Sub-Perception and Supra-Perception Spinal Cord Stimulation in Chronic Pain Syndrome: A Randomized, Semi-Double-Blind, Crossover, Placebo-Controlled Trial
Abstract
:1. Introduction
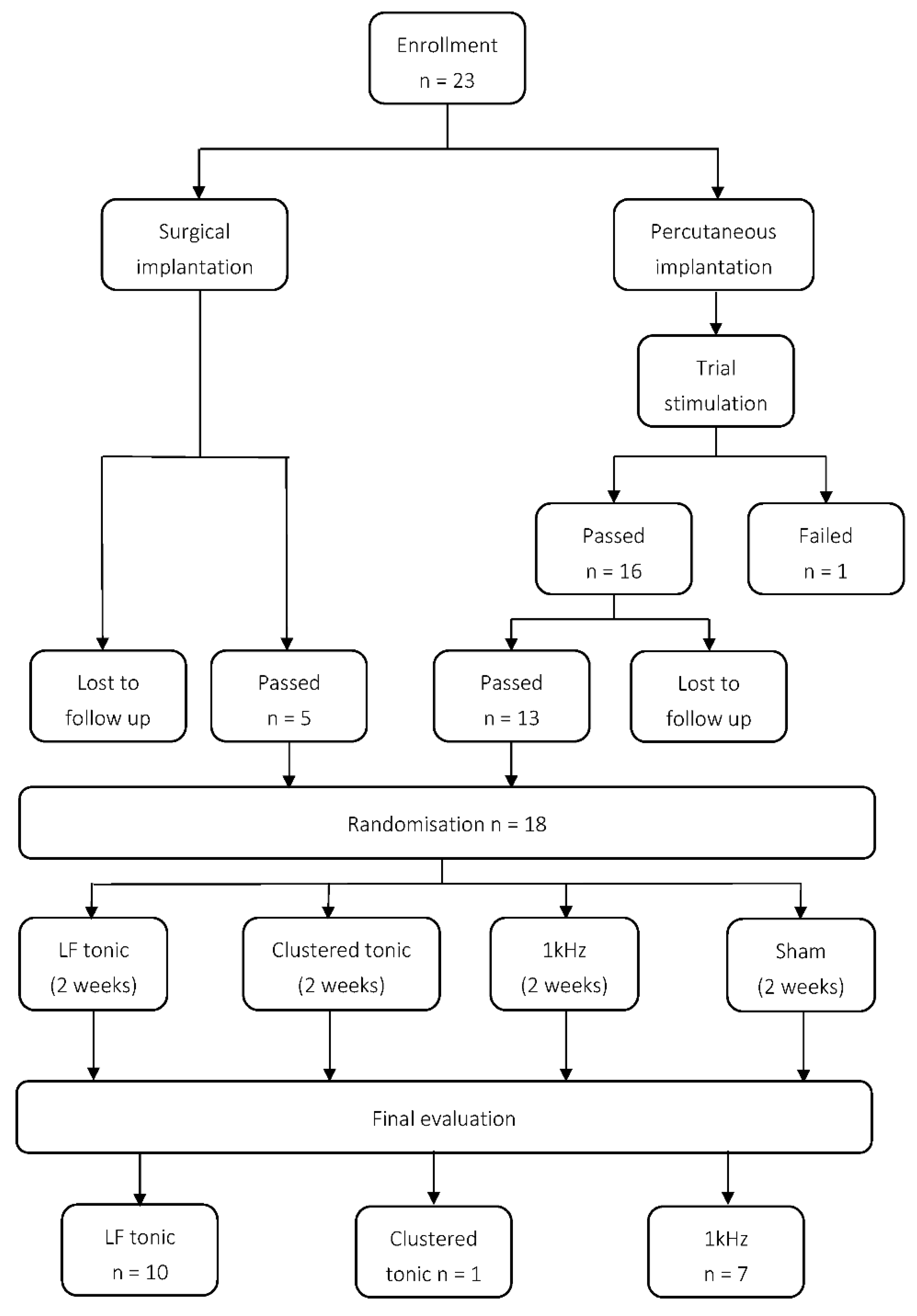
2. Experimental Section
2.1. Materials and Methods
2.2. Implantations
2.3. Programming and Randomisation
2.4. Data Analyses
3. Results
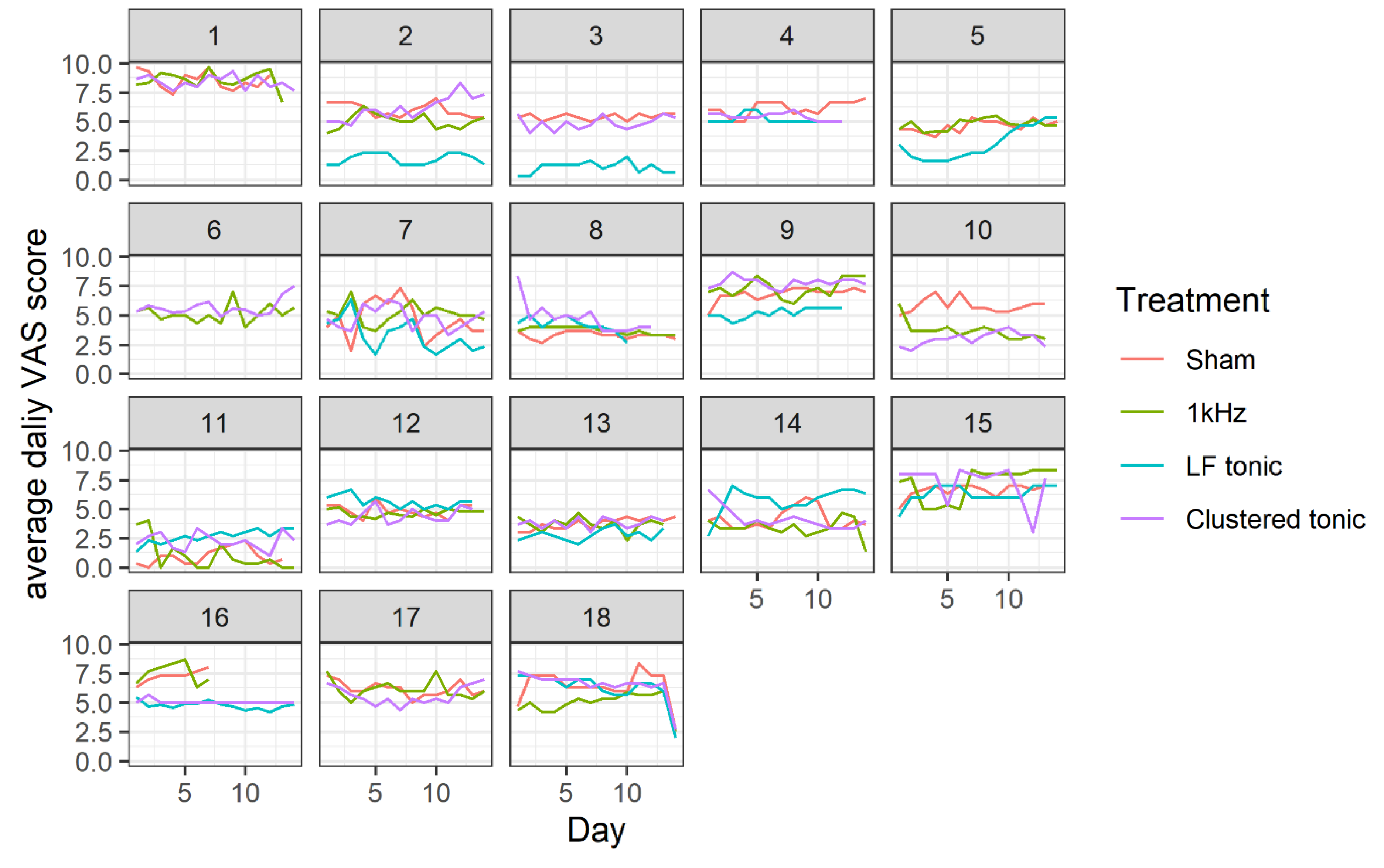
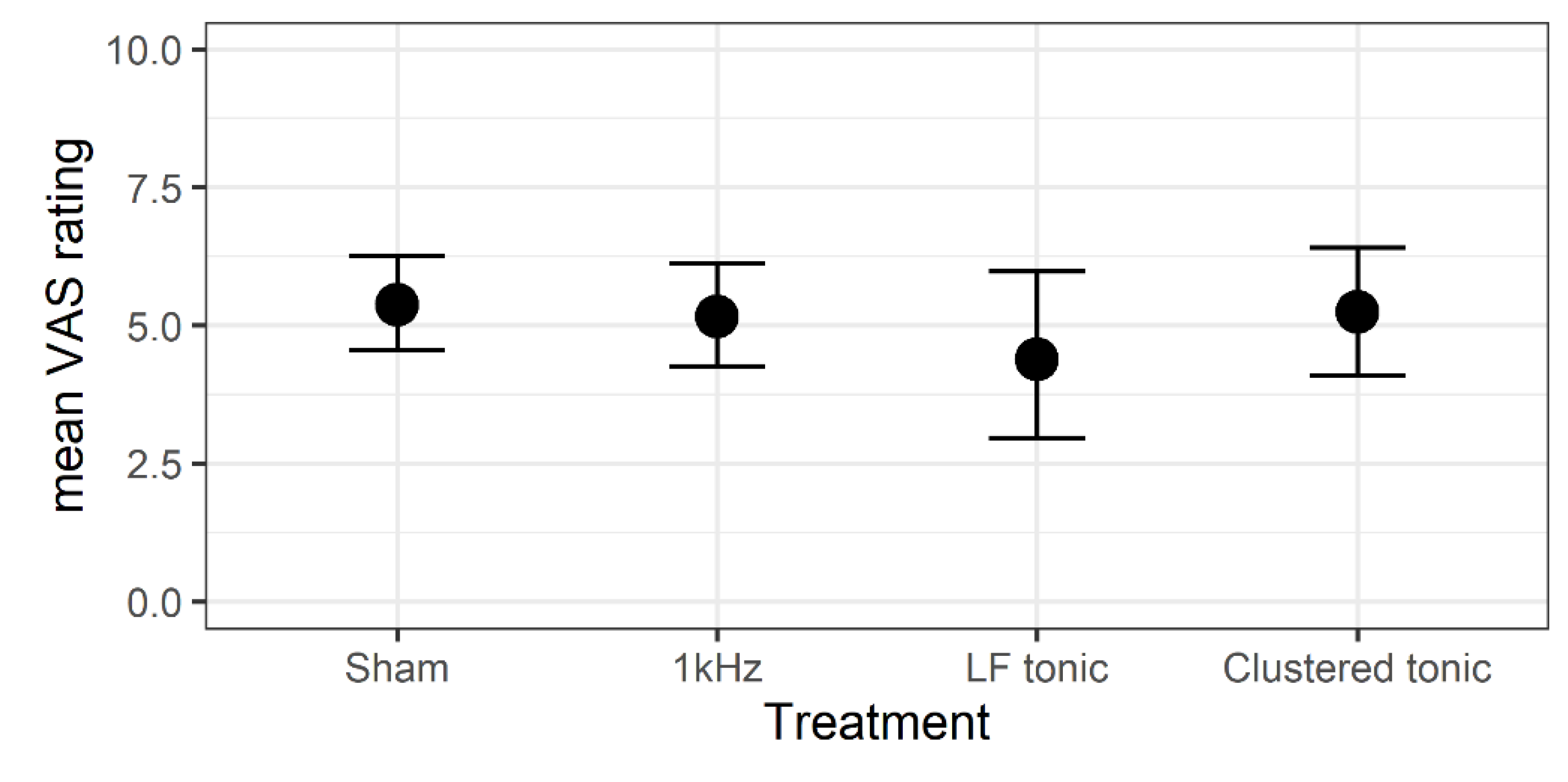
3.1. Effects of Treatment on Daily Pain Levels
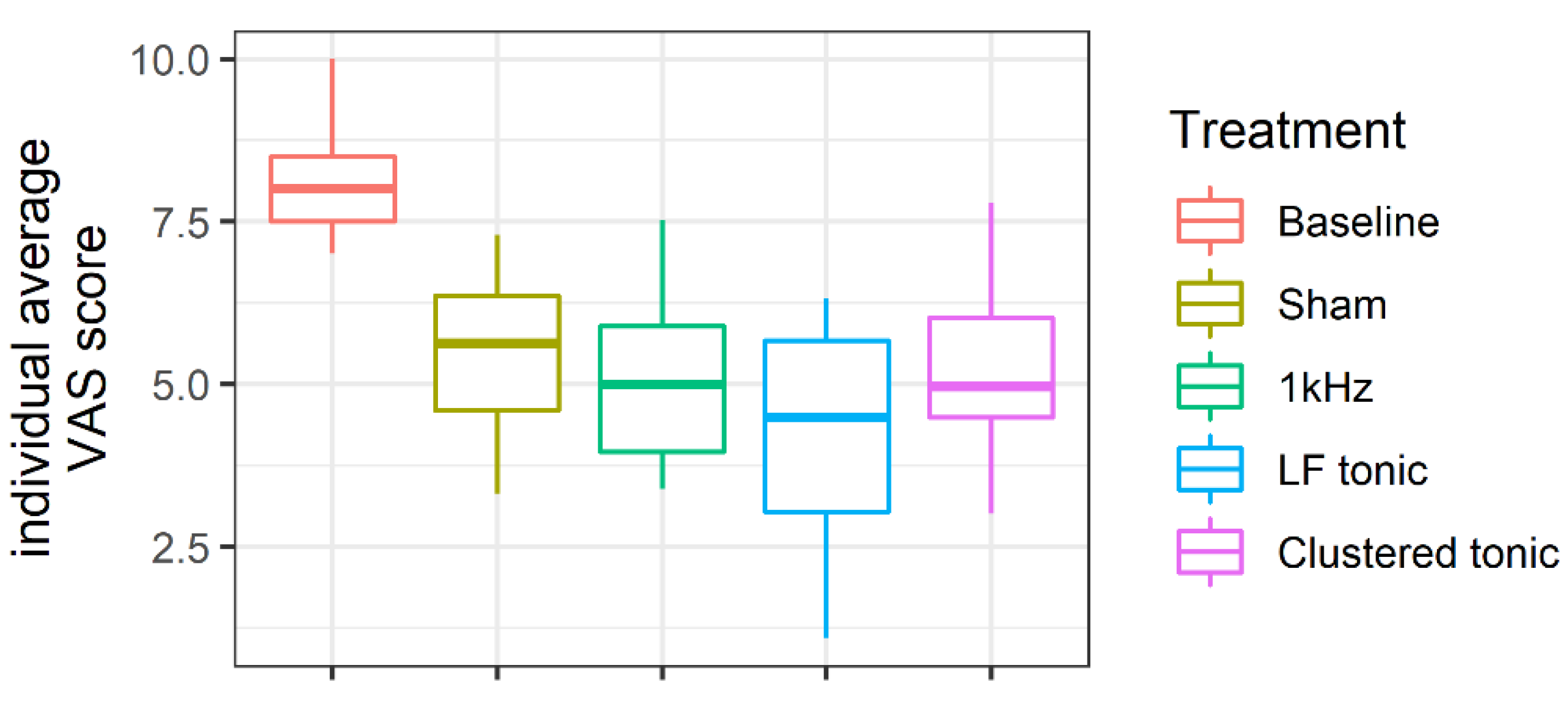
3.2. Change in Pain Levels from Baseline
3.3. Effects of Treatments on the Number and Type of Medications
3.4. Oswestry Scores
3.5. Complications
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Details of the Sampling Procedure
Appendix B. Parameter Estimates of the Regression Models
| Parameter | Estimate | SE | 95% CI | ||
|---|---|---|---|---|---|
| LI | UI | ||||
| Population-Level Effects | |||||
| β | Intercept (Sham) | 5.38 | 0.42 | 4.57 | 6.21 |
| 1 kHz | −0.17 | 0.22 | −0.59 | 0.26 | |
| LF tonic | −0.99 | 0.56 | −2.14 | 0.15 | |
| Clustered tonic | −0.03 | 0.37 | −0.75 | 0.71 | |
| Individual-level effects | |||||
| τ | Intercept (Sham) | 1.73 | 0.32 | 1.24 | 2.47 |
| 1 kHz | 0.68 | 0.21 | 0.34 | 1.17 | |
| LF tonic | 2.04 | 0.45 | 1.35 | 3.04 | |
| Clustered tonic | 1.41 | 0.31 | 0.94 | 2.15 | |
| Day | 0.04 | 0.01 | 0.01 | 0.07 | |
| 1 kHz:Day | 0.07 | 0.02 | 0.02 | 0.11 | |
| LF tonic:Day | 0.12 | 0.03 | 0.07 | 0.2 | |
| Clustered tonic:Day | 0.09 | 0.02 | 0.05 | 0.15 | |
| Parameter | Estimate | SE | 95% CI | ||
|---|---|---|---|---|---|
| LI | UI | ||||
| β | Intercept | 3.32 | 0.29 | 2.75 | 3.89 |
| NSAID | −0.73 | 0.26 | −1.28 | −0.24 | |
| Opioids | −0.94 | 0.28 | −1.49 | −0.41 | |
| z | Intercept (Anticon.) | 1.42 | 0.3 | 0.89 | 2.01 |
| NSAID | −2.35 | 0.41 | −3.18 | −1.57 | |
