Racial Disparities-Associated COVID-19 Mortality among Minority Populations in the US
Abstract
:1. Introduction
2. Disproportionate Impact of Coronavirus Disease 2019 (COVID-19) on Minority Communities in the US
3. Clinical Risk Factors for COVID-19 Acquisition and Adverse Disease That Are Disproportionate among Minority Populations in the US
3.1. Diabetes and COVID-19 Patients
3.2. Hypertension and COVID-19 Patients
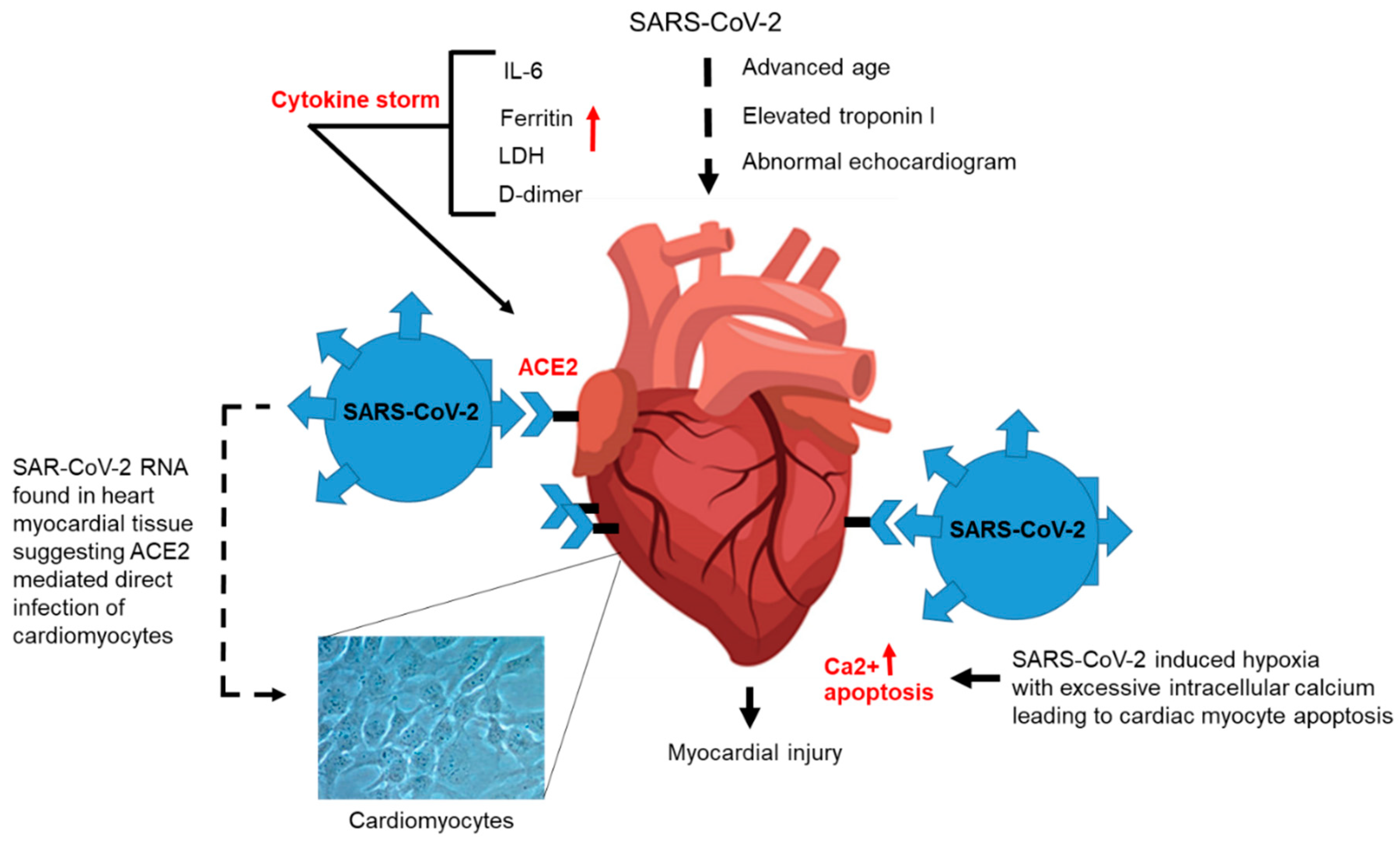
3.3. Cardiovascular Disease and COVID-19 Patients
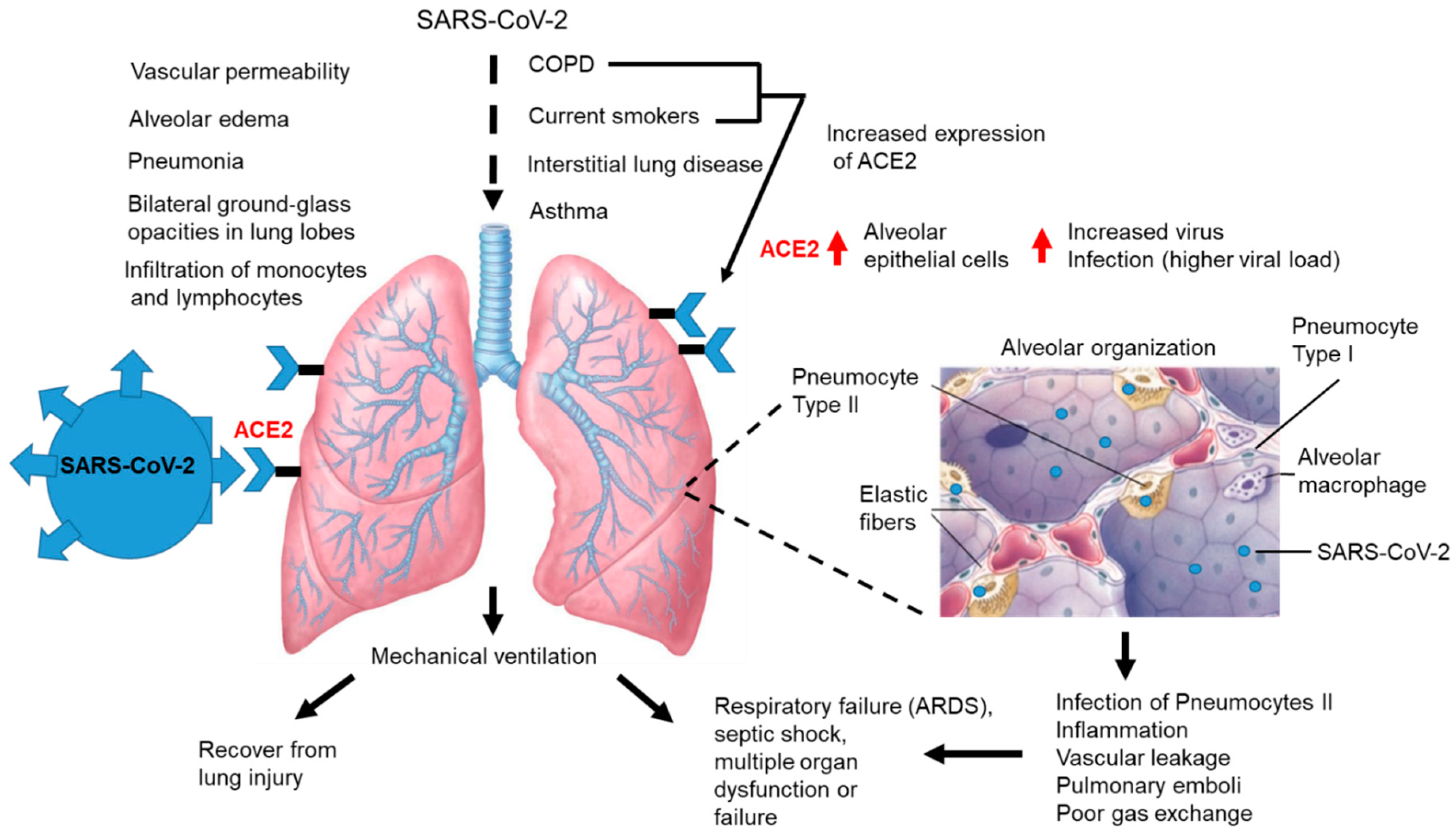
3.4. Pulmonary Disease and COVID-19
4. Interventions to Mitigate Clinical Factors Contributing to COVID-19 Morbidity and Mortality among Minority Populations in the US
4.1. Diabetes and COVID-19 Mitigation Strategies
4.2. Hypertension and COVID-19 Mitigation Strategies
4.3. Cardiovascular Disease and COVID-19 Mitigation Strategies
4.4. Chronic Obstructive Pulmonary Disease and COVID-19 Mitigation Strategies for Underserved Populations
5. Policy Changes to Combat Health Inequities during COVID-19 Crisis and Beyond
6. Practices That May Enhance the Effectiveness of Clinicians When They Engage Disparity Populations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bassetti, M.; Vena, A.; Giacobbe, D.R. The novel Chinese coronavirus (2019-nCoV) infections: Challenges for fighting the storm. Eur. J. Clin. Investig. 2020, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Faculty Opinions recommendation of Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. Faculty Opinions—Post-Publication Peer Review of the Biomedical Literature. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Woo, P.C.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef] [Green Version]
- Chairez, R.; Hollinger, B.; Melnick, J.L.; Dreesman, G.R.; Ghendon, Y.; Porubel, L.; Sasaki, Y.; Sasaki, R.; Cohen, G.H.; Pizer, L.I.; et al. International Committee on Taxonomy of Viruses. Virus Taxonomy: 2017 Release. Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 13 October 2017).
