Pleural Pressure Pulse in Patients with Pleural Effusion: A New Phenomenon Registered during Thoracentesis with Pleural Manometry
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Patients
2.3. Echocardiography and Pulmonary Function Tests
2.4. Thoracentesis and Pleural Manometry
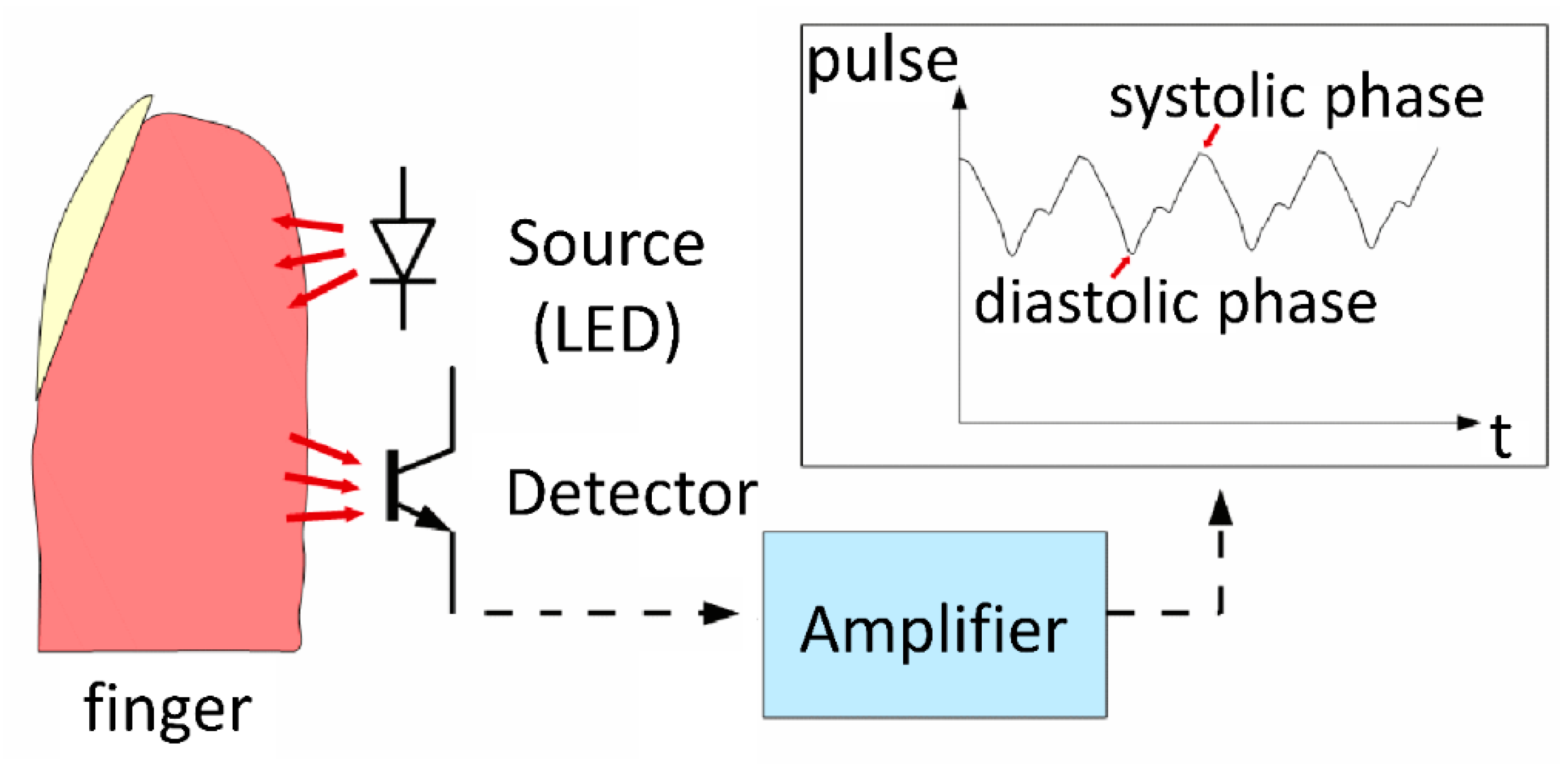
2.5. Pleural Pressure Pulse Assessment
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
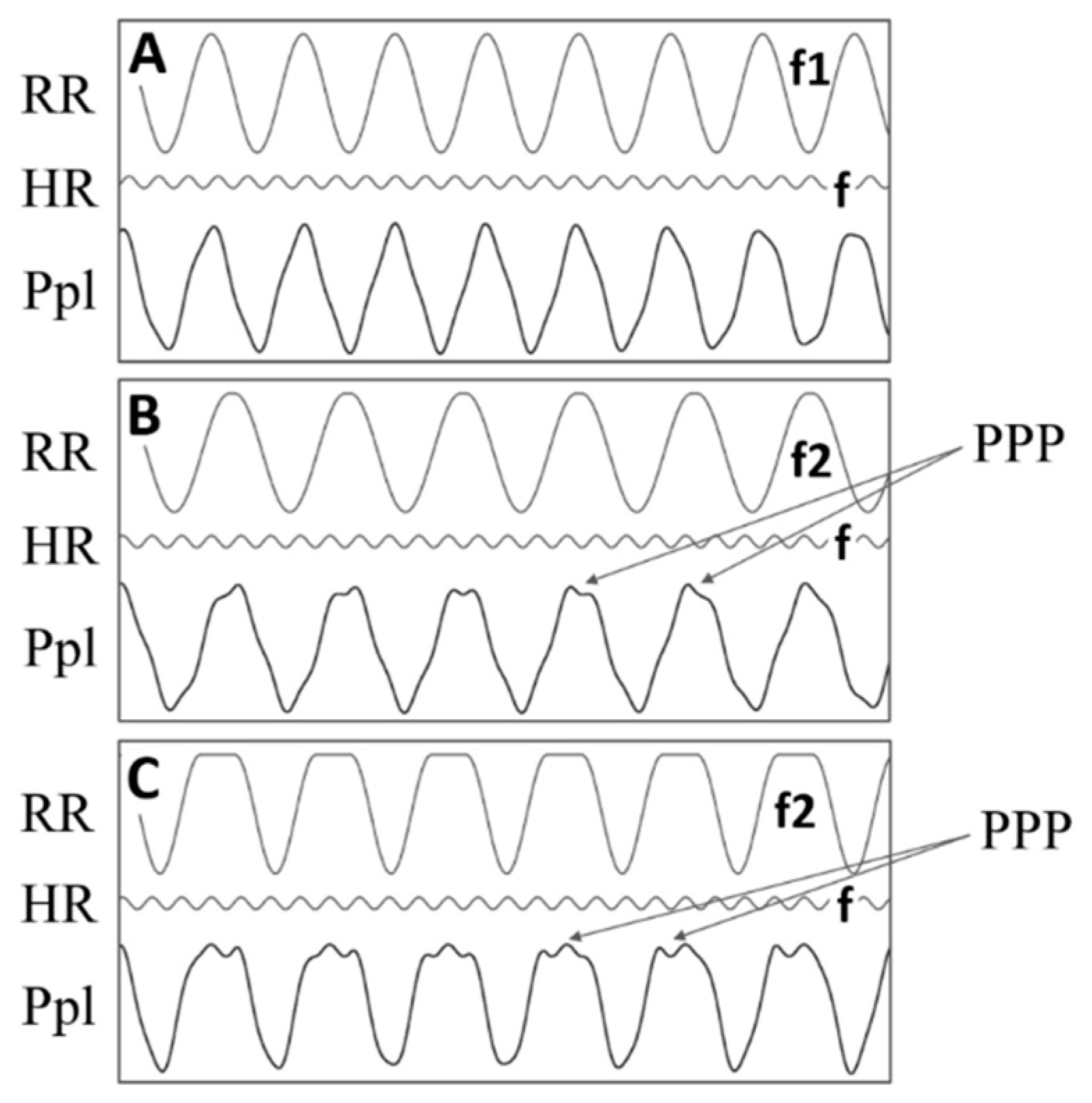
3.2. Pleural Pressure Pulse Characteristics and Origin
3.3. Parameters Characterizing Pleural Effusion, Lung Expandability, and Vital Signs in the Studied Subgroups
3.4. Relation between PPP and Echocardiographic Parameters
3.5. Association of PPP with Pulmonary Function and Arterial Blood Gases
3.6. Consistency of PPP during Thoracentesis and Pleural Fluid Withdrawal
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levett, J.; Agarwal, G. The first man/machine interaction in medicine: The pulsilogium of Sanctorius. Med. Instrum. 1979, 13, 61–63. [Google Scholar] [PubMed]
- De Grijs, R.; Vuillermin, D. Measure of the Heart: Santorio Santorio and the Pulsilogium. arXiv 2017, arXiv:1702.05211. [Google Scholar]
- Lichtenstein, D.A.; Lascols, N.; Prin, S.; Mezière, G. The “lung pulse”: An early ultrasound sign of complete atelectasis. Intensive Care. Med. 2003, 29, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, M.R.; Lo, A.K.C.; Ng, A.C.T.; Bashirzadeh, F.; Wang, W.Y.S.; Fielding, D.I.K. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest 2014, 146, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Bjorn, F.; Sahar, A. Ultrasound as a noninvasive tool to diagnose trapped lung. Am. J. Respir. Cit. Care Med. 2017, 195, A6518. [Google Scholar]
- Blancas Gómez-Casero, R.; Alonso Fernández, M.Á.; Ballesteros Ortega, D.; Martínez González, Ó. Subpleural artifact in lung pulse evidenced by M-mode ultrasound in a patient with atelectasis. Rev. Esp. Anestesiol. Reanim. 2016, 63, 127. [Google Scholar] [CrossRef] [PubMed]
