Impact of Cover Crops on Insect Community Dynamics in Organic Farming
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Cover Crop Treatments and Experimental Design
2.3. Planting and Termination
2.4. Insect Community Collection
2.5. Cash Crop Planting & Pest Damage
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Herbivores | Order | Family |
|---|---|---|
| White fly (Bemisia argentifolii [Bellows & Perring]) | Hemiptera | Aleyrodidae |
| Leaf hopper (Empoasca fabae (Harris]) | Hemiptera | Cicadellidae |
| Lygus bugs (Lygus lineolaris) | Hemiptera | Miridae |
| Flea beetle (Chaetocnema hortensis) | Coleoptera | Chrysomelidae |
| Armyworm (Pseudaletia unipuncta [Haworth]) | Lepidoptera | Noctuidae |
| Looper (Trichoplusia ni [Hübner]) | Lepidoptera | Noctuidae |
| Predators | ||
| Ladybug beetle (Hippodamia convergens [Guérin-Méneville]) | Coleoptera | Coccinellidae |
| Minute pirate bug (Orius sp.) | Hemiptera | Anthocoridae |
| Big eyed bug (Geocoris sp.) | Hemiptera | Geocoridae |
| Spiders (multiple species) | Araneae | |
| Green lacewing (Chrysoperla sp.) | Neuroptera | Chrysopidae |
| Damsel bug (Nabis sp.) | Hemiptera | Nabidae |
| Assassin bug (Zelus sp.) | Hemiptera | Reduviidae |
| Parasitoids | ||
| Parasitoid Wasps (Cotesia sp.) | Hymenoptera | Braconidae |
References
- Bale, J.; Van Lenteren, J.C.; Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.; Blaauw, B.R.; Isaacs, R. Manipulation of natural enemies in agroecosystems: Habitat and semiochemicals for sustainable insect pest control. In Integrated Pest Management and Pest Control—Current and Future Tactics, 1st ed.; Larramendy, M.L., Soloneski, S., Eds.; InTech: Rijeka, Croatia, 2012; pp. 89–126. [Google Scholar]
- Kariyat, R.; Mauck, K.E.; De Moraes, C.M.; Stephenson, A.G.; Mescher, M.C. Inbreeding alters volatile signalling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.). Ecol. Lett. 2012, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Reicosky, D.C.; Forcella, F. Cover crop and soil quality interactions in agroecosystems. J. Soil Water Conserv. 1998, 53, 224–229. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Bailey, B.A. Role of cover crops in improving soil and row crop productivity. Commun. Soil Sci. Plant Anal. 2005, 36, 2733–2757. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Watkins, K.B.; Teasdale, J.R.; Abdul-Baki, A.A. Cover crops in sustainable food production. Food Rev. Int. 2000, 16, 121–157. [Google Scholar] [CrossRef]
- Bugg, R.L.; Wäckers, F.L.; Brunson, K.E.; Dutcher, J.D.; Phatak, S.C. Cool-season cover crops relay intercropped with cantaloupe: Influence on a generalist predator, geocoris punctipes (Hemiptera: Lygaeidae). J. Econ. Èntomol. 1991, 84, 408–416. [Google Scholar] [CrossRef]
- Bugg, R.L.; Waddington, C. Using cover crops to manage arthropod pests of orchards: A review. Agric. Ecosyst. Environ. 1994, 50, 11–28. [Google Scholar] [CrossRef]
- Creamer, N.G.; Baldwin, K.R. An evaluation of summer cover crops for use in vegetable production systems in North Carolina. HortScience 2000, 35, 600–603. [Google Scholar] [CrossRef]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Fergen, J.K. The effects of a winter cover crop on diabrotica virgifera (Coleoptera: Chrysomelidae) populations and beneficial arthropod communities in No-Till maize. Environ. Èntomol. 2010, 39, 1816–1828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicholls, C.I.; Parrella, M.; Altieri, M.A. The effects of a vegetational corridor on the abundance and dispersal of insect biodiversity within a northern California organic vineyard. Landsc. Ecol. 2001, 16, 133–146. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36. [Google Scholar] [CrossRef]
