Environmental Effects on Yield and Composition of Essential Oil in Wild Populations of Spike Lavender (Lavandula latifolia Medik.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetable Samples
2.2. Soils
2.3. Climatic Data
2.4. Analysis and Quantification of Chemical Parameters
2.5. Statistical Analysis
3. Results
3.1. EO Yield and Composition
3.2. Climate
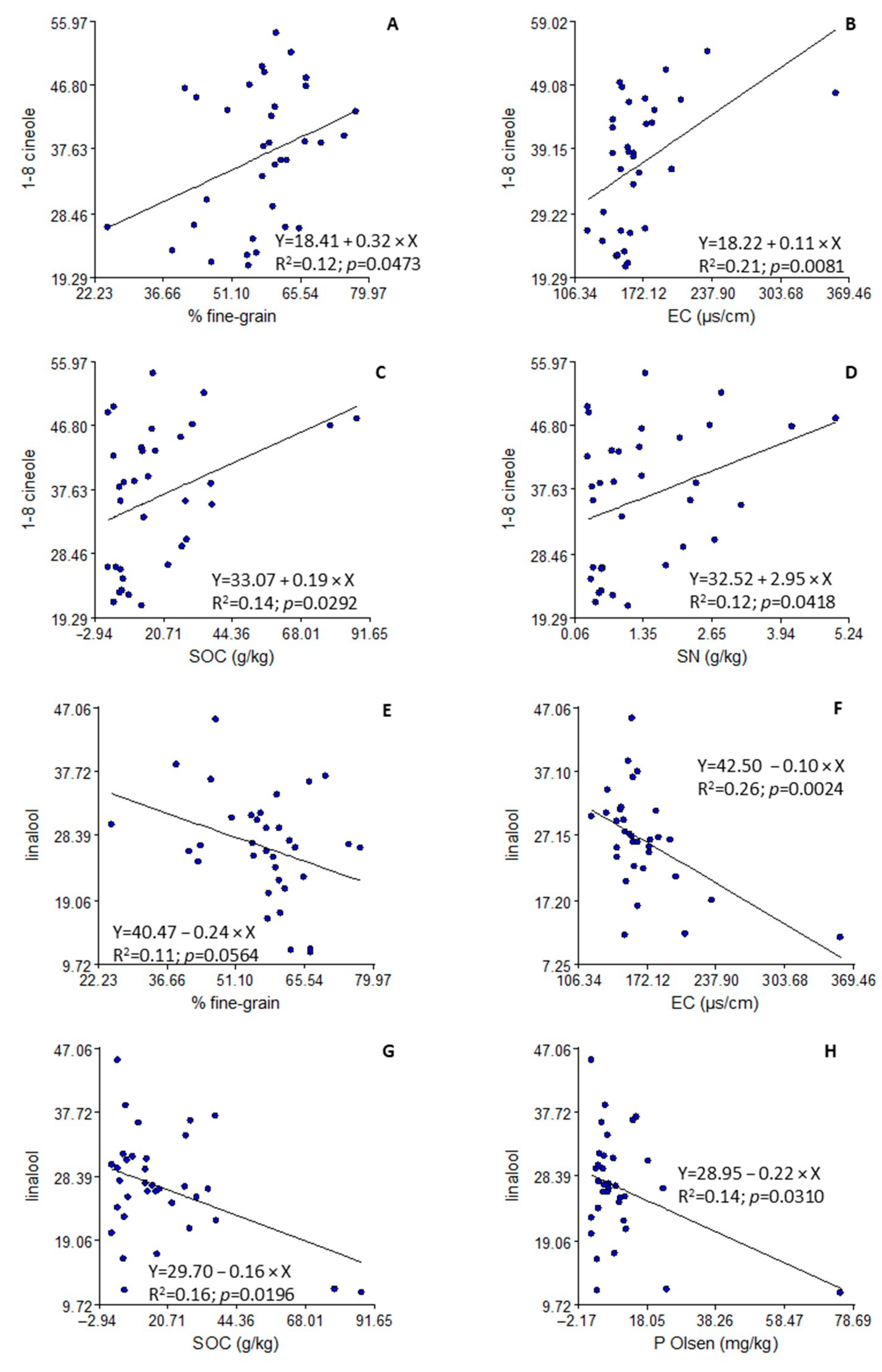
3.3. Edaphic Characteristics
