The Evaluation of Wheat Cultivar Resistance and Yield Loss Thresholds in Response to Barley Yellow Dwarf Virus-PAV Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Assessment of Wheat Cultivar Resistance against BYDV-PAV
2.2. Evaluation of the Effects of BYDV-PAV Infection on Yield and Qualitative Parameters
2.3. Statistical Analysis
3. Results
3.1. Assessment of Wheat Cultivar Resistance Against BYDV-PAV
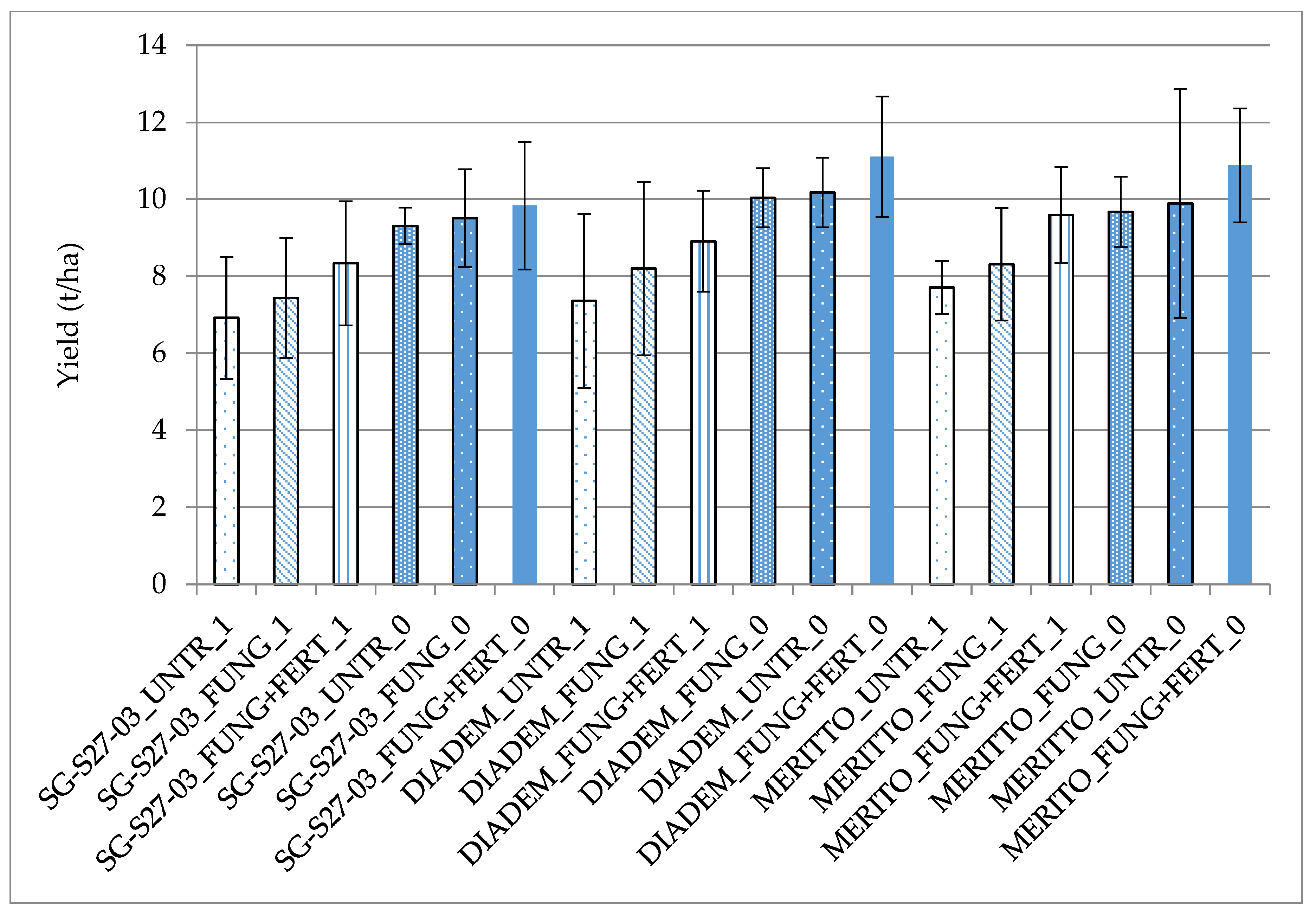
3.2. Evaluation of the Effects of BYDV-PAV Infection on Yield and Qualitative Traits
3.3. The Economic Effects of BYDV-PAV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gray, S.; Gildow, F.E. Luteovirus–Aphid Interactions. Annu. Rev. Phytopathol. 2003, 41, 539–566. [Google Scholar] [CrossRef] [PubMed]
- Perry, K.L.; Kolb, F.L.; Sammons, B.; Lawson, C.; Cisar, G.; Ohm, H. Yield effects of Barley yellow dwarf virus in soft red winter wheat. Phytopathology 2000, 90, 1043–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheurer, K.S.; Friedt, W.; Huth, W.; Waugh, R.; Ordon, F. QTL analysis of tolerance to a German strain of BYDV-PAV in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2001, 103, 1074–1083. [Google Scholar] [CrossRef]
- Kundu, J.K.; Jarošová, J.; Gadiou, S.; Červená, G. Discrimination of three BYDV species by one-step RT-PCR-RFLP and sequence-based methods in cereal plants from the Czech Republic. Cereal Res. Commun. 2009, 37, 541–550. [Google Scholar] [CrossRef]
- Jarošová, J.; Chrpová, J.; Šíp, V.; Kundu, J.K. A comparative study of the Barley yellow dwarf virus species PAV and PAS: Distribution, accumulation and host resistance. Plant Pathol. 2013, 62, 436–443. [Google Scholar] [CrossRef]
- Power, A.; Gray, S. Aphid transmission of barley yellow dwarf viruses interactions between viruses, vectors, and host plants. In Barley Yellow Dwarf: Forty Years of Progress; D’Arcy, J.C., Burnett, P.A., Eds.; APS Press: Saint Paul, MN, USA, 1995; pp. 255–291. [Google Scholar]
- Beoni, E.; Chrpová, J.; Jarošová, J.; Kundu, J.K. Survey of Barley yellow dwarf virus incidence in winter cereal crops, and assessment of wheat and barley resistence to the virus. Crop Pasture Sci. 2016, 67, 1054–1063. [Google Scholar] [CrossRef]
- Cooper, J.I.; Jones, A.T. Responses of plants to viruses—Proposal for the use of terms. Phytopathology 1983, 73, 127–128. [Google Scholar] [CrossRef] [Green Version]
- Jarošová, J.; Beoni, E.; Kundu, J.K. Barley yellow dwarf virus resistance in cereals: Approaches, strategies and prospects. Field Crop. Res. 2016, 198, 200–214. [Google Scholar] [CrossRef]
- Singh, R.P.; Burnett, P.A.; Albarran, M.; Rajaram, S. Bdv1: A gene for tolerance to barley yellow dwarf virus in bread wheat. Crop Sci. 1993, 33, 231–234. [Google Scholar] [CrossRef]
