Weed Infestation and Health of the Soybean Crop Depending on Cropping System and Tillage System
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Experiment and Soil and Climatic Conditions
2.2. Experimental Design and Agronomic Practices
- I.
- CS—cropping system: CR—soybean grown in crop rotation after winter wheat (crop rotation: soybean—winter wheat—winter oilseed rape—winter wheat), CM—soybean monoculture cropping.
- II.
- TS—tillage system: CT—conventional tillage, NT—no-tillage.
2.3. Scope of Study and Statistical Analysis
3. Results
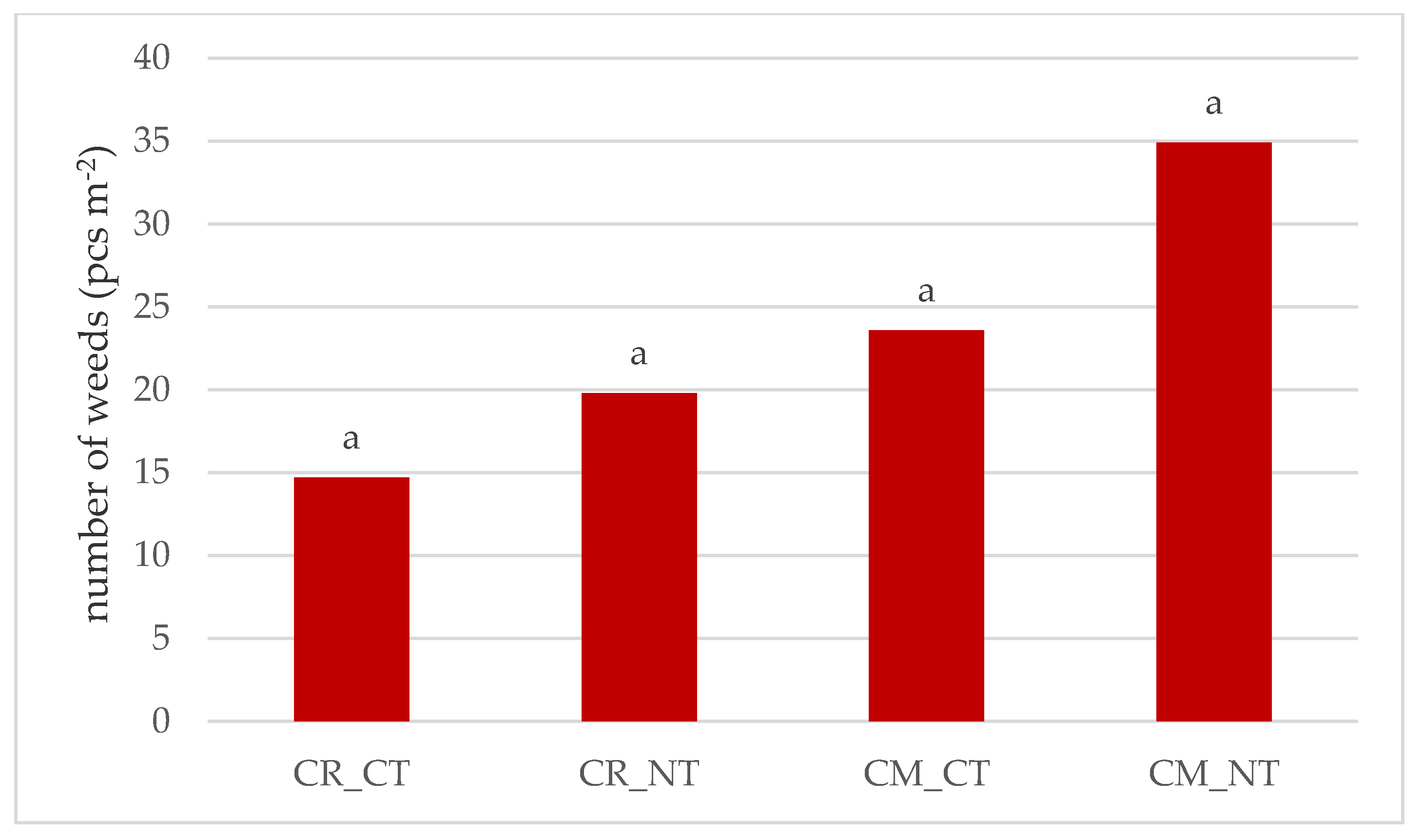
3.1. Weed Infestation of Soybean Crops
3.2. Infection of Soybean Plants with Diseases
4. Discussion
5. Conclusions
- The cultivation of the soybean after it promotes weed infestation. The number and dry weight of weeds was proven to be higher in the monoculture (CM) than in crop rotation (CR), respectively, by 69.4% and 28.6%.
