar] [CrossRef]
- Fezza, F.; Bisogno, T.; Minassi, A.; Appendino, G.; Mechoulam, R.; Di Marzo, V. Noladin ether, a putative novel endocannabinoid: Inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002, 513, 294–298. [Google Scholar] [CrossRef]
- Porter, A.C.; Sauer, J.M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B.; et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Grabiec, U.; Dehghani, F. N-Arachidonoyl Dopamine: A Novel Endocannabinoid and Endovanilloid with Widespread Physiological and Pharmacological Activities. Cannabis Cannabinoid Res. 2017, 2, 183–196. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar]
- Cifelli, P.; Ruffolo, G.; De Felice, E.; Alfano, V.; van Vliet, E.A.; Aronica, E.; Palma, E. Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? Int. J. Mol. Sci. 2020, 21, 723. [Google Scholar] [CrossRef] [PubMed]
- Niesink, R.J.M.; van Laar, M.W. Does Cannabidiol Protect against Adverse Psychological Effects of THC? Front. Psychiatry 2013, 4, 130. [Google Scholar] [CrossRef]
- Bolognini, D.; Rock, E.M.; Cluny, N.L.; Cascio, M.G.; Limebeer, C.L.; Duncan, M.; Stott, C.G.; Javid, F.A.; Parker, L.A.; Pertwee, R.G. Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br. J. Pharmacol. 2013, 168, 1456–1470. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar]
- Izzo, A.A.; Capasso, R.; Aviello, G.; Borrelli, F.; Romano, B.; Piscitelli, F.; Gallo, L.; Capasso, F.; Orlando, P.; Di Marzo, V. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br. J. Pharmacol. 2012, 166, 1444–1460. [Google Scholar] [CrossRef]
- Miliano, C.; Serpelloni, G.; Rimondo, C.; Mereu, M.; Marti, M.; De Luca, M.A. Neuropharmacology of New Psychoactive Substances (NPS): Focus on the Rewarding and Reinforcing Properties of Cannabimimetics and Amphetamine-Like Stimulants. Front. Neurosci. 2016, 10, 153. [Google Scholar]
- Bilici, R. Synthetic cannabinoids. North. Clin. Istanb. 2014, 1, 121–126. [Google Scholar] [CrossRef]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Magid, L.; Heymann, S.; Elgali, M.; Avram, L.; Cohen, Y.; Liraz-Zaltsman, S.; Mechoulam, R.; Shohami, E. Role of CB(2) Receptor in the Recovery of Mice after Traumatic Brain Injury. J. Neurotrauma 2019, 36, 1836–1846. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Cai, S.; Li, R.; Cao, G. Cannabinoid receptor 2 agonist attenuates blood-brain barrier damage in a rat model of intracerebral hemorrhage by activating the Rac1 pathway. Int. J. Mol. Med. 2018, 42, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yun, D.; Zhang, Y.; Tao, Y.; Tan, Q.; Qiao, F.; Luo, B.; Liu, Y.; Fan, R.; Xian, J.; et al. A cannabinoid receptor 2 agonist reduces blood-brain barrier damage via induction of MKP-1 after intracerebral hemorrhage in rats. Brain Res. 2018, 1697, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, M.N.; Baban, B.; et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. BrainBehav. Immun. 2018, 68, 224–237. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef]
- Liu, Y.; McAfee, S.S.; Guley, N.M.; Del Mar, N.; Bu, W.; Heldt, S.A.; Honig, M.G.; Moore II, B.M.; Reiner, A. Abnormalities in Dynamic Brain Activity Caused by Mild Traumatic Brain Injury Are Partially Rescued by the Cannabinoid Type-2 Receptor Inverse Agonist SMM-189. Eneuro 2017, 4. [Google Scholar] [CrossRef]
- Nissinen, J.; Andrade, P.; Natunen, T.; Hiltunen, M.; Malm, T.; Kanninen, K.; Soares, J.I.; Shatillo, O.; Sallinen, J.; Ndode-Ekane, X.E.; et al. Disease-modifying effect of atipamezole in a model of post-traumatic epilepsy. Epilepsy Res. 2017, 136, 18–34. [Google Scholar] [CrossRef]
- Bu, W.; Ren, H.; Deng, Y.; Del Mar, N.; Guley, N.M.; Moore, B.M.; Honig, M.G.; Reiner, A. Mild Traumatic Brain Injury Produces Neuron Loss That Can Be Rescued by Modulating Microglial Activation Using a CB2 Receptor Inverse Agonist. Front. Neurosci. 2016, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.; Khan, M.; Craig, L.; Nakanishi, S.T. Cannabinoid agonist rescues learning and memory after a traumatic brain injury. Ann. Clin. Transl. Neurol. 2015, 2, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Tchantchou, F.; Tucker, L.B.; Fu, A.H.; Bluett, R.J.; McCabe, J.T.; Patel, S.; Zhang, Y. The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology 2014, 85, 427–439. [Google Scholar] [CrossRef]