- Banks, P.; Davidson, J.; Bariana, H.; Larkin, P. Effects of Barley yellow dwarf virus on the yield of winter wheat. Aust. J. Agric. Res. 1995, 46, 935–946. [Google Scholar] [CrossRef]
- Kong, L.; Anderson, J.M.; Ohm, H.W. Segregation distortion in commonwheat of a segment of Thinopyrum intermedium chromosome 7E carrying Bdv3and development of a Bdv3 marker. Plant Breed. 2009, 128, 591–597. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Z.; Xin, Z. Research progress in BYDV resistance genes derived from wheat and its wild relatives. J. Genet. Genom. 2009, 36, 567–573. [Google Scholar] [CrossRef]
- Ayala, L.; van Ginkel, M.; Khairallah, M.; Keller, B.; Henry, M. Expression of Thinopyrum intermedium—Derived Barley yellow dwarf virus resistance in elite bread wheat. Phytopathology 2001, 91, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosová, K.; Chrpová, J.; Šíp, V. Recent advances in breeding of cereals for resistance to barley yellow dwarf virus-A review. Czech J. Genet. Plant Breed. 2008, 44, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Veškrna, O.; Chrpová, J.; Šíp, V.; Sedláček, T.; Horčička, P. Reaction of wheat varieties to infection with barley yellow dwarf virus and prospects for resistance breeding. Czech J. Genet. Plant Breed. 2009, 45, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Van Ginkel, M.; Henrey, M. Breeding for BYDV tolerance/resistance in CIMMYT bread wheats targeted to developing countries. In Barley Yellow Dwarf Disease: Recent Advances and Future Strategies, Proceedings of the An Internation Symposium, Texcoco, Mexico, 1–5 September 2002; CIMMYT: Texcoco, Mexico, 2002; pp. 93–96. [Google Scholar]
- Šíp, V.; Vacke, J.; Škorpík, M. Response of Czech and Slowak winter wheat varieties to the infection with Barley yellow dwarf virus. Genet. Šlechtění 1995, 31, 253–266. (In Czech) [Google Scholar]
- Vacke, J.; Šíp, V.; Škorpík, M. Response of selected spring wheat varieties to the infection with barley yellow dwarf virus. Genet. Šlechtění 1996, 32, 95–106. (In Czech) [Google Scholar]
- Clark, A.G.; Adams, A.N. Characteristics of microplate methods of enzyme-linked immunosorbentassay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Schaller, C.W.; Qualset, C.O. Breeding for resistance to barley yellow dwarf virus. In Proceedings of the Third International Wheat Conference, Madrid, Spain, 22 May–3 June 1980; USDA (University of Nebraska Agricultural Experiment Station): Lincoln, NE, USA, 1980; pp. 528–541. [Google Scholar]
- Choudhury, S.; Al-Shammari, D.; Hu, H.; Meinke, H.; Westmore, G.; Birchall, C.; Larkin, P.; Zhoua, M. A screening method to detect BYDV-PAV resistance in cereals under glasshouse conditions. Plant Pathol. 2018, 67, 1987–1996. [Google Scholar] [CrossRef]
