Structural and Physicochemical Characterization of Chitosan Obtained by UAE and Its Effect on the Growth Inhibition of Pythium ultimum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biologic Material
2.2. Chitin Extraction
2.3. Factorial Design for the UC Obtention
2.4. Physicochemical Characterization
2.4.1. Chitosan Solubility
2.4.2. Total Nitrogen Determination and Protein Content
2.4.3. Ash Content Determination
2.5. Structural Characterization
2.5.1. Degree of Deacetylation (DDA)
2.5.2. Molecular Weight (MW) Determination
2.5.3. X-ray Diffraction Analysis (XRD)
2.6. Inhibitory Effect of the Chitosan
2.7. Statistical Analysis
3. Results
3.1. Chitosan Obtained by UAE
3.2. Physicochemical and Structural Characterization
3.2.1. Determination of the DDA
3.2.2. Determination of the MW and X-ray Diffraction Analysis
3.3. Inhibitory Activity of the Chitosan
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Said Al Hoqani, H.A.; AL-Shaqsi, N.; Hossain, M.A.; Al Sibani, M.A. Isolation and optimization of the method for industrial production of chitin and chitosan from Omani shrimp shell. Carbohydr. Res. 2020, 492, 108001. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Domard, A. A perspective on 30 years research on chitin and chitosan. Carbohydr. Polym. 2011, 84, 696–703. [Google Scholar] [CrossRef]
- Pacheco, N.; Larralde-Corona, C.P.; Sepulveda, J.; Trombotto, S.; Domard, A.; Shirai, K. Evaluation of chitosans and Pichia guillermondii as growth inhibitors of Penicillium digitatum. Int. J. Biol. Macromol. 2008, 43, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Tahtat, D.; Boutrig, H.H.; Khodja, A.N.; Benamer, S.; Hammache, Y.; Mahlous, M. The synergistic effect of gamma irradiation and alkaline soaking at low temperature on the pre-deacetylation of α-chitin: Optimization by design of experiment. Carbohydr. Polym. 2019, 215, 39–46. [Google Scholar] [CrossRef]
- El Knidri, H.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Process Saf. Environ. Prot. 2016, 104, 395–405. [Google Scholar] [CrossRef]
- Ngo, T.H.D.; Ngo, D.N. Effects of low–frequency ultrasound on heterogenous deacetylation of chitin. Int. J. Biol. Macromol. 2017, 104, 1604–1610. [Google Scholar] [CrossRef]
- Birolli, W.G.; Delezuk, J.A.D.M.; Campana-Filho, S.P. Ultrasound-assisted conversion of alpha-chitin into chitosan. Appl. Acoust. 2016, 103, 239–242. [Google Scholar]
- Hafsa, J.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Françaises 2016, 74, 27–33. [Google Scholar] [CrossRef]