- Fujii, M.; Sherchan, P.; Krafft, P.R.; Rolland, W.B.; Soejima, Y.; Zhang, J.H. Cannabinoid type 2 receptor stimulation attenuates brain edema by reducing cerebral leukocyte infiltration following subarachnoid hemorrhage in rats. J. Neurol. Sci. 2014, 342, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Tchantchou, F.; Zhang, Y. Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J. Neurotrauma 2013, 30, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Yeshurun, A.; Willner, D.; Trembovler, V.; Alexandrovich, A.; Mechoulam, R.; Shohami, E.; Leker, R.R. N-arachidonoyl-L-serine (AraS) possesses proneurogenic properties in vitro and in vivo after traumatic brain injury. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2013, 33, 1242–1250. [Google Scholar] [CrossRef]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305. [Google Scholar] [CrossRef]
- Pazos, M.R.; Cinquina, V.; Gómez, A.; Layunta, R.; Santos, M.; Fernández-Ruiz, J.; Martínez-Orgado, J. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 2012, 63, 776–783. [Google Scholar] [CrossRef]
- Schmidt, W.; Schäfer, F.; Striggow, V.; Fröhlich, K.; Striggow, F. Cannabinoid receptor subtypes 1 and 2 mediate long-lasting neuroprotection and improve motor behavior deficits after transient focal cerebral ischemia. Neuroscience 2012, 227, 313–326. [Google Scholar] [CrossRef]
- Elliott, M.B.; Tuma, R.F.; Amenta, P.S.; Barbe, M.F.; Jallo, J.I. Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J. Neurotrauma 2011, 28, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghezi, Z.Z.; Miranda, K.; Nagarkatti, M.; Nagarkatti, P.S. Combination of Cannabinoids, Δ9- Tetrahydrocannabinol and Cannabidiol, Ameliorates Experimental Multiple Sclerosis by Suppressing Neuroinflammation Through Regulation of miRNA-Mediated Signaling Pathways. Front. Immunol. 2019, 10, 1921. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Ahmad, T.K.; Alrushaid, S.; Pozdirca, M.; Ethans, K.; Intrater, H.; Le, T.; Burczynski, F.; Kong, J.; Namaka, M. Therapeutic impact of orally administered cannabinoid oil extracts in an experimental autoimmune encephalomyelitis animal model of multiple sclerosis. Biochem. Biophys. Res. Commun. 2019, 516, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Martet, M.; Feliú, A.; Espejo-Porras, F.; Mecha, M.; Carrillo-Salinas, F.J.; Fernández-Ruiz, J.; Guaza, C.; de Lago, E. The disease-modifying effects of a Sativex-like combination of phytocannabinoids in mice with experimental autoimmune encephalomyelitis are preferentially due to Δ9-tetrahydrocannabinol acting through CB1 receptors. Mult. Scler. Relat. Disord. 2015, 4, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Feliú, A.; Moreno-Martet, M.; Mecha, M.; Carrillo-Salinas, F.J.; de Lago, E.; Fernández-Ruiz, J.; Guaza, C. A Sativex(®) -like combination of phytocannabinoids as a disease-modifying therapy in a viral model of multiple sclerosis. Br. J. Pharmacol. 2015, 172, 3579–3595. [Google Scholar] [CrossRef] [PubMed]
- Buccellato, E.; Carretta, D.; Utan, A.; Cavina, C.; Speroni, E.; Grassi, G.; Candeletti, S.; Romualdi, P. Acute and chronic cannabinoid extracts administration affects motor function in a CREAE model of multiple sclerosis. J. Ethnopharmacol. 2011, 133, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Askari, V.R.; Baradaran Rahimi, V.; Tabatabaee, S.A.; Shafiee-Nick, R. Combination of Imipramine, a sphingomyelinase inhibitor, and β-caryophyllene improve their therapeutic effects on experimental autoimmune encephalomyelitis (EAE). Int. Immunopharmacol. 2019, 77, 105923. [Google Scholar] [CrossRef]
- Alberti, T.B.; Barbosa, W.L.; Vieira, J.L.; Raposo, N.R.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef]
- Shi, Y.; Duan, Y.H.; Ji, Y.Y.; Wang, Z.L.; Wu, Y.R.; Gunosewoyo, H.; Xie, X.Y.; Chen, J.Z. Amidoalkylindoles as Potent and Selective Cannabinoid Type 2 Receptor Agonists with in Vivo Efficacy in a Mouse Model of Multiple Sclerosis. J. Med. Chem. 2017, 60, 7067–7083. [Google Scholar]
- Morales, P.; Gómez-Cañas, M.; Navarro, G.; Hurst, D.P.; Carrillo-Salinas, F.J.; Lagartera, L.; Pazos, R.; Goya, P.; Reggio, P.H.; Guaza, C.; et al. Chromenopyrazole, a Versatile Cannabinoid Scaffold with in Vivo Activity in a Model of Multiple Sclerosis. J. Med. Chem. 2016, 59, 6753–6771. [Google Scholar]