- Du Toit, A. Outbreak of a novel coronavirus. Nat. Rev. Genet. 2020, 18, 123. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef]
- Chan, J.; To, K.K.W.; Tse, H.; Jin, D.-Y.; Yuen, K.-Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef]
- Hui, D.S.; Azhar, E.E.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [Green Version]
- Fehr, A.R.; Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.R.; Navas-Martin, S. Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus. Microbiol. Mol. Boil. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letko, M.C.; Marzi, A.; Munster, V.J. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, P.C.Y.; Lau, S.K.P.; Chu, C.; Chan, K.-H.; Tsoi, H.-W.; Huang, Y.; Wong, B.H.L.; Poon, R.W.S.; Cai, J.J.; Luk, W.-K.; et al. Characterization and Complete Genome Sequence of a Novel Coronavirus, Coronavirus HKU1, from Patients with Pneumonia. J. Virol. 2005, 79, 884–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.; Lau, S.K.P.; To, K.K.-W.; Cheng, V.C.C.; Woo, P.C.; Yuen, K.-Y. Middle East Respiratory Syndrome Coronavirus: Another Zoonotic Betacoronavirus Causing SARS-Like Disease. Clin. Microbiol. Rev. 2015, 28, 465–522. [Google Scholar] [CrossRef] [Green Version]
- Cheng, V.C.C.; Lau, S.K.P.; Woo, P.C.Y.; Yuen, K.-Y. Severe Acute Respiratory Syndrome Coronavirus as an Agent of Emerging and Reemerging Infection. Clin. Microbiol. Rev. 2007, 20, 660–694. [Google Scholar] [CrossRef] [Green Version]
- Zhong, N.S.; Zheng, B.J.; Li, Y.M.; Poon, L.L.M.; Xie, Z.H.; Chan, K.H.; Li, P.H.; Tan, S.Y.; Chang, Q.; Xie Liu, Q.X.; et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February 2003. Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Kuiken, T.; Fouchier, R.A.M.; Schutten, M.; Rimmelzwaan, G.F.; Van Amerongen, G.; Van Riel, D.; Laman, J.D.; De Jong, T.; Van Doornum, G.; Lim, W.; et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 2003, 362, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.; Osterhaus, A.; Fouchier, R. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Van Boheemen, S.; De Graaf, M.; Lauber, C.; Bestebroer, T.M.; Raj, V.S.; Zaki, A.M.; Osterhaus, A.D.; Haagmans, B.L.; Gorbalenya, A.E.; Snijder, E.J.; et al. Genomic Characterization of a Newly Discovered Coronavirus Associated with Acute Respiratory Distress Syndrome in Humans. mBio 2012, 3, e00473–e00512. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, R.D.S.; Valença, I.N.; Ruy, P.D.C.; Ximenez, J.P.B.; da Silva Júnior, W.A.; Covas, D.T.; Kashima, S.; Slavov, S.N.; Covas, D.T. The novel coronavirus SARS-CoV-2: From a zoonotic infection to coronavirus disease 2019. J. Med. Virol. 2020. (Epub ahead of print). [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020, 7, 1346–1351.e2. [Google Scholar] [CrossRef] [PubMed]
- EJordan, R.; Adab, P.; Cheng, K.K. Covid-19: Risk factors for severe disease and death. BMJ 2020, 368, m1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Public Health England. Guidance on Social Distancing for Everyone in the UK. 2020. Available online: https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-forvulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protectingolder-people-and-vulnerable-adults (accessed on 11 May 2020).