- Riedell, W.E.; Kieckhefer, R.W.; Langham, M.A.C.; Hesler, L.S. Root and shoot responses to bird cherry-oat aphids and Barley yellow dwarf virus in spring wheat. Crop Sci. 2003, 43, 1380–1386. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, P.J.; Stoner, W.N. Barley yellow dwarf studies in wheat (Triticum aestivum L.) Yield and quality of hard red winter wheat infected with barley yellow dwarf virus. Crop Sci. 1967, 7, 337–341. [Google Scholar] [CrossRef]
- Šíp, V.; Škorpík, M.; Chrpová, J.; Šottníková, V.; Bártová, Š. Effect of cultivar and cultural practices on grain yield and bread-making quality of winter wheat. Rostl. Výroba 2000, 46, 159–167. (In Czech) [Google Scholar]
- López-Bellido, L.; López-Bellido, R.J.; Castillo, J.E.; López-Bellido, F.J. Effects of long-term tillage, crop rotation and nitrogen fertilization on bread-making quality of hard red spring wheat. Field Crop. Res. 2001, 72, 197–210. [Google Scholar] [CrossRef]
| Rating Scale | Symptoms |
|---|---|
| 0 | No visible symptoms |
| 1 | Trace amounts of yellowing, vigorous plant appearance |
| 2 | Restricted yellowing of leaves, more leaves discolored |
| 3 | Moderate to low amount of yellowing, no sign of dwarfing or reduction in tillering |
| 4 | Moderate to somewhat extensive yellowing, no dwarfing; moderate to good plant vigor |
| 5 | More extensive yellowing, moderate to poor plant vigor, some dwarfing |
| 6 | High level of yellowing, poor plant vigor, apparent dwarfing |
| 7 | Severe yellowing, small spikes, moderate dwarfing, poor plant appearance |
| 8 | Nearly complete yellowing of all leaves, dwarfing, tillering apparently reduced (rosette appearance), reduced spike size with some sterility |
| 9 | Marked dwarfing, complete yellowing, few or no spikes, considerable sterility, forced maturity or drying of the plant before normal maturity is reached |
| Effect | SS | Df | MS | F-Ratio | p-Value |
|---|---|---|---|---|---|
| VSSs | |||||
| Intercept | 2808.652 | 1 | 2808.652 | 9004.891 | 0.000000 |
| CULTIVAR/LINE | 151.296 | 27 | 5.604 | 17.966 | 0.000000 |
| YEAR | 21.831 | 2 | 10.916 | 34.997 | 0.000000 |
| Error | 31.502 | 101 | 0.312 | ||
| GWS-R | |||||
| Intercept | 109028.473 | 1 | 109028.473 | 962.295 | 0.000000 |
| CULTIVAR/LINE | 14867.223 | 27 | 550.638 | 4.860 | 0.000000 |
| YEAR | 3224.314 | 2 | 1612.157 | 14.229 | 0.000004 |
| Error | 11443.350 | 101 | 113.300 | ||
| VSSs (0–9)/GWS-R (%) | |||||
|---|---|---|---|---|---|
| Cultivar/Line | 2015 | 2016 | 2017 | Average | H. g. |
| PSR 3628 | 1.75/14.87 | 1.00/0.00 | 1.00/0.00 | 1.25/4.96 | a/a |
| Sparta | 3.50/20.70 | 5.50/18.95 | 4.50/15.95 | 4.50/18.80 | b/b |
| Bonanza | 4.25/19.24 | 5.50/46.21 | 4.75/34.61 | 4.83/33.35 | bc/bcdef |
| Tilman | 3.75/42.58 | 6.25/40.96 | nt/nt | 5.00/41.77 | bcd/defg |
| Tosca | 4.50/34.06 | 5.50/28.02 | nt/nt | 5.00/31.04 | bcd/bcde |