- Under no-tillage (NT), the number of weeds and dry weight of weeds was, respectively, higher by 42.7% and 36.8% than under conventional tillage (CT).
- The statistical analysis confirmed that growing soybeans in crop rotation, compared to the monoculture, and, in the no-tillage system, increases the percentage of monocotyledonous species in total dry weight of weeds.
- In crop rotation, Echinochloa crus-galli was the most numerous weeds in the soybean crop, whereas, in a monoculture, it was Amaranthus retroflexus. Reducing the numbers of Amaranthus retroflexus, Chenopodium album, Avena fatua, Solanum nigrum, and Elymus repens was observed in crop rotation.
- In both tillage systems, Amaranthus retroflexus was the weed species that occurred in the greatest numbers in the soybean crop. A significantly lower number of Avena fatua and Solanum nigrum was found under conventional tillage (CT) when compared to no-tillage (NT).
- Cropping systems and tillage systems only slightly changed the biological diversity of the weed community in the soybean crop. The Shannon-Wiener index (H′) and the Simpson index (SI) were shown to have similar values in crop rotation (CR) and the soybean monoculture (CM), whereas the biological diversity of the weed community was found to be only slightly greater under the NT system compared to the CT system.
- Throughout the study period, soybeans were infected with Ascochyta blight (Ascochyta sp.), Septoria leaf spot (Septoria glycines), and frogeye leaf spot (Cercospora sojina). The severity of disease symptoms in most of the growing seasons was greater in the seasons characterized by a high amount of rainfall.
- Soybean plants were most infected with the pathogen Septoria glycines and least infected with Ascochyta sp.
- No-tillage and monoculture cropping promoted infection of soybeans with fungal diseases in comparison to the conventional system and crop rotation.
Author Contributions
Funding
Conflicts of Interest
References
- Abbasi Surki, A.; Sharifzade, F.; Tavakkol Afshari, R.; Majnoun Hosseini, N.; Gazor, H.R. Optimization of processing parameters of soybean seeds dried in a constant-bed dryer using response surface methodology. J. Agric. Sci. Tech. Iran. 2010, 12, 409–423. [Google Scholar]
- Filoda, G.; Mrówczyński, M.; Matyjaszczyk, E.; Kierzek, R.; Nawracała, J.; Bubniewicz, P.; Fiedler, Ż.; Kornobis, F.; Matysiak, K.; Pruszyński, G.; et al. Metodyka Integrowanej Ochrony i Produkcji Soi Dla Doradców; Instytut Ochrony Roślin—Państwowy Instytut Badawczy: Poznań, Poland, 2016. (In Polish) [Google Scholar]
- The Act of March 8, 2013 on Crop Protection Products (Dz. U. (Journal of Laws) Item 455, 505. Available online: https://www.gov.pl/web/rolnictwo/akty-prawne3 (accessed on 28 April 2020). (In Polish)
- Anderson, R.L. Impact of subsurface tillage on weed dynamics in the Central Grein Plains. Weed Tech. 2004, 18, 186–192. [Google Scholar] [CrossRef]
- Chokor, J.U.; Ikuenobe, C.E.; Akaelu, I.A. The Effect of tillage and herbicides (rimsulfuron and codal gold) on weed regeneration. Inter. J. Soil Sci. 2008, 3, 164–168. [Google Scholar] [CrossRef]
- Gruber, S.; Pekrun, C.; Möhring, J.; Claupein, W. Long-term yield and weed response to conservation and stubble tillage in SW Germany. Soil Till. Res. 2012, 121, 49–56. [Google Scholar] [CrossRef]
- Morris, N.L.; Miller, P.C.H.; Orson, J.H.; Froud-Williams, R.J. The adoption of noninversion tillage systems in the United Kingdom and the agronomic impact on soil, crops and the environment—A review. Soil Till. Res. 2010, 108, 1–15. [Google Scholar] [CrossRef]