- Long, R.F.; Lamb, C.; Reberg-Horton, S.C.; Chandler, J.; Stimmann, M.; Corbett, A. Beneficial insects move from flowering plants to nearby crops. Calif. Agric. 1998, 52, 23–26. [Google Scholar] [CrossRef]
- Hoffman, M.L.; Weston, L.A.; Snyder, J.C.; Regnier, E.E. Allelopathic influence of germinating seeds and seedlings of cover crops on weed species. Weed Sci. 1996, 44, 579–584. [Google Scholar] [CrossRef]
- Asmah, S.; Ghazali, A.; Syafiq, M.; Yahya, M.S.; Peng, T.L.; Norhisham, A.R.; Puan, C.L.; Lindenmayer, D.; Azhar, B. Effects of polyculture and monoculture farming in oil palm smallholdings on tropical fruit-feeding butterfly diversity. Agric. For. Èntomol. 2016, 19, 70–80. [Google Scholar] [CrossRef]
- Stamps, W.T.; Linit, M.J. Plant diversity and arthropod communities: Implications for temperate agroforestry. Agrofor. Syst. 1997, 39, 73–89. [Google Scholar] [CrossRef]
- E Crews, T.; Carton, W.; Olsson, L. Is the future of agriculture perennial? Imperatives and opportunities to reinvent agriculture by shifting from annual monocultures to perennial polycultures. Glob. Sustain. 2018, 1. [Google Scholar] [CrossRef]
- Costello, M. Abundance, growth rate and parasitism of Brevicoryne brassicae and Myzus persicae (Homoptera: Aphididae) on broccoli grown in living mulches. Agric. Ecosyst. Environ. 1995, 52, 187–196. [Google Scholar] [CrossRef]
- Bryant, A.; Brainard, D.C.; Haramoto, E.R.; Szendrei, Z. Cover crop mulch and weed management influence arthropod communities in strip-tilled cabbage. Environ. Èntomol. 2013, 42, 293–306. [Google Scholar] [CrossRef]
- Legrand, A.; Barbosa, P. Plant morphological complexity impacts foraging efficiency of adult coccinella septempunctata L. (Coleoptera: Coccinellidae). Environ. Èntomol. 2003, 32, 1219–1226. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, M. Using cover crops to mitigate and adapt to climate change. A review. Agron. Sustain. Dev. 2017, 37. [Google Scholar] [CrossRef]
- Kariyat, R.; Hardison, S.; Ryan, A.B.; Stephenson, A.G.; De Moraes, C.M.; Mescher, M.C. Leaf trichomes affect caterpillar feeding in an instar-specific manner. Commun. Integr. Biol. 2018, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Chavana, J.; Soti, P.; Racelis, A.; Kariyat, R. Arbuscular mycorrhizal fungi (AMF) influences growth and insect community dynamics in Sorghum-sudangrass (Sorghum × drummondii). Arthropod-Plant Interact. 2020, 14, 301–315. [Google Scholar] [CrossRef]
- Pare, P.W. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef]
- Kariyat, R.; Scanlon, S.R.; Moraski, R.P.; Stephenson, A.G.; Mescher, M.C.; De Moraes, C.M. Plant inbreeding and prior herbivory influence the attraction of caterpillars (Manduca sexta) to odors of the host plant Solanum carolinense (Solanaceae). Am. J. Bot. 2014, 101, 376–380. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Cover Cropping for Pollinators and Beneficial Insects. Available online: https://www.sare.org/Learning-Center/Bulletins/Cover-Cropping-for-Pollinators-and-Beneficial-Insects/Text-Version (accessed on 30 March 2020).
- Grasela, J.J.; McIntosh, A.H.; Shelby, K.S.; Long, S. Isolation and characterization of a baculovirus associated with the insect parasitoid wasp, cotesiamarginiventris, or its host, Trichoplusia ni. J. Insect Sci. 2008, 8, 1–19. [Google Scholar] [CrossRef]
- Capinera, J.L. Armyworm, Pseudaletia Unipuncta (Haworth) (Insecta: Lepidoptera: Noctuidae); University of Florida: Gainesville, FL, USA, 2012; Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.512.757&rep=rep1&type=pdf (accessed on 29 March 2020).