- Leemans, J.; Dooms, C.; Ninane, V.; Yserbyt, J. Success rate of medical thoracoscopy and talc pleurodesis in malignant pleurisy: A single-center experience. Respirology 2018, 23, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Feller-Kopman, D.; Parker, M.J.; Schwartzstein, R.M. Assessment of pleural pressure in the evaluation of pleural effusions. Chest 2009, 135, 201–209. [Google Scholar] [CrossRef]
- Grabczak, E.M.; Krenke, R.; Zielinska-Krawczyk, M.; Light, R.W. Pleural manometry in patients with pleural diseases - the usefulness in clinical practice. Respir. Med. 2018, 145, 230–236. [Google Scholar] [CrossRef]
- Huggins, J.T.; Maldonado, F.; Chopra, A.; Rahman, N.; Light, R. Unexpandable lung from pleural disease. Respirology 2018, 23, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Vaska, K.; Wann, L.S.; Sagar, K.; Klopfenstein, H.S. Pleural effusion as a cause of right ventricular diastolic collapse. Circulation 1992, 86, 609–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, G.; Tomlinson, C.W.; O’Sullivan, T.; McKelvie, R.S. Right ventricular diastolic collapse without hemodynamic compromise in a patient with large, bilateral pleural effusions. J. Am. Soc. Echocardiogr. 1995, 8, 551–553. [Google Scholar] [CrossRef]
- Sadaniantz, A.; Anastacio, R.; Verma, V.; Aprahamian, N. The incidence of diastolic right atrial collapse in patients with pleural effusion in the absence of pericardial effusion. Echocardiography 2003, 20, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Kopterides, P.; Lignos, M.; Papanikolaou, S.; Papadomichelakis, E.; Mentzelopoulos, S.; Armaganidis, A.; Panou, F. Pleural effusion causing cardiac tamponade: Report of two cases and review of the literature. Heart Lung 2006, 35, 66–67. [Google Scholar] [CrossRef]
- Wemmelund, K.B.; Lie, R.H.; Juhl-Olsen, P.; Frederiksen, C.A.; Hermansen, J.F.; Sloth, E. Pleural effusion decreases left ventricular pre-load and causes haemodynamic compromise: An experimental porcine study. Acta Anaesthesiol. Scand. 2012, 56, 833–839. [Google Scholar] [CrossRef]
- Macintyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.M.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. ATS/ERS Task Force. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef]
- Krenke, R.; Guc, M.; Grabczak, E.M.; Michnikowski, M.; Pałko, K.J.; Chazan, R.; Gólczewski, T. Development of an electronic manometer for intrapleural pressure monitoring. Respiration 2011, 82, 377–385. [Google Scholar] [CrossRef]
- Zielinska-Krawczyk, M.; Grabczak, E.M.; Michnikowski, M.; Zielinski, K.; Korczynski, P.; Stecka, A.; Golczewski, T.; Krenke, R. Patterns of pleural pressure amplitude and respiratory rate changes during therapeutic thoracentesis. BMC Pulm. Med. 2018, 18, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boshuizen, R.C.; Sinaasappel, M.; Vincent, A.D.; Goldfinger, V.; Farag, S.; van den Heuvel, M.M. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: The benefits of high-temporal resolution pleural manometry. J. Bronchol. Interv. Pulmonol. 2013, 20, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Feller-Kopman, D. Therapeutic thoracentesis: The role of ultrasound and pleural manometry. Curr. Opin. Pulm. Med. 2007, 13, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Krawczyk, M.; Krenke, R.; Grabczak, E.M.; Light, R.W. Pleural manometry-historical background, rationale for use and methods of measurement. Respir. Med. 2018, 136, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, F.; Feller-Kopman, D. Should manometry be routinely used during thoracentesis? Yes, but not without some basic physiologic understanding! Expert Rev. Respir. Med. 2016, 10, 1035–1037. [Google Scholar] [CrossRef]
- Zielinska-Krawczyk, M.; Michnikowski, M.; Grabczak, E.M.; Palko, K.J.; Korczynski, P.; Golczewski, T.; Krenke, R. Cough during therapeutic thoracentesis: Friend or foe? Respirology 2015, 20, 166–168. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Chopra, A.; Huggins, J.T.; Nanchal, R. Pleural manometry: Techniques, applications, and pitfalls. J. Thorac. Dis. 2020, 12, 2759–2770. [Google Scholar] [CrossRef]