- Jug, I.; Jug, D.; Sabo, M.; Bojan, S. Winter wheat yield and yield components as affected by soil tillage systems. Turk. J. Agric. For. 2011, 35, 1–7. [Google Scholar]
- Jordan, V.W.L.; Hutcheon, J.A. Influence of Cultivation Practices on Arable Crop Diseases. In Soil Tillage in Agroecosystems; El-Titi, A., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 187–207. [Google Scholar]
- Poštić’s, J.; Cosic, J.; Vrandecic, K.; Jurkovic’s, D.; Saleh, A.A.; Leslie, J.F. Diversity of Fusarium species isolated from weeds and plant debris in Croatia. J. Phytopathol. 2012, 160, 76–81. [Google Scholar] [CrossRef]
- Perez-Brandan, C.; Arzeno, J.L.; Huidobro, J.; Grümberg, B.; Conforto, C.; Hilton, S.; Bending, G.D.; Meriles, J.M.; Vargas-Gil, S. Long term effect of tillage systems on soil microbiological, chemical and physical parameters and the incidence of charcoal rot by Macrophomina phaseolina (Tassi) Goid in soybean. Crop Prot. 2012, 40, 73–82. [Google Scholar] [CrossRef]
- Ohnishi, S.; Miyoshi, T.; Shirai, S. Low temperature stress at different flower developmental stages affects pollen development, pollination, and pod set in soybean. Environ. Exp. Bot. 2010, 69, 56–62. [Google Scholar] [CrossRef]
- Vollmann, J.; Winkler, J.; Fritz, C.N.; Grausgruber, H.; Ruckenbauer, P. Spatial field variations in soybean (Glycine max [L.] Merr.) performance trials affect agronomic characters and seed composition. Eur. J. Agron. 2000, 12, 13–22. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Linquist, B.A.; Lundy, M.E.; Liang, X.; Van Groenigen, K.J.; Lee, J.; Van Gestel, N.; Six, J.; Venterea, R.T.; Van Kessel, C. When does no-till yield more? A global meta-analysis. Field Crops Res. 2015, 183, 156–168. [Google Scholar] [CrossRef]
- Balota, E.L.; Kanashiro, M.; Filho, A.C.; Andrade, D.S.; Dick, R.P. Soil enzyme activities under long-term tillage and crop rotation systems in subtropical agroecosystems. Braz. J. Microbiol. 2004, 35, 300–306. [Google Scholar] [CrossRef]
- Maillard, É.; Angers, D.A.; Chantigny, M.; Lafond, J.; Pageau, D.; Rochette, P.; Lévesque, G.; Leclerc, M.L.; Parent, L.E. Greater accumulation of soil organic carbon after liquid dairy manure application under cereal-forage rotation than cereal monoculture. Agric. Ecosyst. Environ. 2016, 233, 171–178. [Google Scholar] [CrossRef]
- Calzarano, F.; Stagnari, F.; D’Egidio, S.; Pagnani, G.; Galieni, A.; Di Marco, S.; Metruccio, E.G.; Pisante, M. Durum wheat quality, yield and sanitary status under conservation agriculture. Agriculture 2018, 8, 140. [Google Scholar] [CrossRef]
- Sebayang, H.T.; Rifai, A.P. The effect of soil tillage system and weeding time on the growth of weed and yield of soybean (Glycine max (L.) Merril). J. Degrad. Min. Land Manag. 2018, 5, 1237–1243. [Google Scholar] [CrossRef]
- Tillmann, M.; Von Tiedemann, A.; Winter, M. Crop rotation effects on incidence and diversity of Fusarium species colonizing stem bases and grains of winter wheat. J. Plant Dis. Protect. 2017, 124, 121–130. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy, 2015; p. 106. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland a Checklist. In Krytyczna Lista Roślin Naczyniowych Polski. W.; Szafer Institute of Botany, Polish Akademy of Science: Kraków, Poland, 2002; p. 442. [Google Scholar]
- Shannon, C.E. A mathematical theory of communications. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 168, 668. [Google Scholar] [CrossRef]