- Carrillo-Salinas, F.J.; Navarrete, C.; Mecha, M.; Feliú, A.; Collado, J.A.; Cantarero, I.; Bellido, M.L.; Muñoz, E.; Guaza, C. A cannabigerol derivative suppresses immune responses and protects mice from experimental autoimmune encephalomyelitis. PLoS ONE 2014, 9, e94733. [Google Scholar] [CrossRef]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A Cannabigerol Quinone Alleviates Neuroinflammation in a Chronic Model of Multiple Sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Contarini, G.; Franceschini, D.; Facci, L.; Barbierato, M.; Giusti, P.; Zusso, M. A co-ultramicronized palmitoylethanolamide/luteolin composite mitigates clinical score and disease-relevant molecular markers in a mouse model of experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2019, 16, 126. [Google Scholar] [CrossRef]
- Correa, F.; Hernangómez-Herrero, M.; Mestre, L.; Loría, F.; Docagne, F.; Guaza, C. The endocannabinoid anandamide downregulates IL-23 and IL-12 subunits in a viral model of multiple sclerosis: Evidence for a cross-talk between IL-12p70/IL-23 axis and IL-10 in microglial cells. Brain Behav. Immun. 2011, 25, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Yu, F.; Wen, J.; Vana, A.; Zhang, Y. Therapeutic potential of a novel cannabinoid agent CB52 in the mouse model of experimental autoimmune encephalomyelitis. Neuroscience 2013, 254, 427–442. [Google Scholar] [CrossRef]
- Giacoppo, S.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia 2017, 116, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Soundara Rajan, T.; Galuppo, M.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Purified Cannabidiol, the main non-psychotropic component of Cannabis sativa, alone, counteracts neuronal apoptosis in experimental multiple sclerosis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4906–4919. [Google Scholar] [PubMed]
- Giacoppo, S.; Galuppo, M.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2015, 23, 48. [Google Scholar] [CrossRef]
- Rahimi, A.; Faizi, M.; Talebi, F.; Noorbakhsh, F.; Kahrizi, F.; Naderi, N. Interaction between the protective effects of cannabidiol and palmitoylethanolamide in experimental model of multiple sclerosis in C57BL/6 mice. Neuroscience 2015, 290, 279–287. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- De Lago, E.; Moreno-Martet, M.; Cabranes, A.; Ramos, J.A.; Fernández-Ruiz, J. Cannabinoids ameliorate disease progression in a model of multiple sclerosis in mice, acting preferentially through CB1 receptor-mediated anti-inflammatory effects. Neuropharmacology 2012, 62, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011, 163, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Hasseldam, H.; Johansen, F.F. Cannabinoid treatment renders neurons less vulnerable than oligodendrocytes in Experimental Autoimmune Encephalomyelitis. Int. J. Neurosci. 2011, 121, 510–520. [Google Scholar] [CrossRef]
- Mestre, L.; Docagne, F.; Correa, F.; Loría, F.; Hernangómez, M.; Borrell, J.; Guaza, C. A cannabinoid agonist interferes with the progression of a chronic model of multiple sclerosis by downregulating adhesion molecules. Mol. Cell. Neurosci. 2009, 40, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Manterola, A.; Bernal-Chico, A.; Cipriani, R.; Ruiz, A.; Pérez-Samartín, A.; Moreno-Rodríguez, M.; Hsu, K.L.; Cravatt, B.F.; Brown, J.M.; Rodríguez-Puertas, R.; et al. Re-examining the potential of targeting ABHD6 in multiple sclerosis: Efficacy of systemic and peripherally restricted inhibitors in experimental autoimmune encephalomyelitis. Neuropharmacology 2018, 141, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ribeiro, R.; Tanaka, M.; Zhang, Y. Activation of CB2 receptor is required for the therapeutic effect of ABHD6 inhibition in experimental autoimmune encephalomyelitis. Neuropharmacology 2015, 99, 196–209. [Google Scholar] [CrossRef]
- Feliú, A.; Bonilla Del Río, I.; Carrillo-Salinas, F.J.; Hernández-Torres, G.; Mestre, L.; Puente, N.; Ortega-Gutiérrez, S.; López-Rodríguez, M.L.; Grandes, P.; Mecha, M.; et al. 2-Arachidonoylglycerol Reduces Proteoglycans and Enhances Remyelination in a Progressive Model of Demyelination. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 8385–8398. [Google Scholar]