- Thomas, R.; Jenkins, S.; Eastwood, P.R.; Lee, Y.C.; Singh, B. Physiology of breathlessness associated with pleural effusions. Curr. Opin. Pulm. Med. 2015, 21, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Lentz, R.J.; Lerner, A.D.; Pannu, J.K.; Merrick, C.M.; Roller, L.; Walston, C.; Valenti, S.; Goddard, T.; Chen, H.; Huggins, J.T.; et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: A multicentre, single-blind randomised controlled trial. Lancet Respir. Med. 2019, 7, 447–455. [Google Scholar] [CrossRef]
- Wilcken, D.E.L. Physiology of normal heart. Surgery 2018, 36, 48–51. [Google Scholar] [CrossRef]




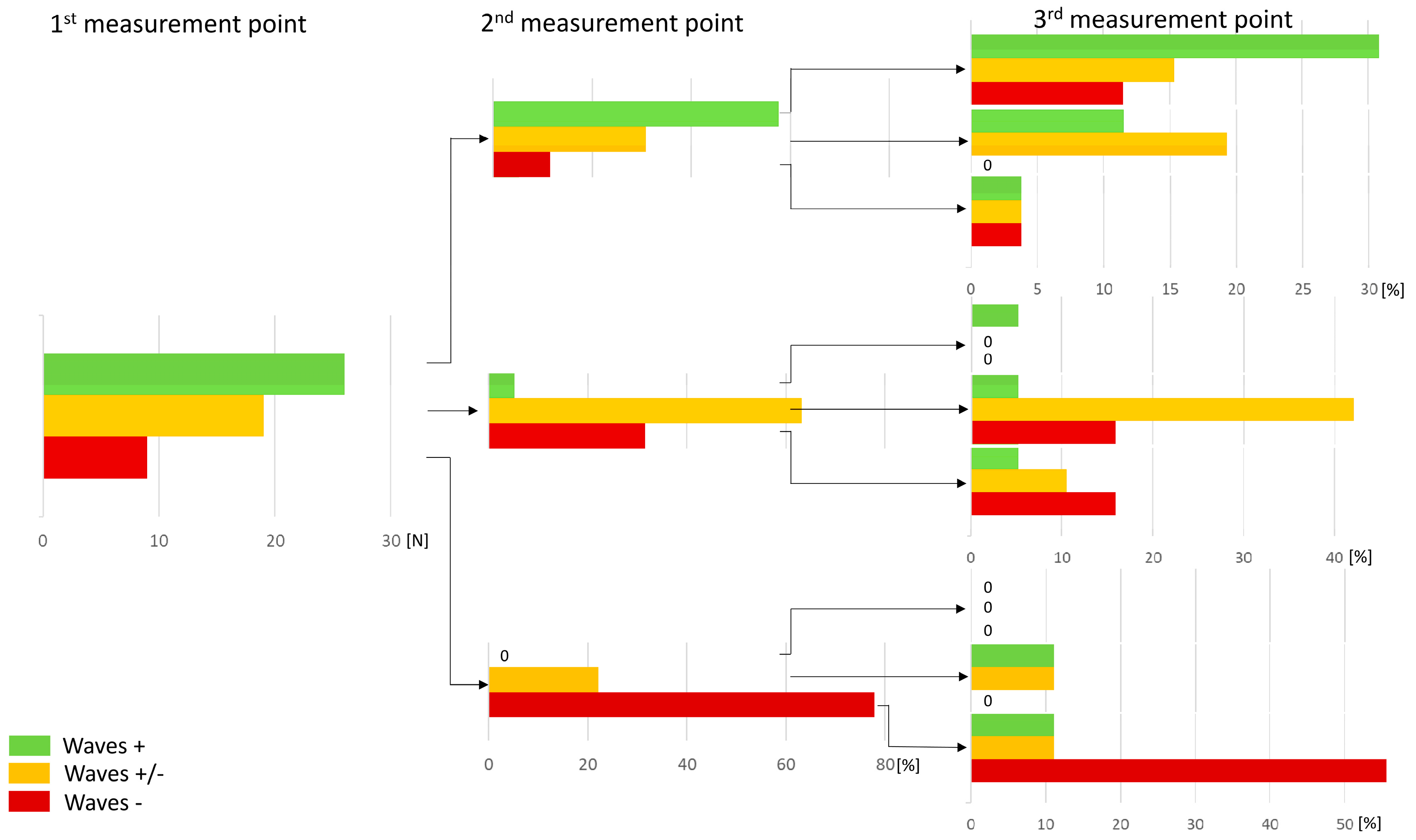
| Parameter | Waves + | Waves − | Waves +/− | p |
|---|---|---|---|---|
| Number of Patients (%) | 26 (48.1) | 9 (16.7) | 19 (35.2) | - |
| Age Years, | 63 (57.2–79.7) | 72 (58–74) | 68 (63.5–77.5) | 0.83 * |
| Gender F/M | 16/10 | 4/5 | 12/7 | 0.61 # |
| BMI kg/m2 | 26 (21.2–28.1) | 25.1 (23.1–28.5) | 25.7 (22.9–27.3) | 0.92 * |
| SBP mmHg | 114 (108–118) | 107 (97–114) | 114 (100–130) | 0.58 * |
| DBP mmHg | 71 (65–75) | 66 (58–71) | 65 (59.5–72) | 0.23 * |
| MBP mmHg | 86 (79.7–92.7) | 77 (74.3–85.3) | 80 (74.5–90) | 0.35 * |
| HR beat/min | 92 (86.5–99.2) | 87.6 (81.1–97.7) | 80.6 (72.1–94.7) | 0.19 * |
| RR per min | 21.7 (18.8–26.9) | 30.5 (27.7–34.6) | 25.37 (23.5–28.5) | 0.008 * |
| HR/RR | 3.9 (3.4–4.9) | 3.1 (2.2–3.5) | 3.3 (2.9–3.7) | 0.003 * |
| Side of pleural effusion R/L | 15/11 | 3/6 | 10/9 | 0.45 # |
Distribution of pleural fluid volume assessed in CXR (%)
| 9 (34.6) 10 (38.5) 7 (26.9) | 4 (44.5) 3 (33.3) 2 (22.2) | 6 (31.6) 9 (47.4) 4 (21) | 0.94 # |
| Volume of withdrawn pleural fluid, mL | 1910 (1500–2712) | 1250 (800–2340) | 1700 (1340–2050) | 0.25 * |
| Initial intrapleural pressure, cmH2O | 2.0 (−0.8–7.7) | 0.7 (−1.9–2.8) | 4.3 (1.8–6.7) | 0.31 * |
