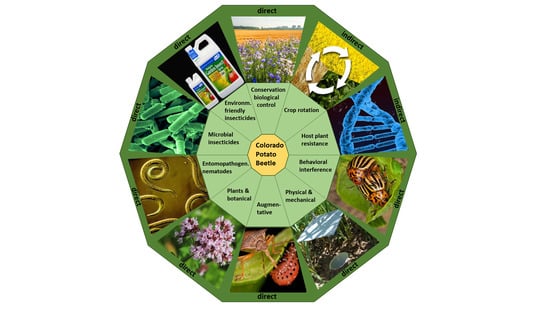
Alternatives to Synthetic Insecticides in the Control of the Colorado Potato Beetle (Leptinotarsa decemlineata Say) and Their Environmental Benefits
Abstract
:1. Introduction
2. Alternative Control Methods
2.1. Indirect Methods for CPB Control
2.1.1. Crop Rotation
2.1.2. Host Plant Resistance
2.2. Direct Methods for CPB Control
2.2.1. Behavioral Interference Methods for CPB
2.2.2. Physical and Mechanical Control
2.2.3. Augmentative Control
2.2.4. Use of the Plant Extracts and Botanical Insecticides
2.2.5. Entomopathogenic Nematodes
2.2.6. Microbial Insecticides
2.2.7. Environmentally Friendly Insecticides, Synergists and Their Combinations with Classical Insecticides
2.2.8. Conservation Biological Control
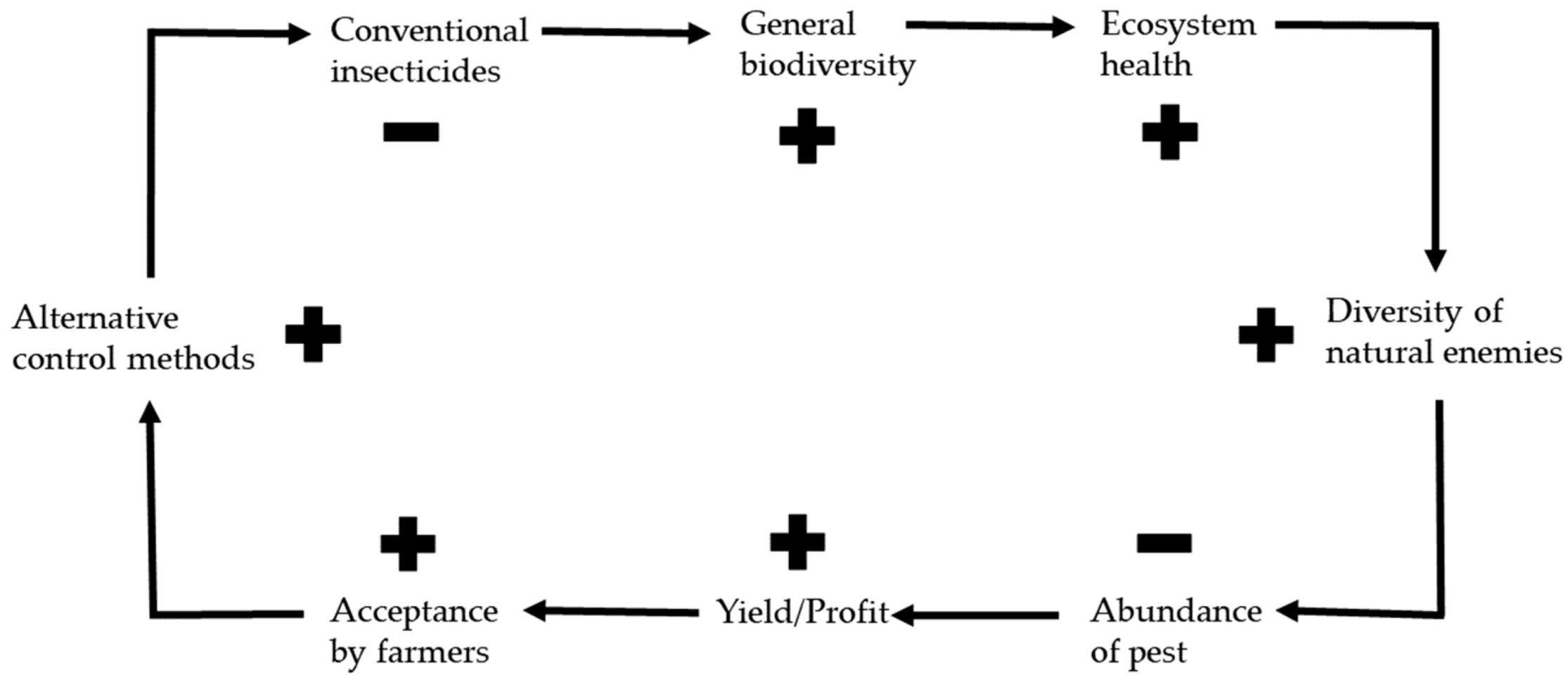
3. Effects of Alternative Control Methods on the Environment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. Sustainable Use of Pesticides. 2020. Available online: https://ec.europa.eu/food/plant/pesticides/sustainable_use_pesticides_en (accessed on 25 November 2020).