- Pacheco, N.; Garnica-Gonzalez, M.; Gimeno, M.; Bárzana, E.; Trombotto, S.; David, L.; Shirai, K. Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules 2011, 12, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Lecumberri, E.; Mateos-Aparicio, I.; Mengíbar, M.; Heras, A. Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr. Polym. 2011, 84, 803–806. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Ultrasound-assisted extraction of chitosan from squid pen: Molecular characterization and fat binding capacity. J. Food Sci. 2019, 84, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Woo, J.Y.; Ahn, C.B.; Je, J.Y. Chitosan-hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Ding, L.; Huang, Y.; Cai, X.X.; Wang, S. Impact of pH, ionic strength and chitosan charge density on chitosan/casein complexation and phase behavior. Carbohydr. Polym. 2019, 208, 133–141. [Google Scholar] [CrossRef]
- Koutsopoulou, E.; Koutselas, I.; Christidis, G.E.; Papagiannopoulos, A.; Marantos, I. Effect of layer charge and charge distribution on the formation of chitosan—Smectite bionanocomposites. Appl. Clay Sci. 2020, 190, 105583. [Google Scholar] [CrossRef]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef]
- Sukmark, T.; Rachtanapun, P.; Rachtanapun, C. Antimicrobial activity of oligomer and polymer chitosan from different sources against foodborne pathogenic bacteria. Kasetsart J. Nat. Sci. 2011, 45, 636–643. [Google Scholar]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Coutinho, T.C.; Ferreira, M.C.; Rosa, L.H.; de Oliveira, A.M.; de Oliveira, J.E.N. Penicillium citrinum and Penicillium mallochii: New phytopathogens of orange fruit and their control using chitosan. Carbohydr. Polym. 2020, 234, 1–3. [Google Scholar] [CrossRef]
- Vanti, G.L.; Masaphy, S.; Kurjogi, M.; Chakrasali, S.; Nargund, V.B. Synthesis and application of chitosan-copper nanoparticles on damping off causing plant pathogenic fungi. Int. J. Biol. Macromol. 2020, 156, 1387–1395. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.; Jones, J.D.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Li, N.; Zhou, Q.; Chang, K.F.; Yu, H.; Hwang, S.F.; Conner, R.L.; Strelkov, S.E.; McLaren, D.L.; Turnbull, G.D. Occurrence, pathogenicity and species identification of Pythium causing root rot of soybean in Alberta and Manitoba, Canada. Crop Prot. 2019, 118, 36–43. [Google Scholar] [CrossRef]
- Zerillo, M.M.; Adhikari, B.N.; Hamilton, J.P.; Buell, C.R.; Lévesque, C.A.; Tisserat, N. Carbohydrate-active enzymes in Pythium and their role in plant cell wall and storage polysaccharide degradation. PLoS ONE 2013, 8, e72572. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Bautista-Baños, S.; Correa-Pacheco, Z.N.; Jiménez-Aparicio, A. Chitosan in the Preservation of Agricultural Commodities Chapter 13—Biological Activity of Chitosan Nanoparticles Against Pathogenic Fungi and Bacteria; Academic Press: Cambridge, MA, USA, 2020; p. 384. [Google Scholar]