| Pleural Elastance, cmH2O/L | 8.1 (7.2–13.3) | 15.8 (5.5–19.1) | 8.4 (4.9–13.3) | 0.61 * |
| Parameter | Waves + (n = 26) ^ | Waves (n = 9) ^ | Waves +/− (n = 19) ^ | p * |
|---|---|---|---|---|
| Blood Gases and Tests | ||||
| SaO2% # | 95.8 (94–96.2) (n = 24) | 96.1 (94.2–97.2) (n = 9) | 93.8 (92.9–94.2) (n = 16) | 0.032 |
| PaO2 mmHg # | 75.5 (72.3–78.7) (n = 24) | 82.3 (71.9–83) (n = 9) | 67.8 (65.8–71.7) (n = 16) | 0.033 |
| NTproBNP pg/mL | 160 (72–338) (n = 25) | 468 (140–1872) (n = 9) | 180 (139–575) (n = 17) | 0.15 |
| Echocardiography | ||||
| LVEDD | 4.2 (4–4.3) (n = 15) | 3.1 (3.7–4.5) (n = 4) | 4.6 (3.9–4.7) (n = 11) | 0.67 |
| TAPSE cm | 1.9 (1.7–2) (n = 16) | 1.6 (1.4–1.7) (n = 4) | 1.6 (1.3–1.9) (n = 11) | 0.037 |
| RV E’ cm/s | 12 (8.5–15.5) (n = 15) | 7 (6–9) (n = 4) | 11 (7–12) (n = 9) | 0.079 |
| LV FS | 37.8 (26.8–43.7) (n = 16) | 28.6 (23.6–33.1) (n = 4) | 41 (33.3–44.1) (n = 11) | 0.13 |
| LV TDI S lat cm/s | 10 (8–12) (n = 13) | 8.5 (7–9.2) (n = 4) | 7 (6–8) (n = 11) | 0.035 |
| LV TDI S med cm/s | 8.5 (6.2–9) (n = 14) | 7 (5.2–8.5) (n = 4) | 6 (4.5–6.5) (n = 11) | 0.031 |
| LV TDI S mean cm/s | 9 (7.1–10.9) (n = 14) | 7.7 (6.1–8.9) (n = 4) | 6 (5.7–7) (n = 11) | 0.076 |
| LV TDI E’ lat cm/s | 9 (8–12) (n = 13) | 7 (6.5–7.7) (n = 4) | 8 (6–8.5) (n = 11) | 0.11 |
| LV TDI E’ med cm/s | 8 (6–8.7) (n = 14) | 6 (5.5–6.2) (n = 4) | 5 (5–5.5) (n = 11) | 0.008 |
| LV TDI E’mean cm/s | 8.2 (6.6–10.1) (n = 14) | 6.5 (6–7) (n = 4) | 6.5 (5.5–7) (n = 11) | 0.081 |
| E/E’ | 7.9 (5.9–12.7) (n = 13) | 10.6 (9.1–11) (n = 4) | 10.6 (9.1–14.2) (n = 11) | 0.21 |
| Pulmonary function tests | ||||
| TLC L | 3.7 (3.5–4.3) (n = 20) | 4.3 (3.6–5.2) (n = 7) | 3.1 (2.9–3.8) (n = 14) | 0.049 |
| TLC% pred | 75.6 (65.8–85) (n = 20) | 79 (66.9–90.3) (n = 7) | 65 (59.7–72.9) (n = 14) | 0.12 |
| DLCO ml/min/mmHg | 13.4 (11.8–15.2) (n = 18) | 12.4 (11.6–13.2) (n = 6) | 10.3 (9.2–11.3) (n = 12) | 0.022 |
| DLCO% pred | 58.1 (53.3–63.6) (n = 18) | 52.1 (44.1–58.5) (n = 6) | 48.2 (44.2–57.4) (n = 12) | 0.047 |