- Łacicowa, B. Metoda laboratoryjna szybkiej oceny odporności jęczmienia na Helminthosporium sativum. Biul. IHAR 1969, 3–4, 61–62. (In Polish) [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Gawęda, D.; Woźniak, A.; Harasim, E. Weed infestations of winter wheat depend on the forecrop and the tillage system. Acta Agrobot. 2018, 71, 1–9. [Google Scholar] [CrossRef]
- Cardina, J.; Herms, C.P.; Doohan, D.J. Crop rotation and tillage system effects on weed seedbanks. Weed Sci. 2002, 50, 448–460. [Google Scholar] [CrossRef]
- Santín-Montanyá, M.I.; Martín-Lammerding, D.; Walter, I.; Zambrana, E.; Tenorio, J.L. Efects of tillage, crop systems and fertilization on weed abundance and diversity in 4-year dry land winter wheat. Eur. J Agron. 2013, 48, 43–49. [Google Scholar] [CrossRef]
- Gawęda, D.; Haliniarz, M.; Cierpiała, R.; Klusek, I. Yield, Weed infestation and seed quality of soybean (Glycine max (L.) Merr.) under different tillage systems. Tarim Bilim. Derg. 2017, 23, 268–275. [Google Scholar]
- Sebayang, H.T.; Fatimah, S. The effect of tillage systems and dosages of cow manure on weed and soybeans yield (Glycine max, Merrill). J. Degrad. Min. Land Manag. 2019, 7, 1959–1963. [Google Scholar] [CrossRef]
- Woźniak, A.; Rachoń, L. Effect of tillage systems on pea crop infestation with weeds. Arch. Agron. Soil Sci. 2019, 65, 877–885. [Google Scholar] [CrossRef]
- Woźniak, A.; Soroka, M. Effects of a 3-year reduced tillage on the yield and quality of grain and weed infestation of spring triticale (Triticosecale Wittmack). J. Plant Prod. 2014, 8, 231–242. [Google Scholar]
- Mahmoodi, S.; Rahimi, A. The critical period of weed control in corn in Birjand region, Iran. Inter. J. Plant Prod. 2009, 3, 91–96. [Google Scholar]
- Mohler, C.L.; Frisch, J.C.; McCulloch, C.E. Vertical movement of weed seed surrogates by tillage implements and natural processes. Soil Till. Res. 2006, 86, 110–122. [Google Scholar] [CrossRef]
- Peigné, J.; Ball, B.C.; Roger-Estrade, J.; David, C. Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 2007, 23, 129–144. [Google Scholar] [CrossRef]
- Ruisi, P.; Frangipane, B.; Amato, G.; Badagliacca, G.; Di Miceli, G.; Plaia, A.; Giambalvo, D. Weed seedbank size and composition in a long-term tillage and crop sequence experiment. Weed Res. 2015, 55, 320–328. [Google Scholar] [CrossRef]
- Małecka, I.; Blecharczyk, A.; Dobrzeniecki, T. Zachwaszczenie zbóż ozimych w zależności od systemu uprawy roli. Prog. Plant Prot. 2006, 46, 253–255. (In Polish) [Google Scholar]
- Chovancova, S.; Illek, F.; Winkler, J. The effect of three tillage treatments on weed infestation in maize monoculture. Pak. J. Bot. 2020, 52, 697–701. [Google Scholar] [CrossRef]
- Małecka-Jankowiak, I.; Blecharczyk, A.; Swędzrzyńska, D.; Sawinska, Z.; Piechota, T. The effect of long-term tillage systems on some soil properties and yield of pea (Pisum sativum L.). Acta Sci. Pol. Agric. 2016, 15, 37–50. [Google Scholar]
- Mancinelli, R.; Muleo, R.; Marinari, S.; Radicetti, E. How Soil Ecological Intensification by Means of Cover Crops Affects Nitrogen Use Efficiency in Pepper Cultivation. Agriculture 2019, 9, 145. [Google Scholar] [CrossRef]
- Velykis, A.; Satkus, A. Weed infestation and changes in field pea (Pisum sativum L.) yield as affected by reduced tillage of a clay loam soil. Zemdirbyste 2010, 97, 73–82. [Google Scholar]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Floristic composition and species diversity of weed community after 10 years of different cropping systems and soil tillage in a Mediterranean environment. Weed Res. 2018, 58, 273–283. [Google Scholar] [CrossRef]