- Adams, M.L.; Katz, D.L.; Grandpre, J. Population-based estimates of chronic conditions affecting risk for complications from coronavirus disease, United States. Emerg. Infect. Dis. 2020, 8, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [Green Version]
- Reyes, C.; Husain, N.; Gutowski, C.; St Clair, S.; Pratt, G. Chicago’s Coronavirus Disparity: Black Chicagoans are Dying at Nearly Six Times the Rate of White Residents, Data Show. Chicago Tribune. Available online: https://www.chicagotribune.com/coronavirus/ct-coronavirus-chicago-coronavirus-deaths-demographics-lightfoot-20200406-77nlylhiavgjzb2wa4ckivh7mu-story.html (accessed on 12 April 2020).
- Thebault, R.; Ba Tran, A.; Williams, V. The Coronavirus is Infecting and Killing Black Americans at an Alarmingly High Rate. Washington Post. Available online: https://www.washingtonpost.com/nation/2020/04/07/coronavirus-is-infecting-killingblack-americans-an-alarmingly-high-rate-postanalysis-shows/ (accessed on 7 April 2020).
- Deslatte, M. Louisiana Data: Virus Hits Blacks, People with Hypertension. US NewsWorld Report. Available online: https://www.usnews.com/news/best-states/louisiana/articles/2020-04-07/louisiana-data-virushits-blacks-people-with-hypertension (accessed on 12 April 2020).
- New York State Department of Health. COVID-19 Fatalities. Available online: https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Fatalities?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n (accessed on 1 May 2020).
- Pan, A.; Liu, L.; Wang, C.; Guo, H.; Hao, X.; Wang, Q.; Huang, J.; He, N.; Yu, H.; Lin, X.; et al. Association of Public Health Interventions With the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA 2020, 323, 1915. [Google Scholar] [CrossRef] [Green Version]
- McBean, A.M.; Li, S.; Gilbertson, D.T.; Collins, A.J. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: Whites, blacks, hispanics, and asians. Diabetes Care 2004, 27, 2317–2324. [Google Scholar] [CrossRef] [Green Version]
- Mokdad, A.H.; Bowman, B.A.; Ford, E.S.; Vinicor, F.; Marks, J.S.; Koplan, J.P. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001, 286, 1195–1200. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.M. Social Determinants of Health and Racial/Ethnic Disparities in Type 2 Diabetes in Youth. Curr. Diab. Rep. 2017, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Pinhas-Hamiel, O.; Zeitler, P.S. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet 2007, 369, 1823–1831. [Google Scholar] [CrossRef]
- Klingensmith, G.J.; Connor, C.G.; Ruedy, K.J.; Beck, R.W.; Kollman, C.; Haro, H.; Wood, J.R.; Lee, J.M.; Willi, S.M.; Cengiz, E.; et al. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr. Diabetes 2015, 17, 266–273. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Umpierrez, G.E. Letter to the Editor: COVID-19 in patients with diabetes: Risk factors that increase morbidity. Metabolism 2020, 108. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Mantzoros, C.; Sowers, J.R. Commentary: COVID-19 in patients with diabetes. Metabolism 2020, 107. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-K.; Feng, Y.; Yuan, M.Y.; Yuan, S.Y.; Fu, H.J.; Wu, B.Y.; Sun, G.Z.; Yang, G.R.; Zhang, X.; Wang, L.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabetes Med. 2006, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Fraussen, J.; Claes, N.; Van Wijmeersch, B.; Van Horssen, J.; Stinissen, P.; Hupperts, R.; Somers, V. B cells of multiple sclerosis patients induce autoreactive proinflammatory T cell responses. Clin. Immunol. 2016, 173, 124–132. [Google Scholar] [CrossRef]
- WHO (World Health Organization). A Global Brief on Hypertension. 2013. Available online: http://ish-world.com/downloads/pdf/global_brief_hypertension.pdf (accessed on 5 June 2016).
- Laurencin, C.T.; McClinton, A. The COVID-19 Pandemic: A Call to Action to Identify and Address Racial and Ethnic Disparities. J. Racial Ethn. Health Disparities 2020, 7, 398–402. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, 00127–00220. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Batlle, D.; Wysocki, J.; Satchell, K. Soluble angiotensin-converting enzyme 2: A potential approach for coronavirus infection therapy? Clin. Sci. 2020, 134, 543–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffrin, E.L.; Flack, J.M.; Ito, S.; Muntner, P.; Webb, R.C. Response to “COVID-19 and ACEI/ARB: Not Associated?”. Am. J. Hypertens. 2020. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Majumdar, S.; Singh, R.; Misra, A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 5, 971–978. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020, 10, 22–20137273. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.; Pfeffer, M.A.; Solomon, S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Tikellis, C.; Thomas, M. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int. J. Pept. 2012, 2012, 256294. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 4, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Ferdinand, K.C.; Yadav, K.; Nasser, S.A.; Od, H.D.C.; Lewin, J.; Cryer, D.R.; Senatore, F.F. Disparities in hypertension and cardiovascular disease in blacks: The critical role of medication adherence. J. Clin. Hypertens. 2017, 19, 1015–1024. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention/National Center for Health Statistics, National Health and Nutrition Examination Survey, 2011–2014. Available online: https://www.cdc.gov/nchs/data/databriefs/db220.pdf (accessed on 1 April 2017).