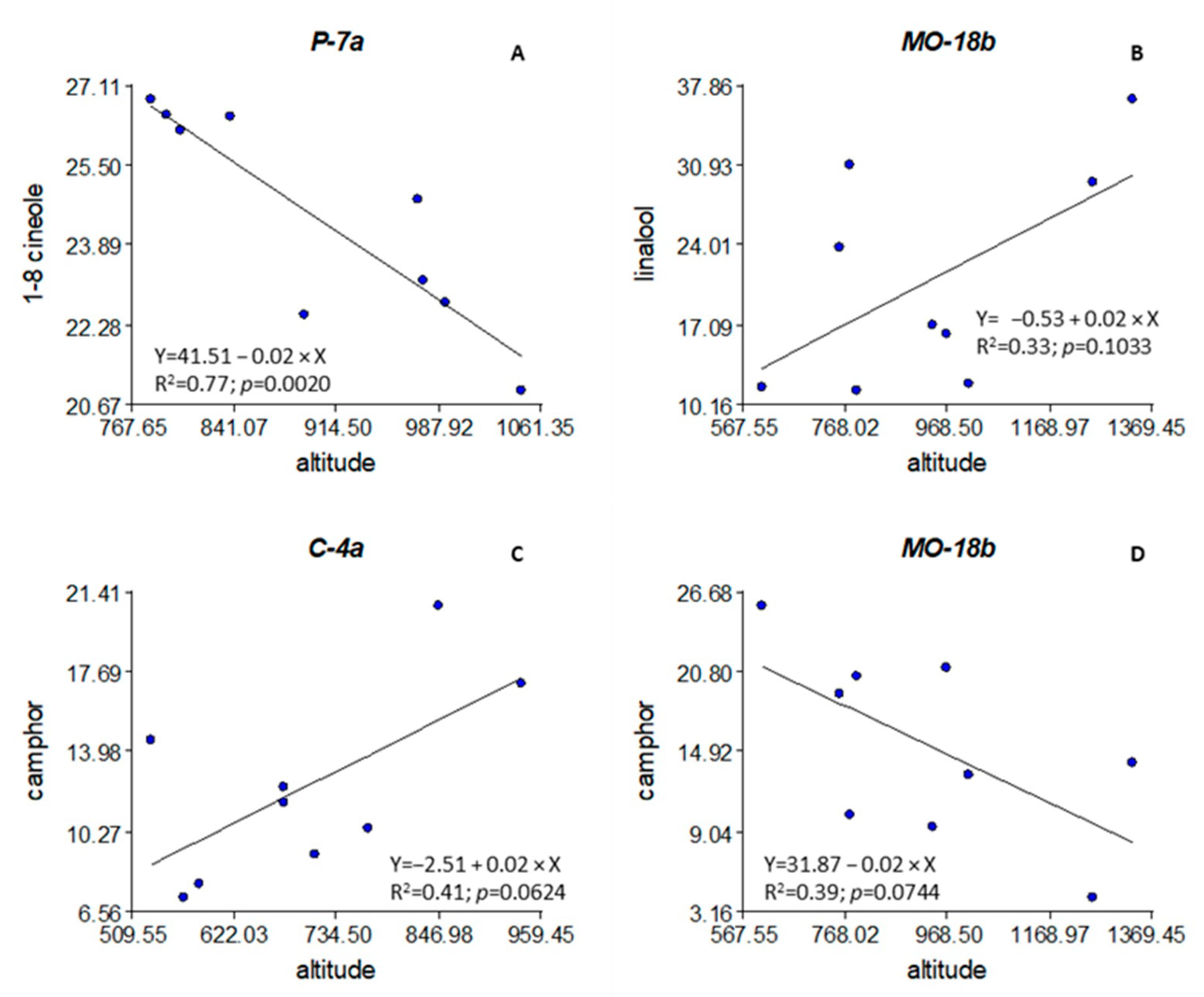
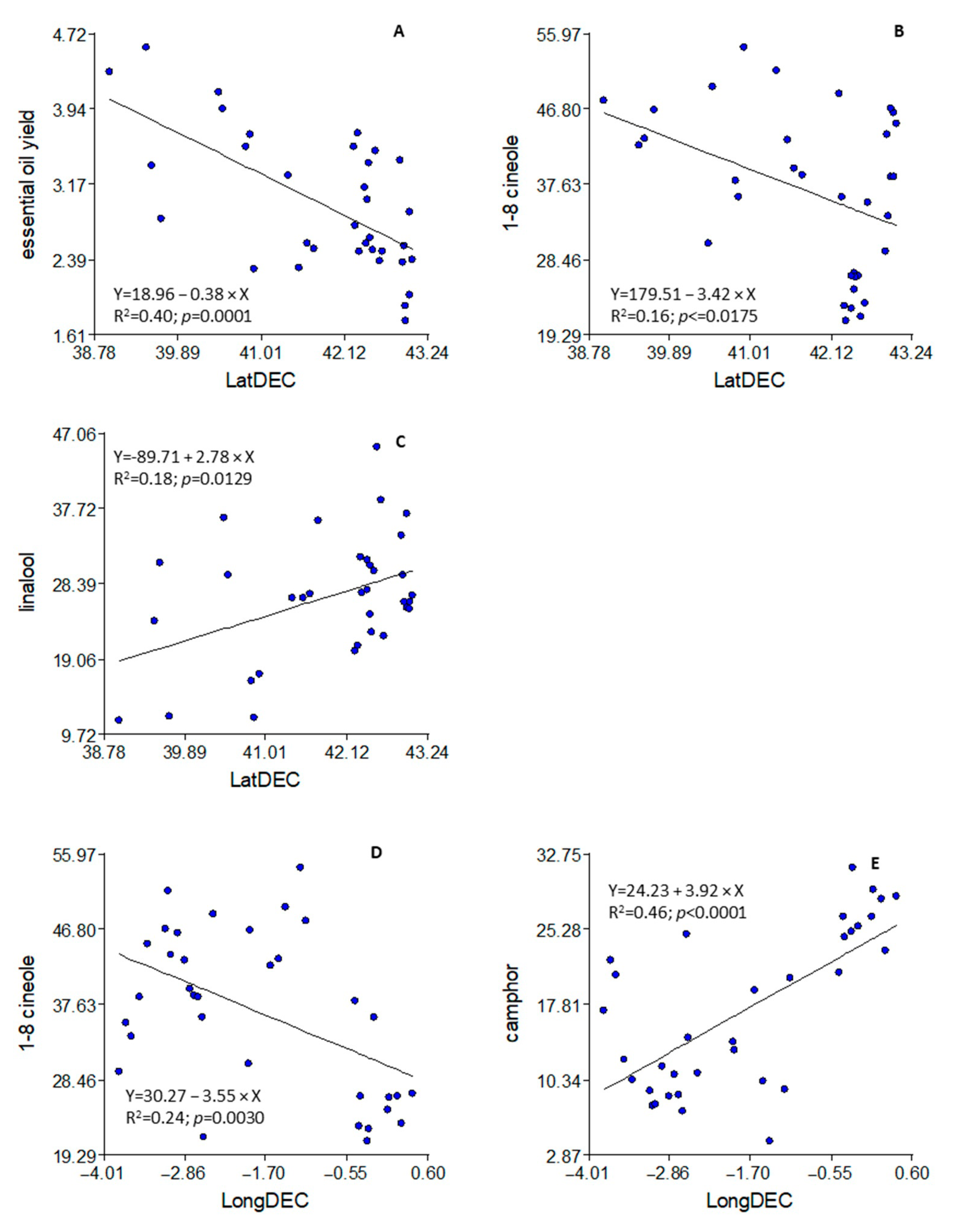
3.4. Altitude, Latitude, and Longitude of the Populations
3.5. Relationship between EO Yield Main Components
4. Discussion
4.1. EO Yield and Composition
4.2. Climate
4.3. Edaphic Characteristics
4.4. Altitude, Latitude, and Longitude of the Populations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morales, R. Lavandula. In Flora Iberica Volume 12; Morales, R., Quintanar, A., Cabezas, F., Pujadas, A.J., Cirujano, S., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 2010; pp. 484–496. [Google Scholar]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Agreste. La statistique, l’évaluation et la prospective du ministère de l’Agriculture et de l’Alimentation. Available online: https://agreste.agriculture.gouv.fr/agreste-web/disaron/SAANR_DEVELOPPE_2/detail/ (accessed on 8 December 2020).