| Opioids | −1.44 | 0.39 | −2.2 | −0.71 | |
| φ | 2.49 | 0.51 | 1.64 | 3.61 | |
| τ | Intercept | 0.71 | 0.19 | 0.42 | 1.13 |
References
- Taylor, R.S. Spinal Cord Stimulation in Complex Regional Pain Syndrome and Refractory Neuropathic Back and Leg Pain/Failed Back Surgery Syndrome: Results of a Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2006, 31, 13–19. [Google Scholar] [CrossRef]
- Hoydonckx, Y.; Costanzi, M.; Bhatia, A. A scoping review of novel spinal cord stimulation modes for complex regional pain syndrome. Can. J. Pain 2019, 3, 33–48. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.S.; Desai, M.J.; Rigoard, P.; Taylor, R.J. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: A systematic review and meta-regression analysis. Pain Pract. 2014, 14, 489–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The neuromodulation appropriateness consensus committee. Neuromodulation 2014, 17, 515–550. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Yong, R.J.; Kaye, A.D.; Urman, R.D. Spinal Cord Stimulation: Comparing Traditional Low-frequency Tonic Waveforms to Novel High Frequency and Burst Stimulation for the Treatment of Chronic Low Back Pain. Curr. Pain Headache Rep. 2019, 23, 23–25. [Google Scholar] [CrossRef]
- Duarte, R.V.; Andronis, L.; Lenders, M.W.P.M.; de Vos, C.C. Quality of life increases in patients with painful diabetic neuropathy following treatment with spinal cord stimulation. Qual. Life Res. 2016, 25, 1771–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robb, L.P.; Cooney, J.M.; McCrory, C.R. Evaluation of spinal cord stimulation on the symptoms of anxiety and depression and pain intensity in patients with failed back surgery syndrome. Ir. J. Med. Sci. 2017, 186, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Szmuda, T.; Słoniewski, P.; Ali, S.; Aleksandrowicz, K. Does Spinal Cord Stimulation Due to Failed Back Surgery Syndrome Lead to Permanent Occupational Disability? Neuromodulation 2019, 23, 653–659. [Google Scholar] [CrossRef]