- Ritchey, M.D.; Chang, A.; Powers, C.; Loustalot, F.; Schieb, L.; Ketcham, M.; Durthaler, J.; Hong, Y. Vital Signs: Disparities in Antihypertensive Medication Nonadherence Among Medicare Part D Beneficiaries—United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 967–976. [Google Scholar] [CrossRef]
- Holmes, H.M.; Luo, R.; Hanlon, J.T.; Elting, L.S.; Suarez-Almazor, M.; Goodwin, J.S. Ethnic disparities in adherence to antihypertensive medications of medicare part D beneficiaries. J. Am. Geriatr. Soc. 2012, 60, 1298–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease 2020. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 5, 259–260. [Google Scholar] [CrossRef] [Green Version]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef]
- Booth, C.M.; Matukas, L.M.; Tomlinson, G.; Rachlis, A.R.; Rose, D.B.; Dwosh, H.A.; Walmsley, S.L.; Mazzulli, T.; Avendano, M.; Derkach, P.; et al. Clinical Features and Short-term Outcomes of 144 Patients With SARS in the Greater Toronto Area. JAMA 2003, 289, 2801. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.P.; Wang, X.; Moura, F.A.; Siddiqi, H.K.; Morrow, D.A.; Bohula, E.A. A current review of COVID-19 for the cardiovascular specialist. Am. Heart J. 2020, 226, 29–44. [Google Scholar] [CrossRef]
- Ren, Z.-L.; Hu, R.; Wang, Z.-W.; Zhang, M.; Ruan, Y.-L.; Wu, Z.-Y.; Wu, H.-B.; Hu, X.-P.; Hu, Z.-P.; Ren, W.; et al. Epidemiologic and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in Wuhan, China: A descriptive survey report. J. Hear. Lung Transplant. 2020, 39, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Guidance for Cardiothoracic Transplant and Mechanical Circulatory Support Centers regarding SARS CoV-2 infection and COVID-19: March 17, 2020. Available online: https://community.ishlt.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=afb06f06-5d63-13d4-c107-d152a9f6cd46 (accessed on 21 March 2020).
- American Society of Transplantation. 2019-nCoV (Coronavirus): FAQs for Organ Transplantation. Updated Feb 29, 2020. Available online: https://www.myast.org/sites/default/files/COVID19%20FAQ%20Tx%20Centers%20030220-1.pdf (accessed on 29 June 2020).
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Schraufnagel, D.E.; Blasi, F.; Kraft, M.; Gaga, M.; Finn, P.W.; Rabe, K.F. An Official American Thoracic Society/European Respiratory Society Policy Statement: Disparities in Respiratory Health. Am. J. Respir. Crit. Care Med. 2013, 188, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Pleasants, R.; Riley, I.L.; Mannino, D.M. Defining and targeting health disparities in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2475–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization Chronic Obstructive Pulmonary Disease (COPD); Fact Sheet. March 2015. Available online: https://www.who.int/mediacentre/factsheets/fs315/en/ (accessed on 23 January 2016).
- World Health Organization Commission on Social Determinants of Health—Final Report. Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of Health. Available online: http://www.who.int/social_determinants/thecommission/finalreport/en/ (accessed on 23 January 2016).