- Cingel, A.; Savić, J.; Lazarević, J.; Ćosić, T.; Raspor, M.; Smigocki, A.; Ninković, S. Extraordinary adaptive plasticity of colorado potato beetle: “Ten-Striped Spearman” in the era of biotechnological warfare. Int. J. Mol. Sci. 2016, 17, 1538. [Google Scholar] [CrossRef] [PubMed]
- Almady, S.; Khelifi, M.; Beaudoin, M.P. Control of the Colorado Potato Beetle, Leptinotarsa decemlineata (Say), Using Predator Insects Released by a Mechanical Prototype. J. Environ. Eng. Sci. 2012, 1, 1279–1287. [Google Scholar]
- Aldrich, J.R.; Cantelo, W.W. Suppression of Colorado potato beetle infestation by pheromone-mediated augmentation of the predatory spined soldier bug, Podisus maculiventris (Say) (Heteroptera: Pentatomidae). Agric. Forest Entomol. 1999, 1, 209–217. [Google Scholar] [CrossRef]
- Gökçe, A.; Whalon, M.E.; Çam, H.I.T.; Yanar, Y.; Demirtaş, İ.I.M.; Gőren, N. Contact and residual toxicities of 30 plant extracts to Colorado potato beetle larvae. Arch. Phytopathol. Pflanzenschutz 2007, 40, 441–450. [Google Scholar] [CrossRef]
- Luckmann, W.H.; Metcalf, R.L. The Pest Management Concept. Introduction to Insect Pest Management, 1st ed.; Wiley: New York, NY, USA, 1994; pp. 1–31. [Google Scholar]
- Alyokhin, A. Colorado potato beetle management on potatoes: Current challenges and future prospects. Fruit Veg. Cereal Sci. Biotechnol. 2009, 3, 10–19. [Google Scholar]
- Alyokhin, A.; Atlihan, R. Reduced fitness of the Colorado potato beetle (Coleoptera: Chrysomelidae) on potato plants grown in manure-amended soil. Environ. Entomol. 2005, 34, 963–968. [Google Scholar] [CrossRef] [Green Version]
- Armer, C.A.; Berry, R.E.; Reed, G.L.; Jepsen, S.J. Colorado potato beetle control by application of the entomopathogenic nematode Heterorhabditis marelata and potato plant alkaloid manipulation. Entomol. Exp. Appl. 2004, 111, 47–58. [Google Scholar] [CrossRef]
- Sablon, L.; Dickens, J.C.; Haubruge, É.; Verheggen, F.J. Chemical ecology of the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), and potential for alternative control methods. Insects 2013, 4, 31–54. [Google Scholar] [CrossRef] [Green Version]
- Wijesinha-Bettoni, R.; Mouillé, B. The Contribution of Potatoes to Global Food Security, Nutrition and Healthy Diets. Am. Potato J. 2019, 96, 139–149. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Zhang, R. Invasion of Colorado potato beetle, Leptinotarsa decemlineata, in China: Dispersal, occurrence, and economic impact. Entomol. Exp. Appl. 2012, 143, 207–217. [Google Scholar] [CrossRef]
- Maharijaya, A.; Vosman, B. Managing the Colorado potato beetle; the need for resistance breeding. Euphytica 2015, 204, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Rusin, M.; Gospodarek, J. The effect of water extracts from Origanum vulgare L. on feeding of Leptinotarsa decemlineata Say. J. Agric. Eng. 2018, 63, 122–127. [Google Scholar]
- Liu, S.Q.; Scott, I.M.; Pelletier, Y.; Kramp, K.; Durst, T.; Sims, S.R.; Arnason, J.T. Dillapiol: A pyrethrum synergist for control of the Colorado potato beetle. J. Econ. Entomol. 2014, 107, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Rowley, D.L.; Greenstone, M.H.; Athanas, M.M. Prey preference and host suitability of the predatory and parasitoid carabid beetle, Lebia grandis, for several species of Leptinotarsa beetles. J. Insect Sci. 2006, 6, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, J. Spinosad effectively controls Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) in organic potato. Acta Agric. Scand. B 2010, 60, 283–286. [Google Scholar] [CrossRef]
- Saroukolai, A.T.; Nouri-Ganbalani, G.; Hadian, J.; Rafiee-Dastjerdi, H. Antifeedant activity and toxicity of some plant essential oils to Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Plant Protect. Sci. 2014, 50, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Moreau, T.L.; Warman, P.R.; Hoyle, J. An evaluation of companion planting and botanical extracts as alternative pest controls for the Colorado potato beetle. Biol. Agric. Hortic. 2006, 23, 351–370. [Google Scholar] [CrossRef]
- Giordanengo, P.; Vincent, C.; Alyokhin, A. Insect Pests of Potato. Global Perspectives on Biology and Management, 1st ed.; Elsevier Inc.: Waltham, MA, USA, 2013. [Google Scholar]
- Huseth, A.S.; Frost, K.E.; Knuteson, D.L.; Wyman, J.A.; Groves, R.L. Effects of landscape composition and rotation distance on Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) abundance in cultivated potato. Environ. Entomol. 2012, 41, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Weisz, R.; Smilowitz, Z.; Christ, B. Distance, rotation, and border crops affect Colorado potato beetle (Coleoptera: Chrysomelidae) colonization and population density and early blight (Alternaria solani) severity in rotated potato fields. J. Econ. Entomol. 1994, 87, 723–729. [Google Scholar] [CrossRef]
- Kuepper, G. Colorado Potato Beetle: Organic Control Options; NCAT Program Specialist Tiffany Nitschke. HTML Production CT, 107, Slot 114; ATTRA: Fayetteville, NC, USA, 2003. [Google Scholar]