- Duarte, R.V.; Nevitt, S.; McNicol, E.; Taylor, R.S.; Buchser, E.; North, R.B.; Eldabe, S. Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain 2020, 161, 24–35. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Plazier, M.; Van Der Loo, E.; Menovsky, T. Burst spinal cord stimulation: Toward paresthesia-free pain suppression. Neurosurgery 2010, 66, 986–990. [Google Scholar] [CrossRef]
- Deer, T.; Slavin, K.V.; Amirdelfan, K.; North, R.B.; Burton, A.W.; Yearwood, T.L.; Tavel, E.; Staats, P.; Falowski, S.; Pope, J.; et al. Success Using Neuromodulation with BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation 2018, 21, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Morgan, D.M.; Brown, L.L.; Yearwood, T.L.; Bundschu, R.; Burton, A.W.; Yang, T.; et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.J.; Tavakkolizadeh, M.; Love-Jones, S.; Patel, N.K.; Gu, J.W.; Bains, A.; Doan, Q.; Moffitt, M. Effects of Rate on Analgesia in Kilohertz Frequency Spinal Cord Stimulation: Results of the PROCO Randomized Controlled Trial. Neuromodulation 2018, 21, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schu, S.; Slotty, P.J.; Bara, G.; Von Knop, M.; Edgar, D.; Vesper, J. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014, 17, 443–450. [Google Scholar] [CrossRef]
- Kriek, N.; Groeneweg, J.G.; Stronks, D.L.; De Ridder, D.; Huygen, F.J.P.M. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: A multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur. J. Pain 2017, 21, 507–519. [Google Scholar] [CrossRef]
- Wolter, T.; Kiemen, A.; Porzelius, C.; Kaube, H. Effects of sub-perception threshold spinal cord stimulation in neuropathic pain: A randomized controlled double-blind crossover study. Eur. J. Pain 2012, 16, 648–655. [Google Scholar] [CrossRef]