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Lian, N.; Deng, Y.; Lin, S. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J. Med. Virol. 2020. (Epub ahead of print). [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Alghamdi, S.M.; Almehmadi, M.; Alqahtani, A.S.; Quaderi, S.; Mandal, S.; Hurst, J.R. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0233147. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.-L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilin, Z.; Yandong, N.; Faguang, J. Role of angiotensin-converting enzyme (ACE) and ACE2 in a rat model of smoke inhalation induced acute respiratory distress syndrome. Burn 2015, 41, 1468–1477. [Google Scholar] [CrossRef]
- Daccord, C.; Touilloux, B.; Von Garnier, C. [Asthma and COPD management during the COVID-19 pandemic]. Rev. Med. Suisse 2020, 16, 933–938. [Google Scholar]
- Lanting, L.C.; Joung, I.M.; MacKenbach, J.P.; Lamberts, S.W.; Bootsma, A.H. Ethnic Differences in Mortality, End-Stage Complications, and Quality of Care Among Diabetic Patients: A review. Diabetes Care 2005, 28, 2280–2288. [Google Scholar] [CrossRef] [Green Version]
- Copeland, K.C.; Zeitler, P.; Geffner, M.; Guandalini, C.; Higgins, J.; Hirst, K.; Kaufman, F.R.; Linder, B.; Marcovina, S.; McGuigan, P.; et al. TODAY Study Group. Characteristics of adolescents and youth with recent-onset type 2 diabetes: The TODAY cohort at baseline. J. Clin. Endocrinol. Metab. 2011, 1, 159–167. [Google Scholar] [CrossRef]
- G. American Diabetes Association. Strategies for Improving Care. Sec. 1 In Standards of Medical Care in Diabetes-2015. Diabetes Care 2015, 38, S1–S94. [Google Scholar]
- Kurian, A.K.; Cardarelli, K.M. Racial and ethnic differences in cardiovascular disease risk factors: A systematic review. Ethn. Dis. 2007, 17, 143–152. [Google Scholar] [PubMed]
- Barker, L.E.; Kirtland, K.A.; Gregg, E.W.; Geiss, L.S.; Thompson, T.J. Geographic Distribution of Diagnosed Diabetes in the U.S. Am. J. Prev. Med. 2011, 40, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, D.J.; Thorpe, R.J.; McGinty, E.E.; Bower, K.; Rohde, C.; Young, J.H.; LaVeist, T.A.; Dubay, L. Disparities in Diabetes: The Nexus of Race, Poverty, and Place. Am. J. Public Health 2014, 104, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.; Artiga, S.; Paradise, J. Health Coverage and Care in the South in 2014 and Beyond; Henry J. Kaiser Family Foundation: Menlo Park, CA, USA, 2014. [Google Scholar]
- Ferdinand, K.C.; Nasser, S.A. Management of Essential Hypertension. Cardiol. Clin. 2017, 35, 231–246. [Google Scholar] [CrossRef]
- CDC. Coronavirus Disease 2019 (COVID-19): People Who Are at Higher Risk for Severe Illness; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html (accessed on 29 June 2020).
- Vasan, R.S.; Beiser, A.; Seshadri, S.; Larson, M.G.; Kannel, W.B.; D’Agostino, R.B.; Levy, D. Residual Lifetime Risk for Developing Hypertension in Middle-aged Women and Men. JAMA 2002, 287, 1003–1010. [Google Scholar] [CrossRef]
- Jean, S.S.; Lee, P.; Hsueh, P.R. Treatment Options for COVID-19: The Reality and Challenges Review. J. Microbiol. Immunol. Infect. 2020, 3, 436–443. [Google Scholar] [CrossRef]
- Bimonte, S.; Crispo, A.; Amore, A.; Celentano, E.; Cuomo, A.; Cascella, M. Potential Antiviral Drugs for SARS-Cov-2 Treatment: Preclinical Findings and Ongoing Clinical Research (Suppl.3). In Vivo 2020, 34, 1597–1602. [Google Scholar] [CrossRef]
- Maddocks, M.; Lovell, N.; Booth, S.; Man, W.D.-C.; Higginson, I. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet 2017, 390, 988–1002. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcendor, D.J. Racial Disparities-Associated COVID-19 Mortality among Minority Populations in the US. J. Clin. Med. 2020, 9, 2442. https://doi.org/10.3390/jcm9082442
Alcendor DJ. Racial Disparities-Associated COVID-19 Mortality among Minority Populations in the US. Journal of Clinical Medicine. 2020; 9(8):2442. https://doi.org/10.3390/jcm9082442
Chicago/Turabian StyleAlcendor, Donald J. 2020. "Racial Disparities-Associated COVID-19 Mortality among Minority Populations in the US" Journal of Clinical Medicine 9, no. 8: 2442. https://doi.org/10.3390/jcm9082442
APA StyleAlcendor, D. J. (2020). Racial Disparities-Associated COVID-19 Mortality among Minority Populations in the US. Journal of Clinical Medicine, 9(8), 2442. https://doi.org/10.3390/jcm9082442