- Yasar, B.; Güngör, M.A. Determination of life table and biology of Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae), feeding on five different potato varieties in Turkey. Appl. Entomol. Zool. 2005, 40, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Sinden, S.L.; Sanford, L.L.; Cantelo, W.W.; Deahl, K.L. Bioassays of segregating plants. J. Chem. Ecol. 1988, 14, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Cappelle, K.; de Oliveira, C.F.R.; Van Eynde, B.; Christiaens, O.; Smagghe, G. The involvement of clathrin-mediated endocytosis and two Sid-1-like transmembrane proteins in double-stranded RNA uptake in the Colorado potato beetle midgut. Insect Mol. Biol. 2016, 25, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef]
- Visal, S.; Taylor, M.A.; Michaud, D. The proregion of papaya proteinase IV inhibits Colorado potato beetle digestive cysteine proteinases. FEBS Lett. 1998, 434, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Panevska, A.; Hodnik, V.; Skočaj, M.; Novak, M.; Modic, Š.; Pavlic, I.; Podržaj, S.; Zarić, M.; Resnik, N.; Maček, P.; et al. Pore-forming protein complexes from Pleurotus mushrooms kill western corn rootworm and Colorado potato beetle through targeting membrane ceramide phosphoethanolamine. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dickens, J.C. Plant volatiles moderate response to aggregation pheromone in Colorado potato beetle. J. Appl. Entomol. 2006, 130, 26–31. [Google Scholar] [CrossRef]
- Dickens, J.C.; Oliver, J.E.; Hollister, B.; Davis, J.C.; Klun, J.A. Breaking a paradigm: Male-produced aggregation pheromone for the Colorado potato beetle. J. Exp. Biol. 2002, 205, 1925–1933. [Google Scholar]
- Kuhar, T.P.; Mori, K.; Dickens, J.C. Potential of a synthetic aggregation pheromone for integrated pest management of Colorado potato beetle. Agric. Forest Entomol. 2006, 8, 77–81. [Google Scholar] [CrossRef]
- Kuhar, T.P.; Hitchner, E.M.; Youngman, R.R.; Mori, K.; Dickens, J.C. Field response of Colorado potato beetle to enantiomeric blends of CPB I aggregation pheromone. Int. J. Agric. Sci. 2012, 3, 896–899. [Google Scholar] [CrossRef]
- Martel, J.W.; Alford, A.R.; Dickens, J.C. Laboratory and greenhouse evaluation of a synthetic host volatile attractant for Colorado potato beetle, Leptinotarsa decemlineata (Say). Agric. Forest Entomol. 2005, 7, 71–78. [Google Scholar] [CrossRef]
- Dickens, J.C. Orientation of Colorado potato beetle to natural and synthetic blends of volatiles emitted by potato plants. Agric. Forest Entomol. 2000, 2, 167–172. [Google Scholar] [CrossRef]
- Gontariu, I.; Enea, I.-C. Protection against the proliferation of the Colorado beetle (Leptinotarsa decemlineata Say) in the concept of organic culture at the potato. Food Environ. Saf. J. 2017, 11, 91–96. [Google Scholar]
- Moore, J. Sweeping fields controls some pests. Am. Veg. Grow. 1990, 1, 10–11. [Google Scholar]
- Boiteau, G.; Singh, R.P.; McCarthy, P.C.; MacKinley, P.D. Wood ash potential for Colorado potato beetle control. Am. Potato J. 2012, 89, 129–135. [Google Scholar] [CrossRef]
- Laguë, C.; Khelifi, M.; Gill, J.; Lacasse, B. Pneumatic and thermal control of Colorado potato beetle. Can. Agric. Eng. 1999, 41, 53–58. [Google Scholar]
- Laguë, C.; Khelif, M.; Lacasse, B. Evaluation of a four-row prototype machine for pneumatic control of Colorado potato beetle. Can. Agric. Eng. 1999, 41, 47–52. [Google Scholar]
- Moyer, D.D.; Derksen, R.C.; McLeod, M.J. Development of a propane flamer for Colorado potato beetle control. Am. Potato J. 1992, 69, 599–600. [Google Scholar]
- Hicks, J.; Couturier, M.; Pelletier, Y. Insect scorcher for the control of the Colorado potato beetle. Can. Agric. Eng. 1999, 41, 227–232. [Google Scholar]
- Pelletier, Y.; Misener, G.C.; McMillan, L.P. Steam as an alternative control method for the management of Colorado potato beetles. Can. Agric. Eng. 1998, 40, 17–22. [Google Scholar]
- Couturier, M.; Hicks, J.B.; Rouison, D.; Pelletier, Y. Thermal initiation of thanatosis to improve the pneumatic removal of the Colorado potato beetle. Can. Biosyst. Eng. 2005, 47, 2.5–2.12. [Google Scholar]
- Boiteau, G.; Pelletier, Y.; Misener, G.C.; Bernard, G. Development and evaluation of a plastic trench barrier for protection of potato from walking adult Colorado potato beetles (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1994, 87, 1325–1331. [Google Scholar] [CrossRef]
- Otálora-Luna, F.; Dickens, J.C. Spectral preference and temporal modulation of photic orientation by Colorado potato beetle on a servosphere. Entomol. Exp. Appl. 2011, 138, 93–103. [Google Scholar] [CrossRef]
- Otálora-Luna, F.; Dickens, J.C. Multimodal stimulation of Colorado potato beetle reveals modulation of pheromone response by yellow light. PLoS ONE 2011, 6, e20990. [Google Scholar] [CrossRef]
- De Ladurantaye, Y.; Khelifi, M.; Cloutier, C.; Coudron, T.A. Short-term storage conditions for transport and farm delivery of the stink bug Perillus bioculatus for the biological control of the Colorado potato beetle. Can. Biosyst. Eng. 2010, 52, 1–4. [Google Scholar]