- Legaspi, J.; Nordlund, D.; Legaspi, B. Tri-trophic interactions and predation rates in Chrysoperla spp. Attacking the silverleaf whitefly. Southwest. Entomol. 1996, 21, 33–42. [Google Scholar]
- Inbar, M.; Gerling, D. Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annu. Rev. Èntomol. 2008, 53, 431–448. [Google Scholar] [CrossRef]
- Mansion-Vaquié, A.; Ferrer, A.; Ramon-Portugal, F.; Wezel, A.; Magro, A. Intercropping impacts the host location behaviour and population growth of aphids. Èntomol. Exp. Appl. 2019, 168, 41–52. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Herbivore induced plant volatiles: Their role in plant defense for pest management. Plant Signal. Behav. 2011, 6, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Himanen, S.J.; Bui, T.N.T.; Maja, M.M.; Holopainen, J.K. Utilizing associational resistance for biocontrol: Impacted by temperature, supported by indirect defence. BMC Ecol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Leslie, A.W.; Hamby, K.A.; McCluen, S.R.; Hooks, C.R.R. Evaluating a Push-Pull Tactic for Management of Epilachna Varivestis Mulsant and Enhancement of Beneficial Arthropods in Phaseolus lunatus L. Available online: https://www.sciencedirect.com/science/article/pii/S0925857419303842 (accessed on 1 April 2020).
- Ninkovic, V.; Dahlin, I.; Vucetic, A.; Petrovič-Obradovič, O.; Glinwood, R.; Webster, B. Volatile exchange between undamaged plants—A new mechanism affecting insect orientation in intercropping. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Mansoer, Z.; Reeves, D.; Wood, C.W. Suitability of sunn hemp as an alternative late-summer legume cover crop. Soil Sci. Soc. Am. J. 1997, 61, 246–253. [Google Scholar] [CrossRef]
- Taiwo, M.A.; Akinjogunla, O.J. Cowpea viruses: Quantitative and qualitative effects of single and mixed viral infections. Afr. J. Biotechnol. 2006, 5, 1749–1756. [Google Scholar]
- Hickman, P.L. Cover Crops as an Integrated Approach for Pest Suppression and Pollinator Promotion in Arkansas. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2019. [Google Scholar]