| FEV1 L | 1.3 (1–1.4) (n = 22) | 1.5 (1.1–1.6) (n = 7) | 1 (0.8–1.2) (n = 14) | 0.064 |
| FEV1% pred | 51.1 (41.7–62.7) (n = 22) | 53.2 (49.5–62.3) (n = 7) | 43 (33.9–47.2) (n = 14) | 0.02 |
| FVC L | 1.7 (1.5–2) (n = 22) | 1.8 (1.5–2.2) (n = 8) | 1.4 (1.2–1.9) (n = 14) | 0.24 |
| FVC% pred | 55.9 (45.2–70) (n = 22) | 62.5 (49.7–66.2) (n = 8) | 51.2 (42.2–54) (n = 14) | 0.26 |
| Parameter | First Alternative Subgroup Division | Second Alternative Subgroup Division | ||||
|---|---|---|---|---|---|---|
| Waves + (n = 26) ^ | Waves − and Waves +/− (n = 28) ^ | p * | Waves + and Waves +/− (n = 45) ^ | Waves − (n = 9) ^ | p * | |
| Effusion and Pleura | ||||||
| Volume ml | 1910 (1500–2712.5) | 1665 (1253.7–2117.5) | 0.13 | 1800 (1350–2300) | 1250 (800–2340) | 0.21 |
| Initial Ppl cmH2O | 2 (−0.8–7.7) | 3.8 (−0.3–6) | 0.88 | 3.6 (−0.1–7.6) | 0.7 (−1.9–2.8) | 0.19 |
| Pleural elastance cmH2O/L | 8.1 (7.2–13.3) | 8.7 (5.2–16.9) | 0.99 | 8.2 (6.2–13.4) | 15.8 (5.5–19.1) | 0.38 |
| Vital Parameters | ||||||
| SBP mmHg | 114 (108–118) | 113 (98.5–130) | 0.65 | 114 (105–123) | 107 (97–114) | 0.31 |
| DBP mmHg | 71 (65–75) | 65 (58.7–71.7) | 0.089 | 69 (62–74.2) | 66 (58–71) | 0.48 |
| HR beats/min | 92 (86.5–99.2) | 83 (74.8–96.9) | 0.14 | 90.3 (77.8–97.5) | 87.6 (81.1–97.7) | 0.79 |
| RR per min | 21.7 (18.8–26.9) | 26.7 (24.2–30.7) | 0.010 | 24.3 (19.8–28.3) | 30.5 (27.7–34.6) | 0.005 |
| HR/RR | 3.9 (3.4–4.9) | 3.2 (2.8–3.6) | 0.001 | 3.7 (3.2–4.7) | 3.1 (2.2–3.5) | 0.030 |
| Blood Gases and Tests | ||||||
| SaO2%# | 95.8 (94–96.2) (n = 24) | 94.1 (92.9–95.7) (n = 25) | 0.18 | 94.4 (93.2–95.9) (n = 40) | 96.1 (94.2–97.2) (n = 9) | 0.18 |
| PaO2 mmHg# | 75.5 (72.3–78.7) (n = 24) | 69.8 (66.3–75.7) (n = 25) | 0.19 | 72.7 (66.7–76.4) (n = 40) | 82.3 (71.9–83) (n = 9) | 0.17 |
| NTproBNP pg/mL | 160 (72–338) (n = 25) | 278 (140–606.7) (n = 26) | 0.081 | 180(90.2–485.2) (n = 42) | 468 (140–1872) (n = 9) | 0.12 |
| Echocardiography | ||||||