- Hough-Goldstein, J.; McPherson, D. Comparison of Perillus bioculatus and Podisus maculiventris (Hemiptera: Pentatomidae) as potential control agents of the Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1996, 89, 1116–1123. [Google Scholar] [CrossRef]
- Greenstone, M.H.; Szendrei, Z.; Payton, M.E.; Rowley, D.L.; Coudron, T.C.; Weber, D.C. Choosing natural enemies for conservation biological control: Use of the prey detectability half-life to rank key predators of Colorado potato beetle. Entomol. Exp. Appl. 2010, 136, 97–107. [Google Scholar] [CrossRef]
- Tarla, S.; Tarla, G. Detection of Perillus bioculatus (F.) (Heteroplera: Pentatomidae) on a New Host in Anatolia. Can. Entomol. 2018, 127, 195–212. [Google Scholar]
- Cloutier, C.; Jean, C. Synergism between natural enemies and biopesticides: A test case using the stinkbug Perillus bioculatus (Hemiptera: Pentatomidae) and Bacillus thuringiensis tenebrionis against Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1998, 91, 1096–1108. [Google Scholar] [CrossRef]
- O’Neil, R. Functional response search strategy of Podisus maculiventris (Heteroptera: Pentatomidae) attacking Colorado potato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 1997, 26, 1183–1190. [Google Scholar] [CrossRef]
- Hazzard, R.V.; Ferro, D.N.; Van Driesche, R.G.; Tuttle, A.F. Mortality of eggs of Colorado potato beetle (Coleoptera: Chrysomelidae) from predation by Coleomegilla maculata (Coleoptera: Coccinellidae). Environ. Entomol. 1991, 20, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Mallampalli, N.; Gould, F.; Barbosa, P. Predation of Colorado potato beetle eggs by a polyphagous ladybeetle in the presence of alternate prey: Potential impact on resistance evolution. Entomol. Exp. Appl. 2005, 114, 47–54. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Srinivasan, R.; Cervantes, F.A. Occurrence of the carabid beetle, Pterostichus melanarius (Illiger), in potato ecosystems of Idaho and its predatory potential on the Colorado potato beetle and aphids. Am. Potato J. 2013, 90, 83–92. [Google Scholar] [CrossRef]
- Sablon, L.; Haubruge, E.; Verheggen, F.J. Consumption of immature stages of Colorado potato beetle by Chrysoperla carnea (Neuroptera: Chrysopidae) larvae in the laboratory. Am. Potato J. 2013, 90, 51–57. [Google Scholar] [CrossRef]
- Groden, E.; Drummond, F.A.; Casagrande, R.A.; Lashomb, J.H. Estimating parasitism of Colorado potato beetle eggs, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae), by Edovum puttleri (Hymenoptera: Eulophidae). Great Lakes Entomol. 1989, 22, 47–54. [Google Scholar]
- Boiteau, G. Insect pest control on potato: Harmonization of alternative and conventional control methods. Am. Potato J. 2010, 87, 412–419. [Google Scholar] [CrossRef]
- De Ladurantaye, Y.; Khelifi, M. Design of a mechanical release system of Perillus bioculatus to control the Colorado potato beetle, Leptinotarsa decemlineata (Say). In Proceedings of the 17th World Congress of the International Commission of Agricultural and Biosystems Engineering (CIGR), Québec City, QC, Canada, 13–17 June 2010. [Google Scholar]
- Chiasson, H.; Vincent, C.; Bostanian, N.J. Insecticidal properties of a Chenopodium-based botanical. J. Econ. Entomol. 2004, 97, 1378–1383. [Google Scholar] [CrossRef]
- Thacker, J.R. An Introduction to Arthropod Pest Control, 1st ed.; Cambridge University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Pascual-Villalobos, M.J.; Robledo, A. Anti-insect activity of plant extracts from the wild flora in southeastern Spain. Biochem. Syst. Ecol. 1999, 27, 1–10. [Google Scholar] [CrossRef]
- Nitao, J.K. Test for toxicity of coniine to a polyphagous herbivore, Heliothis zea (Lepidoptera: Noctuidae). Environ. Entomol. 1987, 16, 656–659. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. J. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Mordue, A.J.; Nisbet, A.J. Azadirachtin from the Neem tree Azadirachta indica: Its actions against insects. An. Soc. Entomol. Brasil 2000, 29, 615–632. [Google Scholar]
- Hassan, E.; Gökçe, A. Production and consumption of biopesticides. In Advances in Plant Biopesticides, 1st ed.; Springer: New Delhi, India, 2014; pp. 361–379. [Google Scholar]
- Roahk, R.C. Present status of rotenone and rotenoids. J. Econ. Entomol. 1941, 34, 684–692. [Google Scholar] [CrossRef]
- Zehnder, G.W. Timing of insecticides for control of Colorado potato beetle (Coleoptera: Chrysomelidae) in eastern Virginia based on differential susceptibility of life stages. J. Econ. Entomol. 1986, 79, 851–856. [Google Scholar] [CrossRef]
- EC (European Commission). Concerning the Non-Inclusion of Rotenone, Extract from Equisetum and Chinin-Hydrochlorid in Annex I to Council Directive 91/414/EEC and the Withdrawal of Authorisations for Plant Protection Products Containing These Substances. EC 2008/317, Published 10 April 2008. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:108:0030:0032:EN:PDF (accessed on 28 October 2020).
- DG SanCo (European Union Director General for Health and Consumers). Available online: https://ec.europa.eu/info/departments/health-and-food-safety_en (accessed on 28 October 2020).