| Faunus | nt/nt | 6.00/57.88 | 4.25/16.54 | 5.13/37.21 | bcd/cdefg |
| Replik | 4.50/24.72 | 6.50/13.98 | nt/nt | 5.17/29.17 | bcde/bcde |
| Annie | 4.50/27.18 | 6.00/40.30 | nt/nt | 5.25/33.74 | bcde/bcdefg |
| Bernstein | 5.50/29.53 | 5.75/50.61 | 4.50/27.13 | 5.25/35.76 | bcde/cdefg |
| Frisky | 4.75/25.91 | 6.00/51.38 | 5.00/17.49 | 5.25/31.60 | bcde/bcde |
| Pankratz | 5.25/25.38 | 5.75/37.04 | 4.75/34.56 | 5.25/32.33 | bcde/bcde |
| Rumor | 4.50/18.09 | 6.00/35.98 | nt/nt | 5.25/27.03 | bcde/bcd |
| Sailor | 4.50/33.20 | 6.00/44.94 | nt/nt | 5.25/39.07 | bcde/cdefg |
| Artist | 5.00/36.28 | 5.75/47.29 | nt/nt | 5.38/41.78 | bcde/defg |
| Athlon | 5.00/30.76 | 5.75/17.57 | nt/nt | 5.38/24.17 | bcde/bc |
| Julie | 4.75/20.82 | 6.00/33.51 | nt/nt | 5.38/27.17 | bcde/bcd |
| Balitus | 5.50/39.08 | 6.00/56.39 | 5.00/13.19 | 5.50/36.22 | cde/cdefg |
| Florus | 5.00/17.74 | 6.00/49.93 | nt/nt | 5.50/33.84 | cde/bcdefg |
| RGT Matahari | 5.25/35.22 | 6.00/33.21 | 5.25/31.40 | 5.50/33.28 | cde/bcdef |
| Partner | nt/nt | 5.75/48.85 | 5.50/50.63 | 5.63/49.74 | cde/gh |
| Genius | 5.50/33.56 | 6.00/34.76 | nt/nt | 5.75/34.16 | cdef/cdefg |
| Vlada | 6.00/36.94 | 6.00/50.23 | 5.50/45.80 | 5.83/44.33 | def/efg |
| Grizzly | 5.50/31.21 | 6.25/36.58 | nt/nt | 5.88/33.90 | def/bcdefg |
| Tobak | 6.00/44.64 | 6.00/52.44 | nt/nt | 6.00/48.54 | defg/fgh |
| Nordika | 6.50/27.62 | 6.00/26.52 | nt/nt | 6.25/27.07 | efg/bcd |
| Gordian | 6.50/33.11 | 7.00/52.44 | nt/nt | 6.75/42.78 | fgh/defg |
| Avenue | 6.50/22.94 | 7.50/47.39 | nt/nt | 7.00/35.17 | gh/cdefg |
| SG-S 27-03 | 7.25/62.44 | 7.25/50.00 | 7.25/66.78 | 7.25/59.74 | h/h |
| Average | 5.06a/30.30a | 5.89b/39.41b | 4.77a/29.51a | 5.40/34.37 | |
| Protein Content | SS | DF | MS | F-Ratio | p-Value |
|---|---|---|---|---|---|
| Intercept | 17421.15 | 1 | 17421.15 | 25618.86 | 0.000000 |
| Year | 273.59 | 2 | 136.80 | 201.17 | 0.000000 |
| Treatment | 2.69 | 2 | 1.34 | 1.98 | 0.144296 |
| Cultivar | 9.76 | 2 | 4.88 | 7.18 | 0.001267 |
| BYDV | 10.25 | 1 | 10.25 | 15.08 | 0.000194 |
| Treatment × cultivar | 6.69 | 4 | 1.67 | 2.46 | 0.050852 |
| Treatment × BYDV | 2.98 | 2 | 1.49 | 2.19 | 0.117260 |
| Cultivar × BYDV | 2.13 | 2 | 1.06 | 1.56 | 0.214864 |
| Error | 62.56 | 92 | 0.68 | ||
| Yield | |||||
| Intercept | 8884.884 | 1 | 8884.884 | 6531.290 | 0.000000 |
| Year | 95.216 | 2 | 47.608 | 34.997 | 0.000000 |
| Treatment | 28.852 | 2 | 14.426 | 10.604 | 0.000072 |
| Cultivar | 13.979 | 2 | 6.989 | 5.138 | 0.007668 |
| BYDV | 103.663 | 1 | 103.663 | 76.203 | 0.000000 |
| Treatment × cultivar | 1.327 | 4 | 0.332 | 0.244 | 0.912653 |
| Treatment × BYDV | 3.466 | 2 | 1.733 | 1.274 | 0.284628 |
| Cultivar × BYDV | 2.040 | 2 | 1.020 | 0.750 | 0.475406 |
| Error | 125.153 | 92 | 1.360 | ||
| Test weight | |||||