| LVEDD | 4.2 (4–4.3) (n = 15) | 4.3 (3.8–4.7) (n = 15) | 0.65 | 4.2 (4–4.6) (n = 26) | 4.1 (3.7–4.5) (n = 4) | 0.62 |
| TAPSE cm | 1.9 (1.7–2) (n = 16) | 1.6 (1.3–1.8) (n = 15) | 0.019 | 1.9 (1.6–2) (n = 27) | 1.6 (1.4–1.7) (n = 4) | 0.062 |
| RV E’cm/s | 12 (8.5–15.5) (n = 15) | 9 (7–12) (n = 13) | 0.052 | 11 (8–14) (n = 24) | 8.5 (7–9.2) (n = 4) | 0.070 |
| LV FS | 37.8 (26.8–43.7) (n = 16) | 35.6 (32.2–43) (n = 15) | 0.98 | 38.1 (32.1–44.1) (n = 27] | 28.6 (23.6–33.1) (n = 4) | 0.062 |
| LV TDI S lat cm/s | 10 (8–12) (n = 13) | 8 (6–8.5) (n = 15) | 0.011 | 8 (7–10) (n = 24) | 8 (8–9) (n = 4) | 0.73 |
| LV TDI S med cm/s | 8.5 (6.2–9) (n = 14) | 6 (4.5–7) (n = 15) | 0.012 | 6 (5.5–9) (n = 25) | 6 (6–8) (n = 4) | 0.78 |
| LV TDI S mean cm/s | 9 (7.1–10.9) (n = 14) | 7 (5.7–7.5) (n = 15) | 0.029 | 7 (6–9.5) (n = 25) | 7.7 (6.1–8.9) (n = 4) | 0.83 |
| LV TDI E’ lat cm/s | 9 (8–12) (n = 13) | 7 (6–8.5) (n = 15) | 0.041 | 8 (6.7–10.2) (n = 24) | 7 (6.5–7.7) (n = 4) | 0.32 |
| LV TDI E’ med cm/s | 8 (6–8.7) (n = 14) | 5 (5–6) (n = 15) | 0.002 | 6 (5–8) (n = 25) | 6 (5.5–6.2) (n = 4) | 0.65 |
| LV TDI E’ mean cm/s | 8.2 (6.6–10.1) (n = 14) | 6.5 (5.5–7) (n = 15) | 0.026 | 7 (6–9) (n = 25) | 6.5 (6–7) (n = 4) | 0.48 |
| E/E’ | 7.9 (5.9–12.7) (n = 13) | 10.6 (9.1–12.6) (n = 15) | 0.12 | 9.4 (7.8–13.6) (n = (n = 24) | 10.6 (9.1–11) (n = 4) | 0.87 |
| Pulmonary Tests | ||||||
| TLC L | 3.7 (3.5–4.3) (n = 20) | 3.5 (3–4.1) (n = 21) | 0.26 | 3.6 (3–4) (n = 34) | 4.3 (3.6–5.2) (n = 7) | 0.15 |
| TLC% pred | 75.6 (65.8–85) (n = 20) | 68.3 (61.1–78.2) (n = 21) | 0.32 | 69.8 (61.6–80.5) (n = 34) | 79 (66.9–90.3) (n = 7) | 0.27 |
| DLCO ml/min/mmHg | 13.4(11.8–15.2) (n = 18) | 10.9 (9.7–12.8) (n = 18) | 0.011 | 11.9 (10.6–14.4) (n = 30) | 12.4 (11.6–13.2) (n = 6) | 0.92 |
| DLCO%pred | 58.1 (53.3–63.6) (n = 18) | 49.7 (42.7–57.5) (n = 18) | 0.014 | 54.9 (48.6–62.6) (n = 30) | 52.1 (44.1–58.5) (n = 6) | 0.56 |
| FEV1 L | 1.3 (1–1.4) (n = 22) | 1.1 (0.8–1.4) (n = 21) | 0.23 | 1.1 (0.9–1.3) (n = 36) | 1.5 (1.1–1.6) (n = 7) | 0.22 |