- Kesdek, M.; Kordali, S.; Usanmaz, A.; Ercisli, S. The toxicity of essential oils of some plant species against adults of colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Tome 2015, 68, 127–136. [Google Scholar]
- Ebadollahi, A.; Geranmayeh, J.; Kamrani, M. Colorado potato beetle (Leptinotarsa decemlineata Say) control potential of essential oil isolated from iranian Cymbopogon citratus Stapf. Nat. Prod. 2017, 23, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Gökçe, A.; Isaacs, R.; Whalon, M.E. Behavioural response of Colorado potato beetle (Leptinotarsa decemlineata) larvae to selected plant extracts. Pest Manag. Sci. 2006, 62, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Alkan, M.; Gökçe, A.; Kara, K. Contact Toxicity of Six Plant Extracts to Different Larval Stages of Colorado Potato Beetle (Leptinotarsa decemlineata Say (Col: Chrysomelidae)). J. Agric. Sci. 2017, 23, 309–316. [Google Scholar]
- Ertürk, Ö.; Sarıkaya, A. Effects of Various Plant Extracts on the Development of the Potato Beetle under Laboratory and Field Conditions: A Combined Study. J. Entomol. Res. Soc. 2017, 19, 101–112. [Google Scholar]
- Bădeanu, M.; Şuteu, D.; Chiorescu, E.; Filipov, F. The use of medicinal and aromatic plant extracts against Colorado beetle species-Leptinotarsa decemlineata (Coleoptera-Chrysomelidae). Rev. Bot. 2017, 14, 101–104. [Google Scholar]
- Scott, I.M.; Jensen, H.; Scott, J.G.; Isman, M.B.; Arnason, J.T.; Philogene, B.J.R. Botanical insecticides for controlling agricultural pests: Piperamides and the Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Arch. Insect Biochem. 2003, 54, 212–225. [Google Scholar] [CrossRef]
- Scott, I.M.; Jensen, H.R.; Philogène, B.J.; Arnason, J.T. A review of Piper spp.(Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem. Rev. 2008, 7, 65. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J.; Biniaś, B. The effect of water extract from wild thyme on Colorado potato beetle feeding. Ecol. Eng. 2016, 17, 197–202. [Google Scholar] [CrossRef]
- Gabaston, J.; El Khawand, T.; Waffo-Teguo, P.; Decendit, A.; Richard, T.; Mérillon, J.M.; Pavela, R. Stilbenes from grapevine root: A promising natural insecticide against Leptinotarsa decemlineata. J. Pest Sci. 2018, 91, 897–906. [Google Scholar] [CrossRef]
- Trdan, S.; Cirar, A.; Bergant, K.; Andjus, L.; Kač, M.; Vidrih, M.; Rozman, L. Effect of temperature on efficacy of three natural substances to Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Acta Agric. Scand. B 2007, 57, 293–296. [Google Scholar] [CrossRef]
- Bohinc, T.; Vučajnk, F.; Trdan, S. The efficacy of environmentally acceptable products for the control of major potato pests and diseases. Zemdirbyste 2019, 106, 135–142. [Google Scholar] [CrossRef]
- Banken, J.A.; Stark, J.D. Multiple routes of pesticide exposure and the risk of pesticides to biological controls: A study of neem and the sevenspotted lady beetle (Coleoptera: Coccinellidae). J. Econ. Entomol. 1998, 91, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Murray, K. Utilization of a neem product in a reduced synthetic chemical insecticidal management program for Colorado potato beetle. Sustain. Agric. Netw. 1997, 24, 1275–1283. [Google Scholar]
- Bezjak, S.; Igrc Barčić, J.; Bažok, R. Efficacy of botanical insecticides in Colorado potato beetle (Leptinotarsa decemlineata, Say., Coleoptera:Chrysomelidae) control. Fragm. Phytomed. Herbol. 2006, 29, 13–24. [Google Scholar]
- Ebrahimi, L.; Niknam, G.; Lewis, E.E. Lethal and sublethal effects of Iranian isolates of Steinernema feltiae and Heterorhabditis bacteriophora on the Colorado potato beetle, Leptinotarsa decemlineata. BioControl 2011, 56, 781–788. [Google Scholar] [CrossRef]
- Toba, H.H.; Lindegren, J.E.; Turner, J.E.; Vail, P.V. Susceptibility of the Colorado potato beetle and the sugarbeet wireworm to Steinernema feltiae and S. glaseri. J. Nematol. 1983, 15, 597. [Google Scholar]
- Kepenekci, I. Infectivity of Native Entomopathogenic Nematodes Applied as Infected-Host Cadavers against the Colorado Potato Beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Egypt. J. Biol. Pest Control 2016, 26, 173. [Google Scholar]
- Laznik, Ž.; Tóth, T.; Lakatos, T.; Vidrih, M.; Trdan, S. Control of the Colorado potato beetle (Leptinotarsa decemlineata [Say]) on potato under field conditions: A comparison of the efficacy of foliar application of two strains of Steinernema feltiae (Filipjev) and spraying with thiametoxam. J. Plant Dis. Protect 2010, 117, 129–135. [Google Scholar] [CrossRef]
- Trdan, S.; Vidrih, M.; Andjus, L.; Laznik, Ž. Activity of four entomopathogenic nematode species against different developmental stages of Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera, Chrysomelidae). Helminthologia 2009, 46, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Trdan, R.E.; Liu, J.; Reed, G. Comparison of endemic and exotic entomopathogenic nematode species for control of Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1997, 90, 1528–1533. [Google Scholar]
- Ghassemi-Kahrizeh, A.; Aramideh, S. Sub-lethal effects of Bacillus thuringiensis Berliner on larvae of Colorado potato beetle, Leptinotarsa decemlineata (say) (Coleoptera: Chrysomelidae). Arch. Phytopathol. Pflanzenschutz 2015, 48, 259–267. [Google Scholar] [CrossRef]
- Wraight, S.P.; Ramos, M.E. Synergistic interaction between Beauveria bassiana-and Bacillus thuringiensis tenebrionis-based biopesticides applied against field populations of Colorado potato beetle larvae. J. Invertebr. Pathol. 2005, 90, 139–150. [Google Scholar] [CrossRef]
- Weinzierl, R.; Henn, T.; Koehler, P.G.; Tucker, C.L. Microbial Insecticides, Cooperative Extension Service; University of Illinois: Gainesville, FL, USA, 1998; Volume 1295, pp. 11–23. [Google Scholar]