- Burillo, J. Investigación y Experimentación de Plantas Aromáticas y Medicinales En Aragón. Cultivo, Transformación y Analítica; Burillo, J., Ed.; Gobierno de Aragón, Departamento de Agricultura, Dirección General de Tecnología Agraria: Zaragoza, Spain, 2003. [Google Scholar]
- Lis-Balchin, M.; Hart, S. Studies on the mode of action of the essential oil of Lavender (Lavandula angustifolia P. Miller). Phyther Res. 1999, 13, 540–542. [Google Scholar] [CrossRef]
- Haig, T.J.; Haig, T.J.; Seal, A.N.; Pratley, J.E.; An, M.; Wu, H. Lavender as a source of novel plant compounds for the development of a natural herbicide. J. Chem. Ecol. 2009, 35, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Santana, O.; Cabrera, R.; Giménez, C.; Sánchez-Vioque, R.; de los Mozos-Pascual, M.; Rodríguez-Conde, M.F.; Laserna-Ruiz, I.; Usano-Alemany, J. Perfil químico y biológico de aceites esenciales de plantas aromáticas de interés agro-industrial en Castilla-La Mancha (España). Grasas y Aceites 2012, 63, 214–222. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Herrero, B.; Pérez-Magariño, S.; Pereira, J.A.; Asensio-S-Manzanera, M.C. By-product of Lavandula latifolia essential oil distillation as source of antioxidants. J. Food Drug Anal. 2015, 23, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Quílez, M.; Ferreres, F.; López-Miranda, S.; Salazar, E.; Jordán, M.J. Seed oil from mediterranean aromatic and medicinal plants of the lamiaceae family as a source of bioactive components with nutritional. Antioxidants 2020, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Boelens, M.H. The essential oil of spike Lavender Lavandula latifolia Vill. (L. spica DC). Perfum. Flavorist 1986, 11, 43–63. [Google Scholar]
- García Vallejo, M.I. Aceites Esenciales De Las Lavandulas Ibericas Ensayo Ide La Quimiotaxonomi; Universidad Complutense de Madrid: Madrid, Spain, 1992; Available online: http://bibdigital.rjb.csic.es/PDF/García-Vallejo_Aceites_esenciales_Lavandulas_ibéricas_Tesis_2002.pdf (accessed on 20 March 2020).
- Lis-Balchin, M. Chemical composition of essential oils from different species, hybrids and cultivars of Lavandula. In Lavender; Taylor & Francis Inc.: New York, NY, USA, 2002; pp. 251–262. [Google Scholar]
- Muñoz-Bertomeu, J.; Arrillaga, I.; Segura, J. Essential oil variation within and among natural populations of Lavandula latifolia and its relation to their ecological areas. Biochem. Syst. Ecol. 2007, 35, 479–488. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phyther. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Kaloustian, J.; Pauli, A.M.; Pastor, J. Evolution of camphor and others components in the essential oils of two labiate species during the biological cycle. Analusis 2000, 28, 308–315. [Google Scholar] [CrossRef]
- Herraiz-Peñalver, D.; Cases, M.Á.; Varela, F.; Navarrete, P.; Sánchez-Vioque, R.; Usano-Alemany, J. Chemical characterization of Lavandula latifolia Medik. essential oil from Spanish wild populations. Biochem. Syst. Ecol. 2013, 46, 59–68. [Google Scholar] [CrossRef]
- Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A. Chemical composition and seasonal variations of spike lavander oil from southern Spain. J. Essent Oil Res. 2004, 16, 206–210. [Google Scholar] [CrossRef]