- Dainese, M.; Martin, E.A.; Aizen, M.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef]
- García, M.J.; Palma-Bautista, C.; Rojano-Delgado, A.M.; Bracamonte, E.; Portugal, J.; Alcántara-de la Cruz, R.; De Prado, R. The triple amino acid substitution TAP-IVS in the EPSPS gene confers high glyphosate resistance to the superweed Amaranthus hybridus. Int. J. Mol. Sci. 2019, 20, 2396. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B. Zmiany bioróżnorodności flory segetalnej w systemie ekologicznym w latach 1996–2007. Res. Appl. Agric. Eng. 2008, 53, 63–68. (In Polish) [Google Scholar]
- Scholes, R.J.; Mace, G.M.; Turner, W.; Geller, G.N.; Jürgens, N.; Larigauderie, A.; Muchoney, D.; Walther, B.A.; Mooney, H.A. Toward a global biodiversity observing system. Science 2008, 321, 1044–1045. [Google Scholar] [CrossRef] [PubMed]
- Rands, M.R.; Adams, W.M.; Bennun, L.; Butchart, S.H.; Clements, A.; Coomes, D.; Entwistle, A.; Hodge, I.; Kapos, V.; Scharlemann, J.P.; et al. Biodiversity conservation: Challenges beyond 2010. Science 2010, 329, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Clements, D.R.; Weise, R.; Swanton, C.J. Integrated weed management and weed species diversity. Phytoprotection 1994, 75, 1–18. [Google Scholar] [CrossRef]
- Suwara, I.; Stępień, W.; Tymińska, A.; Pruska, K. Wpływ wieloletniego nawożenia mineralnego i zmianowania na zachwaszczenie pszenżyta ozimego. Fragm. Agron. 2016, 33, 107–116. (In Polish) [Google Scholar]
- Sekutowski, T.; Domaradzki, K. Bioróżnorodność gatunkowa chwastów w monokulturze pszenicy ozimej w warunkach stosowania uproszczeń w uprawie roli. Fragm. Agron. 2009, 26, 160–169. (In Polish) [Google Scholar]
- Kim, S.I.; Toon, J.S.; Jung, J.W.; Hong, K.B.; Ahn, Y.J.; Kwon, H.W. Toxicity and repellency of origanum essential oil and its components against Tribolium castaneum (Coleoptera: Tenebrionidae) adults. J. Asia Pac. Entomol. 2010, 13, 369–373. [Google Scholar] [CrossRef]
- Hepperly, P.R.; Kirkpatrick, B.L.; Sinclair, J.B. Abutilon theophrasti: Wild host for three fungal parasites of soybean. Phytopathology 1980, 70, 307–310. [Google Scholar] [CrossRef]
- Srinivas, P.; Ramesh Babu, S.; Ratan, V. Role of sclerotia, plant debris and different hosts on survival of rice sheath blight pathogen, Rhizoctonia solani. Int. J. Appl Biol. Pharm. 2014, 5, 29–33. [Google Scholar]
- Nagaraj, B.T.; Sunkad, G.; Pramesh, D.; Naik, M.K.; Patil, M.B. Host range studies of rice sheath blight fungus Rhizoctonia solani (Kuhn). Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3856–3864. [Google Scholar] [CrossRef]
- Chowdhury, A.; Pradhan, S.; Saha, M.; Sanyal, N. Impact of pesticides on soil microbiological parameters and possible bioremediation strategies. Ind. J. Microbiol. 2008, 48, 114–127. [Google Scholar] [CrossRef]
- Feng, P.C.C.; Baley, G.J.; Clinton, W.P.; Bunkers, G.J.; Alibhai, M.F.; Paulitz, T.C.; Kidwell, K.K. Glyphosate inhibits rust diseases in glyphosate-resistant wheat and soybean. Proc. Natl. Acad. Sci. USA 2005, 102, 17290–17295. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.C.C.; Clark, C.; Andrade, G.C.; Balbi, M.C.; Caldwell, P. The control of Asian rust by glyphosate in glyphosate-resistant soybeans. Pest. Manag. Sci. 2008, 64, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.A.; Renner, K.A.; Hammerschmidt, R. Cultivar and herbicide selection affects soybean development and the incidence of Sclerotinia stem rot. Agron. J. 2002, 94, 1270–1281. [Google Scholar] [CrossRef]