- Robles, E.; Salaberria, A.M.; Herrera, R.; Fernandes, S.C.M.; Labidi, J. Self-bonded composite films based on cellulose nanofibers and chitin nanocrystals as antifungal materials. Carbohydr. Polym. 2016, 144, 41–49. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC, 7th ed.; Government Printing Office: Washington, DC, USA, 1997.
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Baxter, A.; Dillon, M.; Anthony Taylor, K.D.; Roberts, G.A.F. Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef]
- Affes, S.; Maalej, H.; Aranaz, I.; Acosta, N.; Heras, Á.; Nasri, M. Enzymatic production of low-Mw chitosan-derivatives: Characterization and biological activities evaluation. Int. J. Biol. Macromol. 2020, 144, 279–288. [Google Scholar] [CrossRef]
- Huang, L.; Bi, S.; Pang, J.; Sun, M.; Feng, C.; Chen, X. Preparation and characterization of chitosan from crab shell (Portunus trituberculatus) by NaOH/urea solution freeze-thaw pretreatment procedure. Int. J. Biol. Macromol. 2020, 147, 931–936. [Google Scholar] [CrossRef]
- Trung, T.S.; Van Tan, N.; Van Hoa, N.; Minh, N.C.; Loc, P.T.; Stevens, W.F. Improved method for production of chitin and chitosan from shrimp shells. Carbohydr. Res. 2020, 489, 107913. [Google Scholar] [CrossRef]
- Perentena, L.; González, C.; Celis, B.; Valbuena, A.; Colina, M. Síntesis de bases de Schiff derivadas del quitosano por reacción con p-dimetilaminobenzaldehído y 4-hidroxi-3-metoxibenzaldehido. Rev. Iberoam. de Polímeros 2015, 16, 1–27. [Google Scholar]
- Shin, C.S.; Kim, D.Y.; Shin, W.S. Characterization of chitosan extracted from Mealworm Beetle (Tenebrio molitor Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef]
- Fiamingo, A.; Delezuk, J.A.D.M.; Trombotto, S.; David, L.; Campana-Filho, S.P. Extensively deacetylated high molecular weight chitosan from the multistep ultrasound-assisted deacetylation of beta-chitin. Ultrason. Sonochem. 2016, 32, 79–85. [Google Scholar] [CrossRef]
- Delezuk, J.A.d.M.; Cardoso, M.B.; Domard, A.; Campana-Filho, S.P. Ultrasound-assisted deacetylation of beta-chitin: Influence of processing parameters. Polym. Int. 2011, 60, 903–909. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rahman, R.M.; Hrdina, R.; Abdel-Mohsen, A.M.; Fouda, M.M.; Soliman, A.Y.; Mohamed, F.K.; Mohsin, K.; Pinto, T.D. Chitin and chitosan from Brazilian Atlantic Coast: Isolation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2015, 80, 107–120. [Google Scholar] [CrossRef]
- Salas, C.; Thompson, Z.; Bhattarai, N. 15—Electrospun chitosan fibers. Electrospun Nanofibers 2017, 371–398. [Google Scholar] [CrossRef]