- Mapa. Ministerio de Agricultura, Pesca y Alimentación. Available online: https://www.mapa.gob.es/es/ (accessed on 8 December 2020).
- Rivas-Martínez, S.; Rivas-Saenz, S. Worldwide Bioclimatic Classification System; Phytosociological Research Center: Madrid, Spain, 2011. [Google Scholar]
- Cases, M.A.; Navarrete, P.; Calvo, R.; López-Ceper, P.; Pérez-Mao, D.; Varela, F. Recolección y caracterización química de espliego (Lavandula latifolia Medik.) en Las Alcarrias (Guadalajara y Cuenca). Primer paso de un programa de selección y mejora. IV Congr Mejor Genética Córdoba 2008, 51, 355–356. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis. Part. 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solid. In Methods of Soil Analysis. Part. 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an ascorbic acid method for determining phosphorous in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. J. 1965, 677–678. [Google Scholar] [CrossRef]
- Pratt, P.F. Availability indexes. Potassium. In Methods of Soil Analysis. Part. 2. American Society of Agronomy; Soil Science Society of America, Inc.: Madison, WI, USA, 1965; pp. 1027–1028. [Google Scholar]
- Bagnouls, F.; Gaussen, H. Saison seche el régime xerothermique, [Dry season and xerothermic regime]. Documents pour les Cartes des Production Vegetates, Sér. Géneralités. Toulouse 1953, 3, 193–239. [Google Scholar]
- Council of Europe. Eurpean Pharmacopoeia, 3rd ed.; Council of Europe: Strasbourg, France, 1996. [Google Scholar]
- International Organization for Standardization. ISO 4719: Oil of Spike Lavender [Lavandula Latifolia (L.f.) Medikus], Spanish Type; International Organization for Standardization: Vernier, Switzerland, 1999. [Google Scholar]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula angustifolia and Lavandula latifolia Essential Oils from Spain: Aromatic Profile and Bioactivities. Planta Med. 2015, 82, 163–170. [Google Scholar] [CrossRef]
- Guillen, M.D.; Cabo, N.; Burillo, J. Characterisation of the essential oils of some cultivated aromatic plants of industrial interest. J. Sci. Food Agric. 1996, 70, 359–363. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Laoutari, S.; Litskas, V.D.; Stavrinides, M.C.; Tzortzakis, N. Effects of water stress on lavender and sage biomass production, essential oil composition and biocidal properties against Tetranychus urticae (Koch). Sci. Hortic. 2016, 213, 96–103. [Google Scholar] [CrossRef]
- Usano-Alemany, J.; Herraiz-Peñalver, D.; Cuadrado Ortiz, J.; De López, B.B.; Ruiz, O.S.; Palá-Paúl, J. Ecological production of lavenders in Cuenca province (Spain). A study of yield production and quality of the essential oils. Bot. Complut. 2011, 35, 147–152. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Drouza, C.; Tzortzakis, N. Optimization of potassium fertilization/nutrition for growth, physiological development, essential oil composition and antioxidant activity of Lavandula angustifolia Mill. J. Soil Sci. Plant. Nutr. 2017, 17, 291–306. [Google Scholar] [CrossRef]

| Bioregion | Component | na | Min. | Mean | S.D. | Max. |
|---|---|---|---|---|---|---|
| All populations | essential oil yield | 34 | 1.75 | 2.95 | 0.71 | 4.58 |
| α-pinene | 34 | 0.85 | 1.73 | 0.53 | 3.13 | |