- Khan, Z.R.; Midega, C.A.O.; Bruce, T.J.A.; Hooper, A.; Pickett, J. Exploiting phytochemicals for developing a ‘push-pull’ crop protection strategy for cereal farmers in Africa. J. Exp. Bot. 2010, 61, 4185–4196. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Jeevanandam, J.; Egbuna, C.; Merghany, R.M.; Akram, M.; Daniyal, M.; Nisar, J.; Sharif, A. Semiochemicals: A green approach to pest and disease control. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 81–89. [Google Scholar]
- Soti, P.G.; Racelis, A. Cover crops for weed suppression in organic vegetable systems in semiarid subtropical Texas. Org. Agric. 2020, 1–8. [Google Scholar] [CrossRef]
- Murrell, E.G.; Ray, S.; Lemmon, M.E.; Luthe, D.; Kaye, J.P. Cover crop species affect mycorrhizae-mediated nutrient uptake and pest resistance in maize. Renew. Agric. Food Syst. 2019, 1–8. [Google Scholar] [CrossRef]
- Bewick, T.A.; Shilling, D.G.; Dusky, J.A.; Williams, D. Effects of celery (apium graveolens) root residue on growth of various crops and weeds. Weed Technol. 1994, 8, 625–629. [Google Scholar] [CrossRef]
- Saha, B.; Devi, C.; Khwairakpam, M.; Kalamdhad, A.S. Vermicomposting and anaerobic digestion-viable alternative options for terrestrial weed management—A review. Biotechnol. Rep. 2018, 17, 70–76. [Google Scholar] [CrossRef] [PubMed]
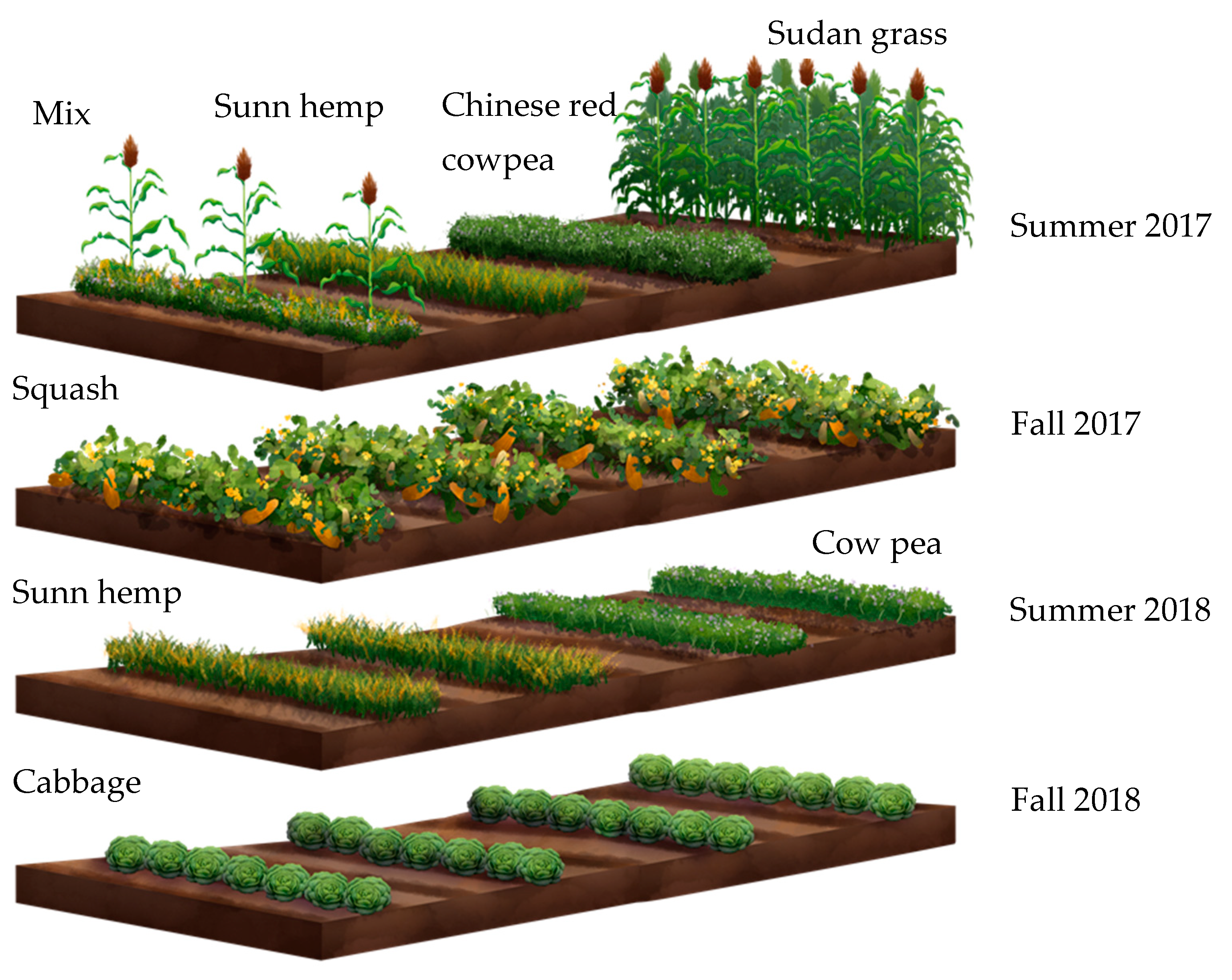








| Cover Crop Treatment | Crop Type | Seeding Rate (kg/ha) |
|---|---|---|
| Sudangrass (SG) | Grass | 45 |
| Cowpea (CP) | Legume | 28 |
| Sunn hemp (SH) | Legume | 45 |
| Mix (Sudangrass + Cowpea + Sunn hemp) | - | 16 + 10 + 16 |
| Control (C) | - | - |
| Year | Season | Crop | Frequency | Data Collected |
|---|---|---|---|---|
| 1 | Summer | Cover crops: CP, SH, SG, Mix | Early and late in season | Herbivores and Beneficial insects |
| Fall | Cash crop: Squash | Early and late in season | Herbivore damage | |
| 2 | Summer | Cover crops: CP and SH | Early and late in season | Plant height, Herbivore damage, Biomass |
| Fall | Cash crop: Cabbage | Early and late in season | Herbivores and Beneficial insects |
| Time | Trait | Test Statistic | P Value |
|---|---|---|---|
| 2017 Cover crop | Total insects (Time) | F = 0.53, df = 2 | 0.598 |