| Intercept | 675,899.3 | 1 | 675,899.3 | 455,276.0 | 0.000000 |
| Year | 426.2 | 2 | 213.1 | 143.5 | 0.000000 |
| Treatment | 10.9 | 2 | 5.4 | 3.7 | 0.029459 |
| Cultivar | 110.3 | 2 | 55.1 | 37.1 | 0.000000 |
| BYDV | 42.4 | 1 | 42.4 | 28.6 | 0.000001 |
| Treatment × cultivar | 11.0 | 4 | 2.7 | 1.8 | 0.126427 |
| Treatment × BYDV | 6.7 | 2 | 3.4 | 2.3 | 0.109144 |
| Cultivar × BYDV | 0.3 | 2 | 0.2 | 0.1 | 0.889468 |
| Error | 136.6 | 92 | 1.5 |
| Variable | Treatment | BYDV | Average | H. g. |
|---|---|---|---|---|
| PROTEIN CONTENT | FUNG | 0 | 12.36582 | a |
| FUNG + FERT | 0 | 12.40240 | a | |
| UNTR | 0 | 12.40943 | a | |
| FUNG + FERT | 1 | 12.55283 | a | |
| UNTR | 1 | 13.20334 | b | |
| FUNG | 1 | 13.27017 | b | |
| YIELD | UNTR | 1 | 7.33339 | a |
| FUNG | 1 | 7.98691 | b | |
| FUNG + FERT | 1 | 8.95098 | c | |
| FUNG | 0 | 9.74385 | d | |
| UNTR | 0 | 9.79630 | d | |
| FUNG + FERT | 0 | 10.60941 | e | |
| TEST WEIGHT | UNTR | 1 | 77.81233 | a |
| FUNG | 1 | 78.43879 | b | |
| FUNG + FERT | 1 | 79.19692 | c | |
| UNTR | 0 | 79.68244 | d | |
| FUNG | 0 | 79.68357 | d | |
| FUNG + FERT | 0 | 79.84349 | d |
| Threshold | Winter Wheat (on Average) | Winter Wheat Cultivar Meritto (Moderately Resistant) Infected with BYDV-PAV | Winter Wheat Cultivar Diadem (Moderately Susceptible) Infected with BYDV-PAV | Spring Wheat |
|---|---|---|---|---|
| Yield (t ha−1) | 6.0 | 4.98 | 4.2 | 5.4 |
| Percent of yield loss compared with non-infected plants (variants) | 0 | 17 | 30 | 10 |
| Yield by commodity price in the Czech Republic (€ ha−1, average price of wheat per ton: 133 € t−1) | 800 | 664 | 560 | 667 |
| Re-cultivation cost (€ ha−1) | 202 | |||
| Economic loss (€ ha−1) | 0 | 136 | 240 | 465 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrpová, J.; Veškrna, O.; Palicová, J.; Kundu, J.K. The Evaluation of Wheat Cultivar Resistance and Yield Loss Thresholds in Response to Barley Yellow Dwarf Virus-PAV Infection. Agriculture 2020, 10, 20. https://doi.org/10.3390/agriculture10010020
Chrpová J, Veškrna O, Palicová J, Kundu JK. The Evaluation of Wheat Cultivar Resistance and Yield Loss Thresholds in Response to Barley Yellow Dwarf Virus-PAV Infection. Agriculture. 2020; 10(1):20. https://doi.org/10.3390/agriculture10010020
Chicago/Turabian StyleChrpová, Jana, Ondřej Veškrna, Jana Palicová, and Jiban Kumar Kundu. 2020. "The Evaluation of Wheat Cultivar Resistance and Yield Loss Thresholds in Response to Barley Yellow Dwarf Virus-PAV Infection" Agriculture 10, no. 1: 20. https://doi.org/10.3390/agriculture10010020
APA StyleChrpová, J., Veškrna, O., Palicová, J., & Kundu, J. K. (2020). The Evaluation of Wheat Cultivar Resistance and Yield Loss Thresholds in Response to Barley Yellow Dwarf Virus-PAV Infection. Agriculture, 10(1), 20. https://doi.org/10.3390/agriculture10010020