- North, J.; Loudermilk, E.; Lee, A.; Sachdeva, H.; Kaiafas, D.; Washabaugh, E.; Sheth, S.; Scowcroft, J.; Mekhail, N.; Lampert, B.; et al. Outcomes of a Multicenter, Prospective, Crossover, Randomized Controlled Trial Evaluating Subperception Spinal Cord Stimulation at ≤1.2 kHz in Previously Implanted Subjects. Neuromodulation 2020, 23, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Duarte, R.V.; McNicol, E.; Colloca, L.; Taylor, R.S.; North, R.B.; Eldabe, S. Randomized Placebo-/Sham-Controlled Trials of Spinal Cord Stimulation: A Systematic Review and Methodological Appraisal. Neuromodulation 2020, 23, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 statement: Extension to randomised crossover trials. BMJ 2019, 366. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 24 April 2020).
- Bürkner, P. Advanced Bayesian Multilevel Modeling with the R Package brms. R J. 2018, 10, 395–411. [Google Scholar] [CrossRef]
- Bürkner, P. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Vehtari, A.; Gelman, A.; Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 2016, 27, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Vehtari, A.; Gelman, A. Practical Bayesian model evaluation using leave-one-out cross-validation and Estimating out-of-sample pointwise predictive accuracy using posterior simulations. arXiv 2016, arXiv:1507.04544. [Google Scholar]
- Kruschke, J. Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Hoffman, M.D.; Gelman, A. The No-U-Turn Sampler: Adaptively Setting Path Lengths in Hamiltonian Monte Carlo. J. Mach. Learn. Res. 2014, 15, 1593–1623. [Google Scholar]
- Carpenter, B.; Gelman, A.; Hoffman, M.D.; Lee, D.; Goodrich, B.; Betancourt, M.; Brubaker, M.A.; Li, P.; Riddell, A. Stan: A Probabilistic Programming Language. J. Stat. Softw. 2017, 76. [Google Scholar] [CrossRef] [Green Version]