- He, X.; Li, K.; Xing, R.; Liu, S.; Hu, L.; Li, P. The production of fully deacetylated chitosan by compression method. Egypt. J. Aquat. Res. 2016, 42, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Kucukgulmez, A.; Celik, M.; Yanar, Y.; Sen, D.; Polat, H.; Kadak, A.E. Physicochemical characterization of chitosan extracted from Metapenaeus stebbingi shells. Food Chem. 2011, 126, 1144–1148. [Google Scholar] [CrossRef] [Green Version]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Antony, R.; Theodore David, S.; Saravanan, K.; Karuppasamy, K.; Balakumar, S. Synthesis, spectrochemical characterisation and catalytic activity of transition metal complexes derived from Schiff base modified chitosan. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2013, 103, 423–430. [Google Scholar] [CrossRef]
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Ben Seghir, B.; Benhamza, M.H. Preparation, optimization and characterization of chitosan polymer from shrimp shells. J. Food Meas. Charact. 2017, 11, 1137–1147. [Google Scholar] [CrossRef]
- Dahmane, E.M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and characterization of chitin and chitosan from parapenaeus longirostris from moroccan local sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of crystalline structural differences between α-and β-chitosan on their nanoparticle formation. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of chitosan: Material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [Green Version]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. de Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef]
- Palma-Guerrero, J.; Jansson, H.B.; Salinas, J.; Lopez-Llorca, L.V. Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J. Appl. Microbiol. 2008, 104, 541–553. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. Characterization and antimicrobial activity of water-soluble N-(4-carboxybutyroyl) chitosans against some plant pathogenic bacteria and fungi. Carbohydr. Polym. 2012, 87, 250–256. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Han, X.; Du, Y. Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pestic. Biochem. Physiol. 2007, 87, 220–228. [Google Scholar] [CrossRef]
- Park, R.D.; Jo, K.J.; Jo, Y.Y.; Jin, Y.L.; Kim, K.Y.; Shim, J.H. Variation of antifungal activities of chitosans on plant pathogens. J. Microbiol. Biotechnol. 2002, 12, 84–88. [Google Scholar]
- Mukhtar Ahmed, K.B.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production-a review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef]
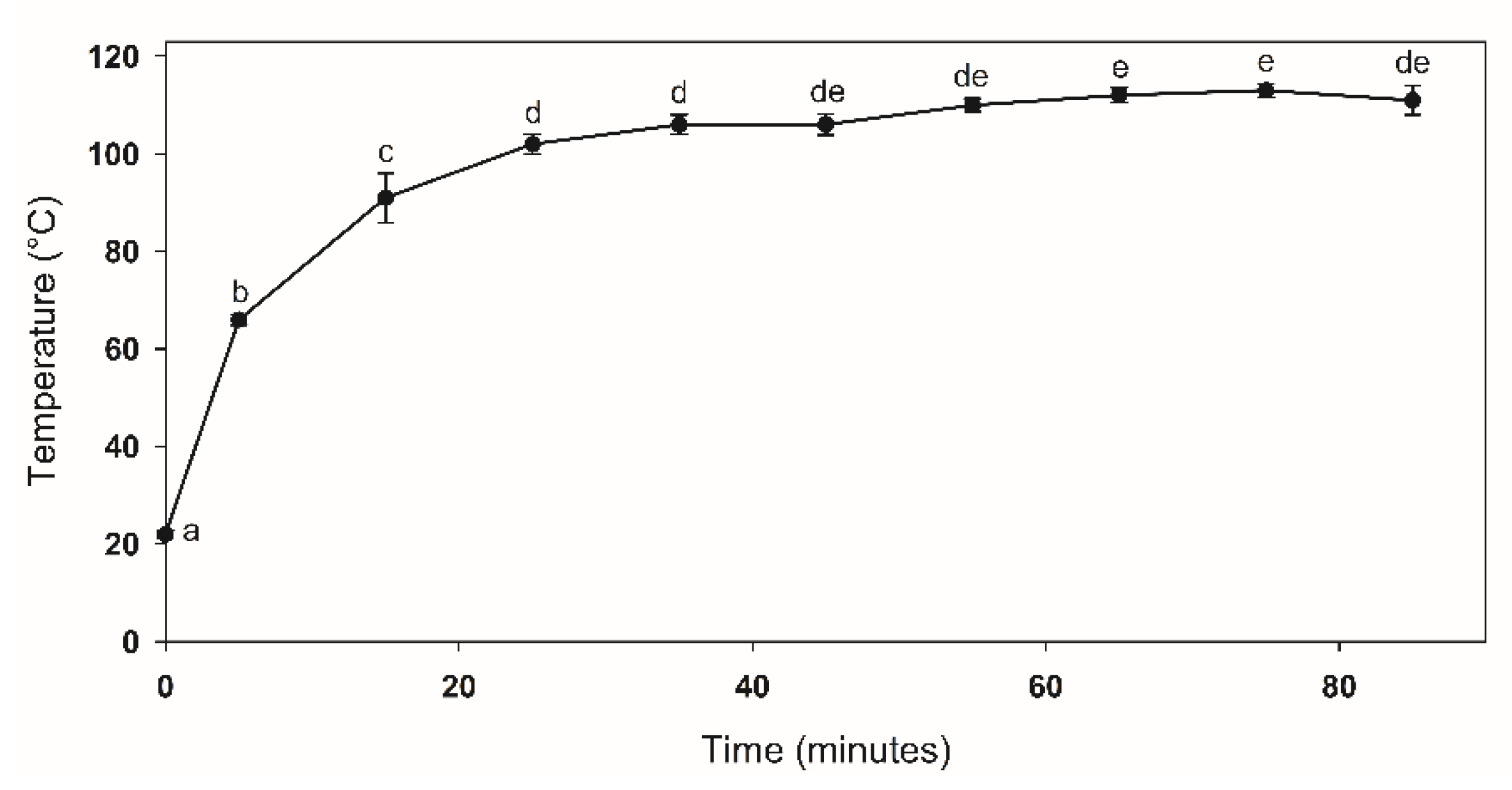

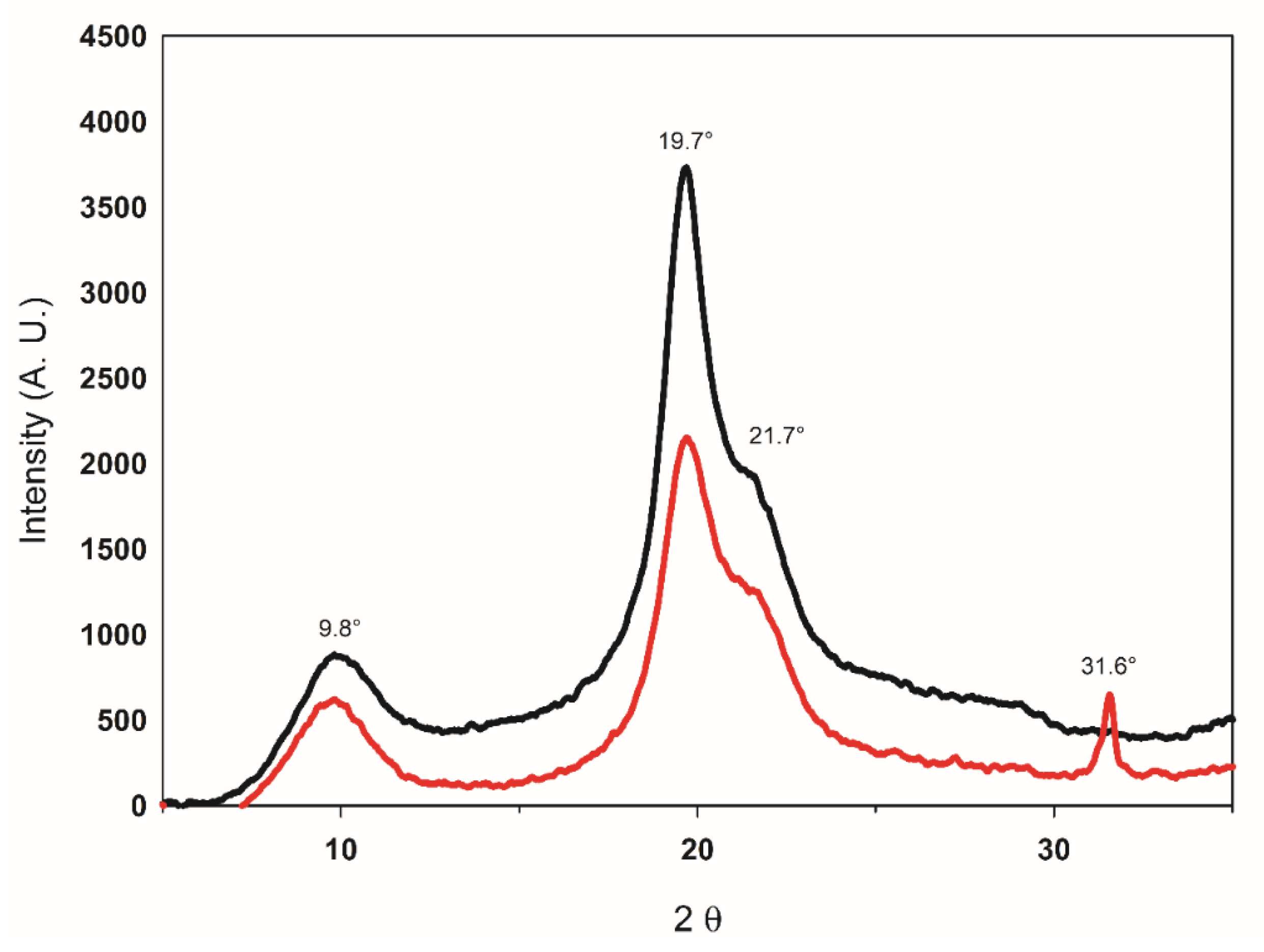

| Treatment (Code) | Factor | Response | |||