- Barčić, J.I.; Bažok, R.; Bezjak, S.; Čuljak, T.G.; Barčić, J. Combinations of several insecticides used for integrated control of Colorado potato beetle (Leptinotarsa decemlineata, Say., Coleoptera: Chrysomelidae). J. Pest Sci. 2006, 79, 223–232. [Google Scholar] [CrossRef]
- Osman, M.A.M. Biological efficacy of some biorational and conventional insecticides in the control of different stages of the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Plant Protect. Sci. 2010, 46, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Bažok, R.; Ðurek, I.; Barčić, J.I.; Čuljak, T.G. Joint action of ecologically acceptable insecticides for the Colorado Potato Beetle (Leptinotarsa decemlineata Say, Coleoptera: Chrysomelidae) control. Fragm. Phytomed. Herbol. 2008, 30, 47–63. [Google Scholar]
- Kovaříková, K.; Pavela, R. United Forces of Botanical Oils: Efficacy of Neem and Karanja Oil against Colorado Potato Beetle under Laboratory Conditions. Plants 2019, 8, 608. [Google Scholar] [CrossRef] [Green Version]
- Trisyono, A.; Whalon, M.E. Toxicity of neem applied alone and in combinations with Bacillus thuringiensis to Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1999, 92, 1281–1288. [Google Scholar] [CrossRef]
- Maliszewska, J.; Tegowska, E. Capsaicin as an organophosphate synergist against Colorado potato beetle (Leptinotarsa decemlineata Say). J. Plant Prot. Res. 2012, 52, 28–34. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Hough-Goldstein, J.A. A survey of arthropod predators of Leptinotarsa decemlineata (Say) in Delaware potato fields. J. Agric. Entom. 1992, 9, 137–142. [Google Scholar]
- Ferro, D.N. Biological control of the Colorado potato beetle. APS 1994, 11, 357–375. [Google Scholar]
- Hilbeck, A.; Kennedy, G.G. Predators feeding on the Colorado potato beetle insecticide-free plots and insecticide-treated commercial potato fields in eastern North Carolina. Biol. Control. 1996, 6, 273–282. [Google Scholar] [CrossRef]
- Snyder, W.E. Give predators a complement: Conserving natural enemy biodiversity to improve biocontrol. Biol. Control 2019, 135, 73–82. [Google Scholar] [CrossRef]
- Radkova, M.; Kalushkov, P.; Chehlarov, E.; Gueorguiev, B.; Naumova, M.; Ljubomirov, T.; Stoichev, S.; Slavov, S.; Djilianov, D. Beneficial arthropod communities in commercial potato fields. Compt. Rend. Acad. Bulg. Sci. 2017, 70, 309–316. [Google Scholar]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Crowder, D.W.; Northfield, T.D.; Strand, M.R.; Snyder, W.E. Organic agriculture promotes evenness and natural pest control. Nature 2010, 466, 109–112. [Google Scholar] [CrossRef]
- Turnbull, L.A.; Hector, A. How to get even with pests. Nature 2010, 466, 36–37. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.M.; Hough-Goldstein, J.A.; Vangessel, M.J. Effects of straw mulch on pest insects, predators, and weeds in watermelons and potatoes. Environ. Entomol. 2004, 33, 1632–1643. [Google Scholar] [CrossRef]
- Dudás, P.; Gedeon, C.; Menyhárt, L.; Ambrus, G.; Tóth, F. The effect of mulching on the abundance and diversity of ground beetle assemblages in two hungarian potato fields. J. Environ. Agric. Sci. 2016, 3, 45–53. [Google Scholar] [CrossRef]
- Nelson, K.L.; Lynch, D.H.; Boiteau, G. Assessment of changes in soil health throughout organic potato rotation sequences. Agric. Ecosyst. Environ. 2009, 131, 220–228. [Google Scholar] [CrossRef]
- Werling, B.P.; Gratton, C. Influence of field margins and landscape context on ground beetle diversity in Wisconsin (USA) potato fields. Agric. Ecosyst. Environ. 2008, 128, 104–108. [Google Scholar] [CrossRef]
- Duan, J.J.; Head, G.; Jensen, A.; Reed, G. Effects of transgenic Bacillus thuringiensis potato and conventional insecticides for Colorado potato beetle (Coleoptera: Chrysomelidae) management on the abundance of ground-dwelling arthropods in Oregon potato ecosystems. Environ. Entomol. 2004, 33, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Meissle, M.; Lang, A. Comparing methods to evaluate the effects of Bt maize and insecticide on spider assemblages. Agric. Ecosyst. Environ. 2005, 107, 359–370. [Google Scholar] [CrossRef]
- Smith, J.; Wolfe, M.; Woodward, L.; Pearce, B.; Lampkin, N.; Marshall, H. Organic Farming and Biodiversity: A Review of the Literature; Organic Center Wales: Aberystwyth, Wales, 2011. [Google Scholar]
- Hald, A.B. Weed vegetation (wild flora) of long established organic versus conventional cereal fields in Denmark. Ann. Appl. Biol. 1999, 134, 307–314. [Google Scholar] [CrossRef]
- Krauss, J.; Gallenberger, I.; Steffan-Dewenter, I. Decreased functional diversity and biological pest control in conventional compared to organic crop fields. PLoS ONE 2011, 6, e19502. [Google Scholar] [CrossRef] [Green Version]
- Fuller, R.J.; Norton, L.R.; Feber, R.E.; Johnson, P.J.; Chamberlain, D.E.; Joys, A.C.; Mathews, F.; Stuart, R.C.; Townsend, M.C.; Manley, W.J.; et al. Benefits of organic farming to biodiversity vary among taxa. Biol. Lett. 2005, 1, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Macfadyen, S.; Gibson, R.; Polaszek, A.; Morris, R.J.; Craze, P.G.; Planqué, R.; Symondson, W.O.; Memmott, J. Do differences in food web structure between organic and conventional farms affect the ecosystem service of pest control? Ecol. Lett. 2009, 12, 229–238. [Google Scholar] [CrossRef]
- Geiger, F.; Hegemann, A.; Gleichman, M.; Flinks, H.; de Snoo, G.R.; Prinz, S.; Tieleman, B.I.; Berendse, F. Habitat use and diet of Skylarks (Alauda arvensis) wintering in an intensive agricultural landscape of the Netherlands. J. Ornithol. 2014, 155, 507–518. [Google Scholar] [CrossRef]
- Sugiyama, A.; Vivanco, J.M.; Jayanty, S.S.; Manter, D.K. Pyrosequencing assessment of soil microbial communities in organic and conventional potato farms. Plant Dis. 2010, 94, 1329–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusch, A.; Bommarco, R.; Ekbom, B. Conservation biological control in agricultural landscapes. In Advances in Botanical Research, 1st ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 81, pp. 333–360. [Google Scholar]