- Veizi, E.; Hayek, S.M.; North, J.; Brent Chafin, T.; Yearwood, T.L.; Raso, L.; Frey, R.; Cairns, K.; Berg, A.; Brendel, J.; et al. Spinal Cord Stimulation (SCS) with Anatomically Guided (3D) Neural Targeting Shows Superior Chronic Axial Low Back Pain Relief Compared to Traditional SCS-LUMINA Study. Pain Med. 2017, 18, 1534–1548. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, K.; Fishman, M.A.; Zuidema, X.; Hunter, C.W.; Levy, R. Mechanism of Action in Burst Spinal Cord Stimulation: Review and Recent Advances. Pain Med. 2019, 20, S13–S22. [Google Scholar] [CrossRef] [Green Version]
- De Ridder, D.; Plazier, M.; Kamerling, N.; Menovsky, T.; Vanneste, S. Burst Spinal Cord Stimulation for Limb and Back Pain. World Neurosurg. 2013, 80, 642–649.e1. [Google Scholar] [CrossRef]
- Kirketeig, T.; Schultheis, C.; Zuidema, X.; Hunter, C.W.; Deer, T. Burst Spinal Cord Stimulation: A Clinical Review. Pain Med. 2019, 20, S31–S40. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, K.; Malayil, R.; Kirketeig, T.; Deer, T. Burst Spinal Cord Stimulation: A Systematic Review and Pooled Analysis of Real-World Evidence and Outcomes Data. Pain Med. 2019, 20, S47–S57. [Google Scholar] [CrossRef] [Green Version]
- Van Buyten, J.P.; Wille, F.; Smet, I.; Wensing, C.; Breel, J.; Karst, E.; Devos, M.; Pöggel-Krämer, K.; Vesper, J. Therapy-Related Explants After Spinal Cord Stimulation: Results of an International Retrospective Chart Review Study. Neuromodulation 2017, 20, 642–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicket, M.C.; Dunn, R.Y.; Ahmed, S.U. High-frequency spinal cord stimulation for chronic pain: Pre-clinical overview and systematic review of controlled trials. Pain Med. 2016, 17, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- Perruchoud, C.; Eldabe, S.; Batterham, A.M.; Madzinga, G.; Brookes, M.; Durrer, A.; Rosato, M.; Bovet, N.; West, S.; Bovy, M.; et al. Analgesic efficacy of high-frequency spinal cord stimulation: A randomized double-blind placebo-controlled study. Neuromodulation 2013, 16, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisy, A.; Palmisani, S.; Pang, D.; Sanderson, K.; Wesley, S.; Tan, Y.; McCammon, S.; Trescott, A. Prospective, Randomized, Sham-Control, Double Blind, Crossover Trial of Subthreshold Spinal Cord Stimulation at Various Kilohertz Frequencies in Subjects Suffering From Failed Back Surgery Syndrome (SCS Frequency Study). Neuromodulation 2018, 21, 457–465. [Google Scholar] [CrossRef]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Yearwood, T.L.; Bundschu, R.; et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results from a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery 2016, 79, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, S.; Roeske, S.; Chaudhry, S.R.; Kinfe, T.M. Burst or High-Frequency (10 kHz) Spinal Cord Stimulation in Failed Back Surgery Syndrome Patients with Predominant Back Pain: One Year Comparative Data. Neuromodulation 2017, 20, 661–667. [Google Scholar] [CrossRef]
- Simopoulos, T.; Aner, M.; Sharma, S.; Ghosh, P.; Gill, J.S. Explantation of Percutaneous Spinal Cord Stimulator Devices: A Retrospective Descriptive Analysis of a Single-Center 15-Year Experience. Pain Med. 2019, 20, 1355–1361. [Google Scholar] [CrossRef]
- Chaudhry, Z.A.; Najib, U.; Bajwa, Z.H.; Jacobs, W.C.; Sheikh, J.; Simopoulos, T.T. Detailed analysis of allergic cutaneous reactions to spinal cord stimulator devices. J. Pain Res. 2013, 6, 617–623. [Google Scholar] [CrossRef] [Green Version]