| Total insects (Crop) | F = 1.41, df = 4 | 0.286 | |
| Beneficial insects (Time) | F = 4.98, df = 2 | 0.025 | |
| Beneficial insects (Crop) | F = 1.13, df = 4 | 0.387 | |
| Herbivores (Time) | F = 1.83, df = 2 | 0.199 | |
| Herbivores (Crop) | F = 3.93, df= 4 | 0.026 | |
| 2017 Cash crop | Damage (Early season) | F = 4.36, df = 4 | 0.015 |
| Damage (Mid season) | F = 1.58, df = 4 | 0.230 | |
| Damage (Late season) | F = 0.26, df = 4 | 0.896 | |
| 2018 Cover crop | Height (Time) | F =117.51, df =1 | 0.000 |
| Height (Crop) | F =617.74, df = 1 | 0.000 | |
| Height (Time* Crop) | F =53.73, df =1 | 0.000 | |
| Damage (Crop) | F = 0.09, df = 1 | 0.762 | |
| Damage (Time* Crop) | F = 225.83, df = 1 | 0.000 | |
| Biomass | t = 3.96, df = 31 | 0.000 | |
| 2018 Cash crop | Biomass | F = 4.6, df = 2 | 0.013 |
| Total insects (Time) | F = 0.37, df = 1 | 0.548 | |
| Total insects (Cover crop) | F = 8.59, df = 2 | 0.002 | |
| Cash crop | F =1.14, df = 2 | 0.337 | |
| Beneficial’s (Time) | F = 0.74, df = 1 | 0.397 | |
| Cover crop | F = 2.6, df = 2 | 0.095 | |
| Cash crop | F = 0.31, df = 2 | 0.735 | |
| Herbivores (Time) | F = 0.92, df = 1 | 0.348 | |
| Herbivores (Cover crops) | F = 9.17, df = 2 | 0.001 | |
| Herbivores (Cash crop) | F = 1.98, df = 2 | 0.160 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, L.; Soti, P.; Kaur, J.; Racelis, A.; Kariyat, R.R. Impact of Cover Crops on Insect Community Dynamics in Organic Farming. Agriculture 2020, 10, 209. https://doi.org/10.3390/agriculture10060209
Martinez L, Soti P, Kaur J, Racelis A, Kariyat RR. Impact of Cover Crops on Insect Community Dynamics in Organic Farming. Agriculture. 2020; 10(6):209. https://doi.org/10.3390/agriculture10060209
Chicago/Turabian StyleMartinez, Lili, Pushpa Soti, Jasleen Kaur, Alexis Racelis, and Rupesh R. Kariyat. 2020. "Impact of Cover Crops on Insect Community Dynamics in Organic Farming" Agriculture 10, no. 6: 209. https://doi.org/10.3390/agriculture10060209
APA StyleMartinez, L., Soti, P., Kaur, J., Racelis, A., & Kariyat, R. R. (2020). Impact of Cover Crops on Insect Community Dynamics in Organic Farming. Agriculture, 10(6), 209. https://doi.org/10.3390/agriculture10060209