- Alfoeldi, T.; Fliessbach, A.; Geier, U.; Kilcher, L.; Niggli, U.; Pfiffner, L.; Stolze, M.; Willer, H. Organic agriculture and the environment. In Organic Agriculture, Environment and Food Security; El-Hage Scialabba, N., Hattam, C., Eds.; Environment and Natural Resources Series 4; Food and Agriculture Organisation of the United Nations (FAO): Rome, Italy, 2002. [Google Scholar]
- Patt, J.M.; Hamilton, G.C.; Lashomb, J.H. Impact of strip-insectary intercropping with flowers on conservation biological control of the Colorado potato beetle. Adv. Hortic. Sci. 1997, 175–181. [Google Scholar]
- Alyokhin, A.; Porter, G.; Groden, E.; Drummond, F. Colorado potato beetle response to soil amendments: A case in support of the mineral balance hypothesis? Agric. Ecosyst. Environ. 2005, 109, 234–244. [Google Scholar] [CrossRef]
- Sidauruk, L.; Sipayung, P. Cropping management on potato field, a strategy to suppress pest by increasing insect diversity and natural enemies. In Proceedings of the International Conference on Agribussines, Food and Agro-Technology, Medan, Indonesia, 19–21 September 2018; IOP Publishing: Bristol, UK, 2018; Volume 205, p. 012026. [Google Scholar]
- Dvorák, P.; Kuchtová, P.; Tomásek, J. Response of surface mulching of potato (Solanum tuberosum) on SPAD value, Colorado potato beetle and tuber yield. Int. J. Agric. Biol. 2013, 15, 798–800. [Google Scholar]
- Brust, G.E. Natural enemies in straw-mulch reduce Colorado potato beetle populations and damage in potato. Biol. Control 1994, 4, 163–169. [Google Scholar] [CrossRef]
- Suja, G.; Sundaresan, S.; John, K.S.; Sreekumar, J.; Misra, R.S. Higher yield, profit and soil quality from organic farming of elephant foot yam. Agron 2012, 32, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.M.; Campbell, K.G.; Lyon, S.R.; Jones, S.S. Evidence of varietal adaptation to organic farming systems. Field Crops Res. 2007, 102, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Alyokhin, A.; Mota-Sanchez, D.; Baker, M.; Snyder, W.E.; Menasha, S.; Whalon, M.; Dively, G.; Moarsi, W.F. The Red Queen in a potato field: Integrated pest management versus chemical dependency in Colorado potato beetle control. Pest Manag. Sci. 2015, 71, 343–356. [Google Scholar] [CrossRef]
- Azfar, S.; Nadeem, A.; Basit, A. Pest detection and control techniques using wireless sensor network: A review. J. Entomol. 2015, 3, 92–99. [Google Scholar]
- Shennan, C. Biotic interactions, ecological knowledge and agriculture. Philos. Trans. R. Soc. B 2008, 363, 717–739. [Google Scholar] [CrossRef] [Green Version]

| Alternative Method | Treatment Example [and Literature Reference Number] | Comments on Treatment Details nd/or Future Prospects |
|---|---|---|
| Crop rotation | Rotation and field distance [10,20,22] | effective when large field distances |
| Host plant resistance | RNA interference [26,27] | applied increasingly, powerful tool, but not for organic farming |
| Resistant potato varieties [28,29] | various results, no 100% protection | |
| Transgenic [10,20,23] | often used successfully, but not for organic farming | |
| Behavioral interference methods | 3,7-dimethyl-2-oxo-oct-6-ene-1,3-diol [31,32] | good potential, also in field studies |
| (Z)-3-hexenyl acetate (þ/–)-linalool [33,34] | good potential, also in field studies | |
| α-mangostin [10] | efficient, also against CPB oviposition | |
| Lactones [10] | efficient, against oviposition and larvae | |
| Limestone dust [34] | strong, especially against larval stages | |
| Limonin [10] | efficient, also against CPB oviposition | |
| Methyl salicylate [34,35] | good potential, also in field studies | |
| Quercus alba L. [10] | efficient, also against CPB oviposition | |
| Pheromone combinations [30] | very efficient, especially in the field | |
| Sesquiterpenes [10] | reduces CPB feeding and oviposition | |
| Terpenoids [10] | efficient, against oviposition and larvae | |
| Physical and mechanical control | Fire and burner [40,41,42] | higher mortality than most insecticides |
| Nets and trenches [45] | good but not very high efficiency in field | |
| Mechanical predator distributor [3,49] | efficient, but depends on predator species | |
| Pneumatic [37,39,43,44] | very efficient, but complicated in field | |
| Traps [46,47] | very high efficiency in field tests | |
| Wood ash [38] | highly efficient, but reduced with moisture | |
| Augmentative control | Chrysoperla carnea (Stephens) [58] | efficient vs. early larvae, field studies needed |
| Coleomegilla maculate Lengi [54,55] | efficient in many field studies since 1980s | |
| Edovum puttleri Grissell [58] | successful control, more field studies needed | |
| Lebia grandis Say [16,50] | strong in laboratory, more field studies needed | |
| Perillus bioculatus (F.) [4,48,49,50,51] | efficient control in laboratory and field studies | |
| P. bioculatus + Bacillus thuringienses Berliner (Bt) [52] | very successful | |
| Podisus maculiventris Say [4,49,53] | successful, not lower in field than laboratory | |
| Pterostichus melanarius (Illiger) [56] | very efficient vs. eggs and larvae | |
| Plant extracts and botanical insecticide | Acanthus dioscoridis L. [74,75] | protects potato leaves 1–2 days in field |
| Achillea millefolium L. [74,75] | protects potato leaves 1–2 days in field | |
| Aesculus hippocastanum L. [76] | successful in field studies | |
| Alnus glutinosa L. [76] | successful in field studies | |
| Arctium lappa L. [74] | efficient, especially mid-high doses | |
| Armoracia rusticana L. [19] | weak effects in field trials | |
| Artemisia absinthium L. [77] | successful in field studies | |
| Artemisia vulgaris L. [5,77] | efficient, also against CPB eggs | |