| nr | Age | Sex | Diagnosis | Duration of Pain Years | Trial | Type of Electrode | Complication | VAS Baseline | Preferred Mode |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | m | FBSS | 5 | yes | P2x1x8 PN | no follow-up/after 10 months removal | 9 | - |
| 2 | 48 | f | FBSS | 15 | yes | P1x8 PN | no | 8 | LF |
| 3 | 43 | f | FBSS | 10 | yes | P1x16/P1x8 PN | replacement after 15 months/dysfunction | 8 | 1kHz |
| 4 | 50 | m | CRPS | 30 | no | S2x8 PN | no | 9 | 1kHz |
| 5 | 52 | m | FBSS | 3 | yes | P1x8 PN | no | 9 | LF |
| 58 | m | FBSS | 4 | no | S2X8 PN | no | 9 | LF | |
| 7 | 35 | m | FBSS | 2 | yes | P1x8 PN/M | replacement after 10 months/battery depletion | 7 | LF |
| 8 | 59 | f | FBSS | 8 | yes | P1x8 PN | no | 8 | LF |
| 9 | 46 | f | FBSS | 4 | yes | P1x8 PN | no | 7 | LF |
| 10 | 62 | m | CRPS | 1 | yes | P1x8 PN | no | 10 | LF |
| 11 | 53 | f | FBSS | 11 | no | S2x8 M | no | 8 | 1kHz |
| 12 | 60 | m | FBSS | 3 | yes | P1x8 PN | no follow-up | ||
| 13 | 53 | f | FBSS | 9 | yes | P2x1x8 PN | no | 8 | LF |
| 14 | 65 | m | FBSS | 5 | yes | P2x1x8 PN | removal after 12 months/no pain relief | 7 | 1kHz |
| 15 | 62 | f | CRPS | 10 | no | S2x8 PN | no | 9 | 1kHz |
| 16 | 62 | f | FBSS | 9 | no | S2x8 PN | no | 8 | Clustered |
| 17 | 74 | f | FBSS | 15 | yes | P2X1X8 PN | removal after 17 months/no pain relief | 7 | 1kHz |
| 18 | 44 | m | FBSS | 3 | yes | P1x8 PN | no | 10 | LF |
| 19 | 74 | f | FBSS | 4 | yes | P2x1x8 PN | removal after 13 months/allergic reaction | 10 | 1kHz |
| 20 | 57 | m | FBSS | 6 | yes | P2x1x8 PN | no | 8 | LF |
| 21 | 62 | m | CRPS | 2 | yes | P1x8 | failed trial | ||
| 22 | 65 | m | CRPS | 10 | no | S2x8 PN | no follow-up | ||
| 23 | 64 | f | FBSS | 8 | yes | P2x1x8 PN | no follow-up |
| VAS | ΔVAS | 95% CI | t | df | p | Cohen’s d | % Pain Reduction | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | LI | UI | ||||||
| Sham | 5.42 | 1.22 | 2.73 | 1.70 | 1.74 | 3.71 | 5.99 | 13 | <0.001 | 1.66 | 0.34 |