|---|---|---|---|---|---|
| Amplitude (%) | NaOH (%) | Interval (min) A 1–B 2 | Solubility (%) 3 | Yield (%) | |
| UC T1 | 75 | 50 | 5–5 | 13.42 ± 3.5 a | <15 ± 3.91 a |
| UC T2 | 75 | 50 | 7.5–5 | 7.37 ± 2.6 a | <15 ± 5.29 a |
| UC T3 | 90 | 50 | 5–5 | 42.8 ± 1.7 c | 20.65 ± 0.82 a |
| UC T4 | 90 | 50 | 7.5–5 | 20.79 ± 0.6 b | 17.26 ± 0.5 a |
| UC T5 | 75 | 65 | 5–5 | 98.0 ± 0.1 f | 80.32 ± 0.08 b |
| UC T6 | 75 | 65 | 7.5–5 | 97.9 ± 0.3 f | 81.05 ± 0.25 b |
| UC T7 | 90 | 65 | 5–5 | 100 ± 0.0 f | 86.89 ± 0.0 c |
| UC T8 | 90 | 65 | 7.5–5 | 94.8 ± 4.2 f | 87.21 ± 3.86 c |
| CC | ND | ND | ND | 100 ± 0.0 f | ND |
| Conventional Chitosan | ND | 50 | 300 | 58.72 ± 0.9 d | 88.35 ± 1.35 c |
| Conventional Chitosan | ND | 60 | 300 | 85.40 ± 0.7 e | 87.5 ± 0.72 c |
| Time (h) | PDA + UC | PDA + CC |
|---|---|---|
| 24 | 98.92% a | 100.00% a |
| 48 | 93.00% b | 100.00% a |
| 72 | 85.98% b | 100.00% a |
| 96 | 77.65% a | 80.00% a |
| 120 | 64.62% b | 72.06% a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-López, H.; Pech-Cohuo, S.C.; Herrera-Pool, E.; Medina-Torres, N.; Cuevas-Bernardino, J.C.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Ramos-Díaz, A.; Trombotto, S.; Pacheco, N. Structural and Physicochemical Characterization of Chitosan Obtained by UAE and Its Effect on the Growth Inhibition of Pythium ultimum. Agriculture 2020, 10, 464. https://doi.org/10.3390/agriculture10100464
Martín-López H, Pech-Cohuo SC, Herrera-Pool E, Medina-Torres N, Cuevas-Bernardino JC, Ayora-Talavera T, Espinosa-Andrews H, Ramos-Díaz A, Trombotto S, Pacheco N. Structural and Physicochemical Characterization of Chitosan Obtained by UAE and Its Effect on the Growth Inhibition of Pythium ultimum. Agriculture. 2020; 10(10):464. https://doi.org/10.3390/agriculture10100464
Chicago/Turabian StyleMartín-López, Héctor, Soledad Cecilia Pech-Cohuo, Emanuel Herrera-Pool, Nelly Medina-Torres, Juan Carlos Cuevas-Bernardino, Teresa Ayora-Talavera, Hugo Espinosa-Andrews, Ana Ramos-Díaz, Stéphane Trombotto, and Neith Pacheco. 2020. "Structural and Physicochemical Characterization of Chitosan Obtained by UAE and Its Effect on the Growth Inhibition of Pythium ultimum" Agriculture 10, no. 10: 464. https://doi.org/10.3390/agriculture10100464
APA StyleMartín-López, H., Pech-Cohuo, S. C., Herrera-Pool, E., Medina-Torres, N., Cuevas-Bernardino, J. C., Ayora-Talavera, T., Espinosa-Andrews, H., Ramos-Díaz, A., Trombotto, S., & Pacheco, N. (2020). Structural and Physicochemical Characterization of Chitosan Obtained by UAE and Its Effect on the Growth Inhibition of Pythium ultimum. Agriculture, 10(10), 464. https://doi.org/10.3390/agriculture10100464