- Brindisi, M.; Maramai, S.; Gemma, S.; Brogi, S.; Grillo, A.; Di Cesare Mannelli, L.; Gabellieri, E.; Lamponi, S.; Saponara, S.; Gorelli, B.; et al. Development and Pharmacological Characterization of Selective Blockers of 2-Arachidonoyl Glycerol Degradation with Efficacy in Rodent Models of Multiple Sclerosis and Pain. J. Med. Chem. 2016, 59, 2612–2632. [Google Scholar] [CrossRef]
- Hernández-Torres, G.; Cipriano, M.; Hedén, E.; Björklund, E.; Canales, Á.; Zian, D.; Feliú, A.; Mecha, M.; Guaza, C.; Fowler, C.J.; et al. A reversible and selective inhibitor of monoacylglycerol lipase ameliorates multiple sclerosis. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 13765–13770. [Google Scholar] [CrossRef]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol Attenuates Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis through Induction of Myeloid-Derived Suppressor Cells. Front. Immunol. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Dash, H.H.; Chavali, S. Management of traumatic brain injury patients. Korean J. Anesth. 2018, 71, 12–21. [Google Scholar] [CrossRef]
- Stocchetti, N.; Carbonara, M.; Citerio, G.; Ercole, A.; Skrifvars, M.B.; Smielewski, P.; Zoerle, T.; Menon, D.K. Severe traumatic brain injury: Targeted management in the intensive care unit. Lancet Neurol. 2017, 16, 452–464. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef]
- Savinainen, J.R.; Saario, S.M.; Laitinen, J.T. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. (Oxf. Engl.) 2012, 204, 267–276. [Google Scholar] [CrossRef]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, A.B.; Siopi, E.; Finn, D.P.; Marchand-Leroux, C.; Garcia-Segura, L.M.; Jafarian-Tehrani, M.; Viveros, M.P. CB1 and CB2 cannabinoid receptor antagonists prevent minocycline-induced neuroprotection following traumatic brain injury in mice. Cereb. Cortex (N.Y. 1991) 2015, 25, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Darlington, C.L.; Smith, P.F.; Ashton, J.C. Antibody testing for brain immunohistochemistry: Brain immunolabeling for the cannabinoid CB₂ receptor. J. Neurosci. Methods 2013, 216, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Højsgaard Chow, H.; Schreiber, K.; Magyari, M.; Ammitzbøll, C.; Börnsen, L.; Romme Christensen, J.; Ratzer, R.; Soelberg Sørensen, P.; Sellebjerg, F. Progressive multiple sclerosis, cognitive function, and quality of life. Brain Behav. 2018, 8, e00875. [Google Scholar] [CrossRef]
- Handel, A.E.; Giovannoni, G.; Ebers, G.C.; Ramagopalan, S.V. Environmental factors and their timing in adult-onset multiple sclerosis. Nat. Rev. Neurol. 2010, 6, 156–166. [Google Scholar] [CrossRef]
- Global, regional, and national burden of motor neuron diseases 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 1083–1097. [CrossRef]
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of Multiple Sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef]
- Nussbaum, L.; Hogea, L.M.; Calina, D.; Andreescu, N.; Gradinaru, R.; Stefanescu, R.; Puiu, M. Modern treatment approaches in psychoses. Pharmacogenetic, neuroimagistic and clinical implications. Farmacia 2017, 65, 75–81. [Google Scholar]
- De Faria, O., Jr.; Moore, C.S.; Kennedy, T.E.; Antel, J.P.; Bar-Or, A.; Dhaunchak, A.S. MicroRNA dysregulation in multiple sclerosis. Front. Genet. 2012, 3, 311. [Google Scholar] [PubMed]
- Centonze, D.; Muzio, L.; Rossi, S.; Furlan, R.; Bernardi, G.; Martino, G. The link between inflammation, synaptic transmission and neurodegeneration in multiple sclerosis. Cell Death Differ. 2010, 17, 1083–1091. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef]
- Cabranes, A.; Venderova, K.; de Lago, E.; Fezza, F.; Sánchez, A.; Mestre, L.; Valenti, M.; García-Merino, A.; Ramos, J.A.; Di Marzo, V.; et al. Decreased endocannabinoid levels in the brain and beneficial effects of agents activating cannabinoid and/or vanilloid receptors in a rat model of multiple sclerosis. Neurobiol. Dis. 2005, 20, 207–217. [Google Scholar] [CrossRef]
- Loría, F.; Petrosino, S.; Mestre, L.; Spagnolo, A.; Correa, F.; Hernangómez, M.; Guaza, C.; Di Marzo, V.; Docagne, F. Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur. J. Neurosci. 2008, 28, 633–641. [Google Scholar] [CrossRef]
- Benito, C.; Romero, J.P.; Tolón, R.M.; Clemente, D.; Docagne, F.; Hillard, C.J.; Guaza, C.; Romero, J. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).