| Bifora radians (M.Bieb) [74,75] | efficient, especially mid-high doses | |
| Brassica napus L. [82] | efficient in laboratory, strong vs. adults | |
| Buxus sempervirens L. [76] | very successful in field studies | |
| Capsaicin extract [19] | more efficient in synergistic use | |
| Cymbopogon citrates Stapf [73] | successful in laboratory vs. larvae | |
| Eugenia caryophyllus (Sprengel) [18] | efficient vs. larvae, adults in laboratory | |
| Garlic extract [20] | weak effects in field trials | |
| Grapevine root extract [80] | toxic especially for larval development | |
| Hedera helix L. [5] | successful in laboratory studies | |
| Heracleum platytaenium Boiss [73] | strong, especially against larval stages | |
| Humulus lupulus L. [5,72,73] | causes very high CPB mortality | |
| Linum usitatissimum L. [19] | weak effects in field trials | |
| Liquidambar orientalis L. [76] | very successful in field studies | |
| Lolium temulentum L. [5] | successful in laboratory studies | |
| Mentha spicata L. [18] | efficient vs. larvae, adults in laboratory | |
| Myrtus communis L. [18] | efficient vs. larvae, adults in laboratory | |
| Neem seed extract [19] | successful in laboratory and field studies | |
| Ocimum basilicum L. [20] | efficient vs. larvae, adults in laboratory | |
| Origanum vulgare L. [14,72] | strong, also in low doses still vs. females | |
| Phlomoides tuberosa L. [75] | efficient in laboratory tests | |
| Pine extract [19] | weak effects in field trials | |
| Piper nigrum L. [78] | efficient, loses function under sunlight | |
| Piper tuberculatum L. [78] | efficient, especially vs. early larval stages | |
| Pyola [23] | successful, not for organic farming if contains GM oilseed rape oil | |
| Pyrethrin [86] | high efficacy in laboratory and field | |
| Rhus coriaria L. [76] | efficient also in field studies | |
| Rotenone [23,69] | efficient, but very toxic, also to mammals, not allowed in EU | |
| Rubia tinctoria L. [5] | efficient in laboratory studies | |
| Salvia officinalis L. [5] | successful in laboratory studies | |
| Sambucus nigra L. [5] | successful in laboratory studies | |
| Satureja hortensis L. [77] | effects on eggs, not CPB adults | |
| Satureja khuzistanica Jamzad [18] | very efficient against larval stages | |
| Slaked lime [84] | efficient against adults in laboratory studies | |
| Tagetes patula L. [19] | successful in laboratory and field studies | |
| Tanacetum vulgare L. [19] | successful in laboratory and field studies | |
| Thymus daenensis Celak [18] | efficient vs. larvae, adults in laboratory | |
| Thymus serpyllum L. [80] | strong vs. larvae in mid-high concentrations | |
| Urtica dioica L. [5] | successful in laboratory studies | |
| Verbascum songaricum Schrenk [5,74] | only vs. larvae with high concentrations | |
| Xanthium strumarium L. [5,74] | efficient, especially vs. early larval stages | |
| Entomopathogenic nematodes | Heterorhabditis bacteriophora Poinar [87,89,91] | good efficiency with high temperatures |
| Heterorhabditis indica Poinar [92] | very efficient in laboratory experiments | |
| Heterorhabditis marelatus Lui & Berry [92] | efficient in laboratory experiments | |
| Heterorhabditis megidis Poinar [91] | good efficiency with high temperatures | |
| Steinernema carpocapsae Weiser [89,91] | good efficiency with high temperatures | |
| Steinernema feltiae Filipjev [88,89,90,91] | good efficiency with high temperatures | |
| Steinernema glaseri Steiner [88] | successful vs. larvae in laboratory studies | |
| Steinernema oregonense Lui & Berry [92] | medium successful in laboratory tests | |
| Steinernema riobrave Cabanillas [92] | medium successful in laboratory tests | |
| Microbial insecticides | Bacillus thuringienses Berliner (Bt) [2,93,94] | very successful |
| Beauveria bassiana (Bals.-Criv.) Vuill. (1912) [94,95] | very successful | |
| Environmentally friendly insecticides, synergists and their combinations with synthetic insecticides | Azadirachtin [82,84] | lethal, but improvement needed |
| Capsaicin + Organophosphate [101] | widely used, successful combination | |
| Avermectin C [96] | especially successful vs. CPB adults | |
| Karanja oil + Azadirachtin [99] | promising, field studies needed | |
| Avermectin B1 + B1b [96] | strong, but less than actara, spinosad | |
| Azadirachtin + Bt [100] | successful, but not for organic farming | |
| Spinosad and combinations [17,96,97,98] | very successful synergistic effects | |
| Conservation biological control | Mulching (wheat or rye straw) [111] | weak, better combined with other methods |
| Natural enemy diversity [52,106,107,108,109,112,113] | very efficient, but complex system |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göldel, B.; Lemic, D.; Bažok, R. Alternatives to Synthetic Insecticides in the Control of the Colorado Potato Beetle (Leptinotarsa decemlineata Say) and Their Environmental Benefits. Agriculture 2020, 10, 611. https://doi.org/10.3390/agriculture10120611
Göldel B, Lemic D, Bažok R. Alternatives to Synthetic Insecticides in the Control of the Colorado Potato Beetle (Leptinotarsa decemlineata Say) and Their Environmental Benefits. Agriculture. 2020; 10(12):611. https://doi.org/10.3390/agriculture10120611
Chicago/Turabian StyleGöldel, Bastian, Darija Lemic, and Renata Bažok. 2020. "Alternatives to Synthetic Insecticides in the Control of the Colorado Potato Beetle (Leptinotarsa decemlineata Say) and Their Environmental Benefits" Agriculture 10, no. 12: 611. https://doi.org/10.3390/agriculture10120611
APA StyleGöldel, B., Lemic, D., & Bažok, R. (2020). Alternatives to Synthetic Insecticides in the Control of the Colorado Potato Beetle (Leptinotarsa decemlineata Say) and Their Environmental Benefits. Agriculture, 10(12), 611. https://doi.org/10.3390/agriculture10120611