| FEV1%pred | 51.1 (41.7–62.7) (n = 22) | 46.6(38.6–52.3) (n = 21) | 0.19 | 46.8 (39–57.9) (n = 36) | 53.2 (49.5–62.3) (n = 7) | 0.11 |
| FVC L | 1.7 (1.5–2) (n = 22) | 1.5 (1.2–2) (n = 22) | 0.37 | 1.6 (1.3–2) (n = 36) | 1.8 (1.5–2.2) (n = 8) | 0.41 |
| FVC%pred | 55.9 (45.2–70) (n = 22) | 52.9 (42.2–62.5) (n = 22) | 0.25 | 53.1 (45–66.2) (n = 36) | 62.5 (49.7–66.2) (n = 8) | 0.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabczak, E.M.; Michnikowski, M.; Styczynski, G.; Zielinska-Krawczyk, M.; Stecka, A.M.; Korczynski, P.; Zielinski, K.; Palko, K.J.; Rahman, N.M.; Golczewski, T.; et al. Pleural Pressure Pulse in Patients with Pleural Effusion: A New Phenomenon Registered during Thoracentesis with Pleural Manometry. J. Clin. Med. 2020, 9, 2396. https://doi.org/10.3390/jcm9082396
Grabczak EM, Michnikowski M, Styczynski G, Zielinska-Krawczyk M, Stecka AM, Korczynski P, Zielinski K, Palko KJ, Rahman NM, Golczewski T, et al. Pleural Pressure Pulse in Patients with Pleural Effusion: A New Phenomenon Registered during Thoracentesis with Pleural Manometry. Journal of Clinical Medicine. 2020; 9(8):2396. https://doi.org/10.3390/jcm9082396
Chicago/Turabian StyleGrabczak, Elzbieta M., Marcin Michnikowski, Grzegorz Styczynski, Monika Zielinska-Krawczyk, Anna M. Stecka, Piotr Korczynski, Krzysztof Zielinski, Krzysztof J. Palko, Najib M. Rahman, Tomasz Golczewski, and et al. 2020. "Pleural Pressure Pulse in Patients with Pleural Effusion: A New Phenomenon Registered during Thoracentesis with Pleural Manometry" Journal of Clinical Medicine 9, no. 8: 2396. https://doi.org/10.3390/jcm9082396
APA StyleGrabczak, E. M., Michnikowski, M., Styczynski, G., Zielinska-Krawczyk, M., Stecka, A. M., Korczynski, P., Zielinski, K., Palko, K. J., Rahman, N. M., Golczewski, T., & Krenke, R. (2020). Pleural Pressure Pulse in Patients with Pleural Effusion: A New Phenomenon Registered during Thoracentesis with Pleural Manometry. Journal of Clinical Medicine, 9(8), 2396. https://doi.org/10.3390/jcm9082396