| 1kHZ | 5.17 | 1.40 | 3.04 | 1.47 | 2.20 | 3.89 | 7.76 | 13 | <0.001 | 2.15 | 0.37 |
| LF tonic | 4.18 | 1.76 | 4.07 | 2.11 | 2.73 | 5.41 | 6.68 | 11 | <0.001 | 2.02 | 0.50 |
| Clustered tonic | 5.27 | 1.33 | 2.80 | 1.63 | 1.86 | 3.74 | 6.42 | 13 | <0.001 | 1.78 | 0.34 |
| Baseline | 8.13 | 0.99 | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokal, P.; Malukiewicz, A.; Kierońska, S.; Murawska, J.; Guzowski, C.; Rudaś, M.; Paczkowski, D.; Rusinek, M.; Krakowiak, M. Sub-Perception and Supra-Perception Spinal Cord Stimulation in Chronic Pain Syndrome: A Randomized, Semi-Double-Blind, Crossover, Placebo-Controlled Trial. J. Clin. Med. 2020, 9, 2810. https://doi.org/10.3390/jcm9092810
Sokal P, Malukiewicz A, Kierońska S, Murawska J, Guzowski C, Rudaś M, Paczkowski D, Rusinek M, Krakowiak M. Sub-Perception and Supra-Perception Spinal Cord Stimulation in Chronic Pain Syndrome: A Randomized, Semi-Double-Blind, Crossover, Placebo-Controlled Trial. Journal of Clinical Medicine. 2020; 9(9):2810. https://doi.org/10.3390/jcm9092810
Chicago/Turabian StyleSokal, Paweł, Agnieszka Malukiewicz, Sara Kierońska, Joanna Murawska, Cezary Guzowski, Marcin Rudaś, Dariusz Paczkowski, Marcin Rusinek, and Mateusz Krakowiak. 2020. "Sub-Perception and Supra-Perception Spinal Cord Stimulation in Chronic Pain Syndrome: A Randomized, Semi-Double-Blind, Crossover, Placebo-Controlled Trial" Journal of Clinical Medicine 9, no. 9: 2810. https://doi.org/10.3390/jcm9092810
APA StyleSokal, P., Malukiewicz, A., Kierońska, S., Murawska, J., Guzowski, C., Rudaś, M., Paczkowski, D., Rusinek, M., & Krakowiak, M. (2020). Sub-Perception and Supra-Perception Spinal Cord Stimulation in Chronic Pain Syndrome: A Randomized, Semi-Double-Blind, Crossover, Placebo-Controlled Trial. Journal of Clinical Medicine, 9(9), 2810. https://doi.org/10.3390/jcm9092810