| camphene | 34 | 0.37 | 0.81 | 0.27 | 1.45 | |
| sabinene+β-pinene | 34 | 1.52 | 2.85 | 0.87 | 4.75 | |
| 1,8-cineole | 34 | 20.96 | 36.62 | 9.89 | 54.30 | |
| linalool | 34 | 11.42 | 26.74 | 7.74 | 45.36 | |
| camphor | 34 | 4.23 | 17.23 | 7.97 | 31.39 | |
| borneol | 34 | 1.05 | 2.36 | 1.00 | 5.97 | |
| α-terpineol | 34 | 0.38 | 0.88 | 0.21 | 1.39 | |
| Cantabroatlantic (C-4a) | essential oil yield | 9 | 1.75 | 2.52 b | 0.63 | 3.51 |
| 1,8-cineole | 9 | 21.51 | 38.20 a | 8.63 | 46.91 | |
| linalool | 9 | 25.29 | 30.69 a | 6.92 | 45.36 | |
| camphor | 9 | 7.23 | 12.34 b | 4.46 | 20.73 | |
| Prepyrenean (P-7a) | essential oil yield | 9 | 2.37 | 2.86 ab | 0.47 | 3.70 |
| 1,8-cineole | 9 | 20.96 | 24.46 b | 2.18 | 26.82 | |
| linalool | 9 | 22.43 | 29.39 ab | 4.73 | 38.81 | |
| camphor | 9 | 23.17 | 27.04 a | 2.58 | 31.39 | |
| Mediterranean Castillian (MC-18a) | essential oil yield | 7 | 2.30 | 2.77 b | 0.46 | 3.55 |
| 1,8-cineole | 7 | 35.45 | 41.84 a | 6.19 | 51.41 | |
| linalool | 7 | 20.01 | 25.60 ab | 5.58 | 36.28 | |
| camphor | 7 | 7.76 | 13.43 b | 7.04 | 24.83 | |
| Mediterranean Oroiberian (MO-18b) | essential oil yield | 9 | 2.28 | 3.63 a | 0.74 | 4.58 |
| 1,8-cineole | 9 | 30.40 | 43.13 a | 7.41 | 54.30 | |
| linalool | 9 | 11.42 | 21.03 b | 9.49 | 36.60 | |
| camphor | 9 | 4.23 | 15.28 b | 6.80 | 25.61 |
| Year | Component | na | Min. | Mean | S.D. | Max. |
|---|---|---|---|---|---|---|
| 2011 | essential oil yield | 33 | 0.83 | 2.77 b | 1.08 | 5.78 |
| 1,8-cineole | 34 | 13.05 | 32.89 b | 8.99 | 50.51 | |
| linalool | 31 | 0.23 | 21.17 b | 15.95 | 48.94 | |
| camphor | 31 | 4.17 | 20.07 a | 10.15 | 36.67 | |
| 2012 | essential oil yield | 34 | 0.97 | 2.06 c | 0.64 | 3.18 |
| 1,8-cineole | 34 | 14.08 | 42.04 a | 13.55 | 66.13 | |
| linalool | 34 | 3.03 | 24.36 b | 11.13 | 49.94 | |
| camphor | 34 | 4.94 | 17.11 ab | 7.83 | 31.97 | |
| 2013 | essential oil yield | 34 | 1.69 | 3.99 a | 1.30 | 6.80 |
| 1,8-cineole | 34 | 17.09 | 34.92 b | 10.67 | 55.03 | |
| linalool | 34 | 5.31 | 34.00 a | 10.18 | 54.08 | |
| camphor | 34 | 3.60 | 15.49 b | 7.65 | 31.82 |
| Year | Bioregion | TRI1 | TRI2 | TRI3 | TRI4 | Annual | * |
|---|---|---|---|---|---|---|---|
| 2011 | C-4a | 239.34 a | 197.07 a | 150.51 a | 119.63 b | 706.54 a | B |
| P-7a | 238.67 a | 157.18 ab | 162.07 a | 177.56 a | 735.47 a | B | |
| MC-18a | 134.01 b | 145.69 ab | 194.06 a | 89.48 bc | 563.24 ab | AB | |
| MO-18b | 128.92 b | 98.8 b | 178.94 a | 49.17 c | 455.84 b | B | |
| 2012 | C-4a | 180.36 a | 196.79 a | 200.73 a | 71.35 bc | 649.23 a | B |
| P-7a | 188.87 a | 40.33 b | 240.69 a | 131.22 a | 601.11 a | C | |
| MC-18a | 108.37 b | 66.15 b | 165.11 b | 76.91 bc | 416.55 b | B | |
| MO-18b | 110.99 b | 36.92 b | 107.67 c | 51.34 c | 306.92 b | B | |
| 2013 | C-4a | 218.28 b | 430.94 a | 281.3 a | 119.72 b | 1,050.25 a | A |
| P-7a | 296.47 a | 232.69 b | 288.2 a | 204.16 a | 1,021.51 a | A | |
| MC-18a | 162.02 bc | 173.29 bc | 257.01 a | 97.52 b | 689.85 b | A | |
| MO-18b | 197.07 c | 95.89 c | 206.58 a | 124.34 b | 623.87 b | A |
| CAT | pH | % Fine-Grain | EC | SOC | SN | K | P | Altitude |
|---|---|---|---|---|---|---|---|---|
| C-4a | ||||||||
| 558 | 8.12 | 46.89 | 157.7 | 3.46 | 0.460 | 158.85 | 1.51 | 530 |
| 559 | 7.56 | 69.92 | 163.4 | 37.09 | 2.360 | 418.97 | 14.95 | 566 |
| 560 | 7.45 | 41.21 | 158.7 | 16.42 | 1.321 | 108.96 | 6.41 | 676 |
| 561 | 7.45 | 60.19 | 143.0 | 13.12 | 1.271 | 391.81 | 5.13 | 584 |
| 562 | 8.04 | 54.91 | 173.7 | 30.56 | 2.615 | 321.50 | 11.32 | 710 |
| 563 | 7.95 | 43.55 | 183.0 | 26.65 | 2.041 | 378.44 | 8.83 | 769 |
| 564 | 8.25 | 58.90 | 143.1 | 7.09 | 0.535 | 86.92 | 10.11 | 676 |
| 565 | 8.04 | 57.62 | 163.4 | 13.94 | 0.958 | 235.93 | 4.99 | 848 |
| 566 | 7.99 | 59.75 | 134.0 | 27.15 | 2.105 | 301.09 | 6.23 | 939 |
| Mean | 7.87 a | 54.77 ab | 157.78 a | 19.50 a | 1.52 a | 266.94 a | 7.72 a | 699.78 b |
| S.D. | 0.30 | 9.22 | 16.62 | 11.37 | 0.79 | 125.15 | 4.01 | 134.79 |
| P-7a | ||||||||
| 530 | 7.81 | 38.51 | 154.3 | 6.34 | 0.555 | 68.38 | 5.55 | 977 |
| 531 | 7.60 | 24.85 | 118.3 | 1.36 | 0.584 | 28.54 | 3.48 | 838 |
| 534 | 7.63 | 43.23 | 174.6 | 22.20 | 1.788 | 102.24 | 9.69 | 781 |
| 535 | 7.46 | 65.38 | 159.9 | 5.83 | 0.550 | 90.91 | 1.57 | 802 |
| 536 | 8.33 | 55.55 | 133.3 | 6.76 | 0.370 | 139.15 | 18.43 | 973 |
| 537 | 7.52 | 54.56 | 155.2 | 12.96 | 1.066 | 61.12 | 6.83 | 1048 |
| 538 | 7.88 | 56.46 | 147.3 | 5.38 | 0.510 | 64.53 | 3.84 | 993 |
| 539 | 8.19 | 62.46 | 151.3 | 4.21 | 0.412 | 83.45 | 3.71 | 793 |
| 540 | 7.59 | 54.27 | 146.7 | 8.58 | 0.773 | 115.77 | 5.42 | 892 |
| Mean | 7.78 a | 50.59 b | 149.00 a | 8.18 a | 0.734 a | 83.79 b | 6.50 a | 899.67 ab |
| S.D. | 0.30 | 12.80 | 16.00 | 6.13 | 0.447 | 32.89 | 5.04 | 100.58 |
| MC-18a | ||||||||
| 552 | 7.52 | 63.76 | 194.0 | 34.54 | 2.830 | 45.63 | 22.66 | 1133 |
| 553 | 7.75 | 77.35 | 174.9 | 17.72 | 0.884 | 36.30 | 6.83 | 940 |
| 554 | 7.87 | 74.80 | 157.5 | 15.27 | 1.335 | 40.96 | 5.40 | 1008 |
| 555 | 7.75 | 66.51 | 158.9 | 10.69 | 0.794 | 24.69 | 4.76 | 1130 |
| 556 | 7.85 | 58.16 | 152.2 | 1.42 | 0.314 | 35.04 | 1.58 | 835 |
| 557 | 7.59 | 61.46 | 199.6 | 28.04 | 2.245 | 186.34 | 11.90 | 879 |
| 567 | 8.10 | 60.21 | 168.4 | 37.26 | 3.215 | 323.31 | 11.11 | 1056 |
| Mean | 7.78 a | 66.04 a | 172.21 a | 20.70 a | 1.66 a | 98.90 ab | 9.18 a | 997.29 a |
| S.D. | 0.19 | 7.38 | 16.44 | 13.10 | 1.11 | 113.80 | 6.95 | 117.76 |
| MO-18b | ||||||||
| 529 | 8.00 | 57.63 | 149.9 | 3.37 | 0.292 | 126.13 | 2.86 | 1255 |
| 544 | 8.42 | 62.58 | 151.0 | 5.70 | 0.417 | 40.87 | 3.42 | 604 |
| 545 | 8.17 | 57.91 | 162.9 | 5.45 | 0.384 | 153.03 | 3.42 | 968 |
| 546 | 7.20 | 60.46 | 234.0 | 17.14 | 1.384 | 154.64 | 8.53 | 941 |
| 547 | 7.65 | 66.78 | 208.0 | 77.98 | 4.164 | 687.71 | 23.66 | 1012 |
| 548 | 8.08 | 50.20 | 181.2 | 13.57 | 0.766 | 144.84 | 7.98 | 777 |
| 549 | 7.82 | 59.54 | 143.2 | 3.32 | 0.298 | 129.83 | 3.71 | 757 |
| 551 | 7.73 | 66.85 | 357.5 | 87.35 | 5.004 | 353.52 | 75.01 | 790 |
| 568 | 7.30 | 45.90 | 631.5 | 28.60 | 2.711 | 417.79 | 13.95 | 1333 |
| Mean | 7.82 a | 58.65 ab | 246.58 a | 26.94 a | 1.71 a | 245.37 ab | 15.84 a | 937.44 a |
| S.D. | 0.40 | 6.97 | 159.12 | 32.71 | 1.81 | 204.13 | 23.20 | 238.49 |
| CAT | Bioregion | Altitude | Essential Oil Yield | 1,8-Cineol | Linalool | Camphor |
|---|---|---|---|---|---|---|
| 558 | C-4a | 530 | 3.51 | 21.51 | 45.36 | 14.52 |
| 559 | 566 | 1.75 | 38.53 | 37.15 | 7.23 | |
| 560 | 676 | 2.01 | 46.36 | 26.19 | 11.60 | |
| 561 | 584 | 2.35 | 43.69 | 29.43 | 7.85 | |
| 562 | 710 | 1.90 | 46.91 | 25.42 | 9.24 | |
| 563 | 769 | 2.38 | 45.09 | 26.89 | 10.37 | |
| 564 | 676 | 2.87 | 38.57 | 25.29 | 12.33 | |
| 565 | 848 | 2.52 | 33.69 | 26.17 | 20.73 | |
| 566 | 939 | 3.41 | 29.41 | 34.31 | 17.18 | |
| Mean | 699.78 b | 2.52 b | 38.20 a | 30.69 a | 12.34 b | |
| S.D. | 134.79 | 0.63 | 8.63 | 6.92 | 4.46 | |
| 530 | P-7a | 977 | 2.37 | 23.16 | 38.81 | 23.17 |
| 531 | 838 | 2.48 | 26.49 | 30.05 | 28.25 | |
| 534 | 781 | 3.38 | 26.82 | 24.54 | 28.61 | |
| 535 | 802 | 2.61 | 26.20 | 22.43 | 29.25 | |
| 536 | 973 | 3.01 | 24.81 | 30.75 | 26.51 | |
| 537 | 1048 | 2.47 | 20.96 | 27.35 | 25.08 | |
| 538 | 993 | 3.70 | 22.74 | 31.65 | 26.58 | |
| 539 | 793 | 3.13 | 26.51 | 27.70 | 24.54 | |
| 540 | 892 | 2.55 | 22.47 | 31.27 | 31.39 | |
| Mean | 899.67 ab | 2.86 b | 24.46 b | 29.39 ab | 27.04 a | |
| S.D. | 100.58 | 0.47 | 2.18 | 4.73 | 2.58 | |
| 552 | MC-18a | 1133 | 3.26 | 51.41 | 26.59 | 7.76 |
| 553 | 940 | 2.30 | 43.07 | 26.56 | 8.64 | |
| 554 | 1008 | 2.55 | 39.51 | 27.07 | 10.87 | |
| 555 | 1130 | 2.50 | 38.78 | 36.28 | 8.76 | |
| 556 | 835 | 3.55 | 48.70 | 20.01 | 10.93 | |
| 557 | 879 | 2.74 | 35.98 | 20.72 | 24.83 | |
| 567 | 1056 | 2.47 | 35.45 | 21.94 | 22.25 | |
| Mean | 997.29 a | 2.77 b | 41.84 a | 25.60 ab | 13.43 b | |
| S.D. | 117.76 | 0.46 | 6.19 | 5.58 | 7.04 | |
| 529 | MO-18b | 1255 | 3.94 | 49.46 | 29.53 | 4.23 |
| 544 | 604 | 3.68 | 35.99 | 11.69 | 25.61 | |
| 545 | 968 | 3.55 | 37.98 | 16.32 | 21.04 | |
| 546 | 941 | 2.28 | 54.30 | 17.10 | 9.39 | |
| 547 | 1012 | 2.80 | 46.72 | 11.89 | 13.23 | |
| 548 | 777 | 3.35 | 43.12 | 30.95 | 10.22 | |
| 549 | 757 | 4.58 | 42.41 | 23.76 | 19.19 | |
| 551 | 790 | 4.33 | 47.82 | 11.42 | 20.44 | |
| 568 | 1333 | 4.11 | 30.40 | 36.60 | 14.15 | |
| Mean | 937.44 a | 3.63 a | 43.13 a | 21.03 b | 15.28 b | |
| S.D. | 238.49 | 0.74 | 7.41 | 9.49 | 6.80 |
| Essential Oil Yield | 1,8-Cineole | Linalool | Camphor | |
|---|---|---|---|---|
| essential oil yield | 1.00 | |||
| 1,8-cineole | −0.02 | 1.00 | ||
| linalool | −0.15 | −0.48 ** | 1.00 | |
| camphor | 0.15 | −0.76 ** | −0.13 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Sestelo, M.; Carrillo, J.M. Environmental Effects on Yield and Composition of Essential Oil in Wild Populations of Spike Lavender (Lavandula latifolia Medik.). Agriculture 2020, 10, 626. https://doi.org/10.3390/agriculture10120626
Fernández-Sestelo M, Carrillo JM. Environmental Effects on Yield and Composition of Essential Oil in Wild Populations of Spike Lavender (Lavandula latifolia Medik.). Agriculture. 2020; 10(12):626. https://doi.org/10.3390/agriculture10120626
Chicago/Turabian StyleFernández-Sestelo, Montserrat, and José M. Carrillo. 2020. "Environmental Effects on Yield and Composition of Essential Oil in Wild Populations of Spike Lavender (Lavandula latifolia Medik.)" Agriculture 10, no. 12: 626. https://doi.org/10.3390/agriculture10120626
APA StyleFernández-Sestelo, M., & Carrillo, J. M. (2020). Environmental Effects on Yield and Composition of Essential Oil in Wild Populations of Spike Lavender (Lavandula latifolia Medik.). Agriculture, 10(12), 626. https://doi.org/10.3390/agriculture10120626