- Nelson, K.A.; Renner, K.A.; Hammerschmidt, R. Effects of Protoporphyrinogen oxidase inhibitors on soybean (Glycine max L.) response, Sclerotinia sclerotiorum disease development, and phytoalexin production by soybean. Weed Technol. 2002, 16, 353–359. [Google Scholar] [CrossRef]
- Corrêa, M.J.P.; Alves, P.L.; Da, C.A. Effects of herbicides application on photochemical efficiency in conventional and genetically modified soybeans. Ciênc. Agrotec. 2010, 34, 1136–1145. [Google Scholar] [CrossRef]
- Katan, J.; Eshel, Y. Interactions between herbicides and plant pathogens. In Residue Reviews; Gunther, F.A., Gunther, J.D., Eds.; Springer: New York, NY, USA, 1973. [Google Scholar]
- Araújo, A.S.F.; Monteiro, R.T.R.; Abarkeli, R.B. Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 2003, 52, 799–804. [Google Scholar] [CrossRef]
- Boyette, C.D.; Reddy, K.N.; Hoagland, R.E. Glyphosate and bioherbicide interaction for con-trolling kudzu (Pueraria lobata), redvine (Brunnichia ovata), and trumpetcreeper (Campsis radicans). Biocontrol. Sci. Tech. 2006, 16, 1067–1077. [Google Scholar] [CrossRef]
- Amin, M.; Melkamu, F. Management of Ascochyta Blight (Ascochyta rabiei) in chickpea using a new fungicide. Res. Plant Biol. 2014, 2, 27–32. [Google Scholar]
- Bretag, T.W.; MacLeod, W.J.; Kimber, R.B.E.; Moore, K.J.; Knights, E.J.C.; Davidson, J.A. Management of ascochyta blight in chickpeas in Australia. Australas. Plant Path. 2008, 37, 486–497. [Google Scholar] [CrossRef]
- Davidson, J.A.; Kimber, R.B.E. Integrated disease management of ascochyta blight in pulse crops. Eur. J. Plant Pathol. 2007, 119, 99–110. [Google Scholar] [CrossRef]
- Sudheesh, S.; Rodda, M.S.; Davidson, J.; Javid, M.; Stephens, A.; Slater, A.T.; Cogan, N.O.I.; Forster, J.W.; Kaur, S. SNP-based linkage mapping for validation of QTLs for resistance to Ascochyta blight in lentil. Front. Plant Sci. 2016, 7, 1604. [Google Scholar] [CrossRef] [PubMed]
- Małecka, I.; Sawinska, Z.; Blecharczyk, A.; Dytman-Hagedorn, M. Zdrowotność pszenicy ozimej w różnych wariantach uprawy roli. Prog. Plant Prot. Post. Ochr. Roślin 2014, 54, 246–250. (In Polish) [Google Scholar]
- Sułek, A.; Podolska, G.; Jaśkiewicz, B. Plonowanie i zdrowotność dwóch podgatunków pszenicy w zależności od udziału zbóż w strukturze zasiewów w warunkach integrowanej produkcji. Pol. J. Agron. 2016, 27, 118–125. (In Polish) [Google Scholar]
- Strom, N.; Hu, W.; Haarith, D.; Chen, S.; Bushley, K. Interactions between soil properties, fungal communities, the soybean cyst nematode, and crop yield under continuous corn and soybean monoculture. Appl. Soil Ecol. 2020, 147, 103388. [Google Scholar] [CrossRef]
- Kraska, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Pałys, E. The effect of tillage systems and catch crops on the yield, grain quality and health of spring wheat. Acta Sci. Pol. Agric. 2014, 13, 21–38. [Google Scholar]
- Supronienė, S.; Mankeviĉienė, A.; Kadžienė, G.; Kaĉergius, A.; Feiza, V.; Feizienė, D.; Semaškienė, R.; Dabkeviĉius, Z.; Tamošiūnas, K. The impact of tillage and fertilization on Fusarium infection and mycotoxin production in wheat grains. Zemdirbyste 2012, 99, 265–272. [Google Scholar]
- Vrandečić, K.; Jug, D.; Ćosić, J.; Stošić, M.; Poštić, J. The impact of tillage and fertilization on soybean grain infection with fungi. Rom. Agric. Res. 2014, 31, 139–145. [Google Scholar]
- Gawęda, D.; Nowak, A.; Haliniarz, M.; Woźniak, A. Yield and economic effectiveness of soybean grown under different cropping systems. Int. J. Plant Prod. 2020, 53, 1–11. Available online: https://link.springer.com/article/10.1007/s42106-020-00098-1#article-info (accessed on 29 April 2020). [CrossRef]




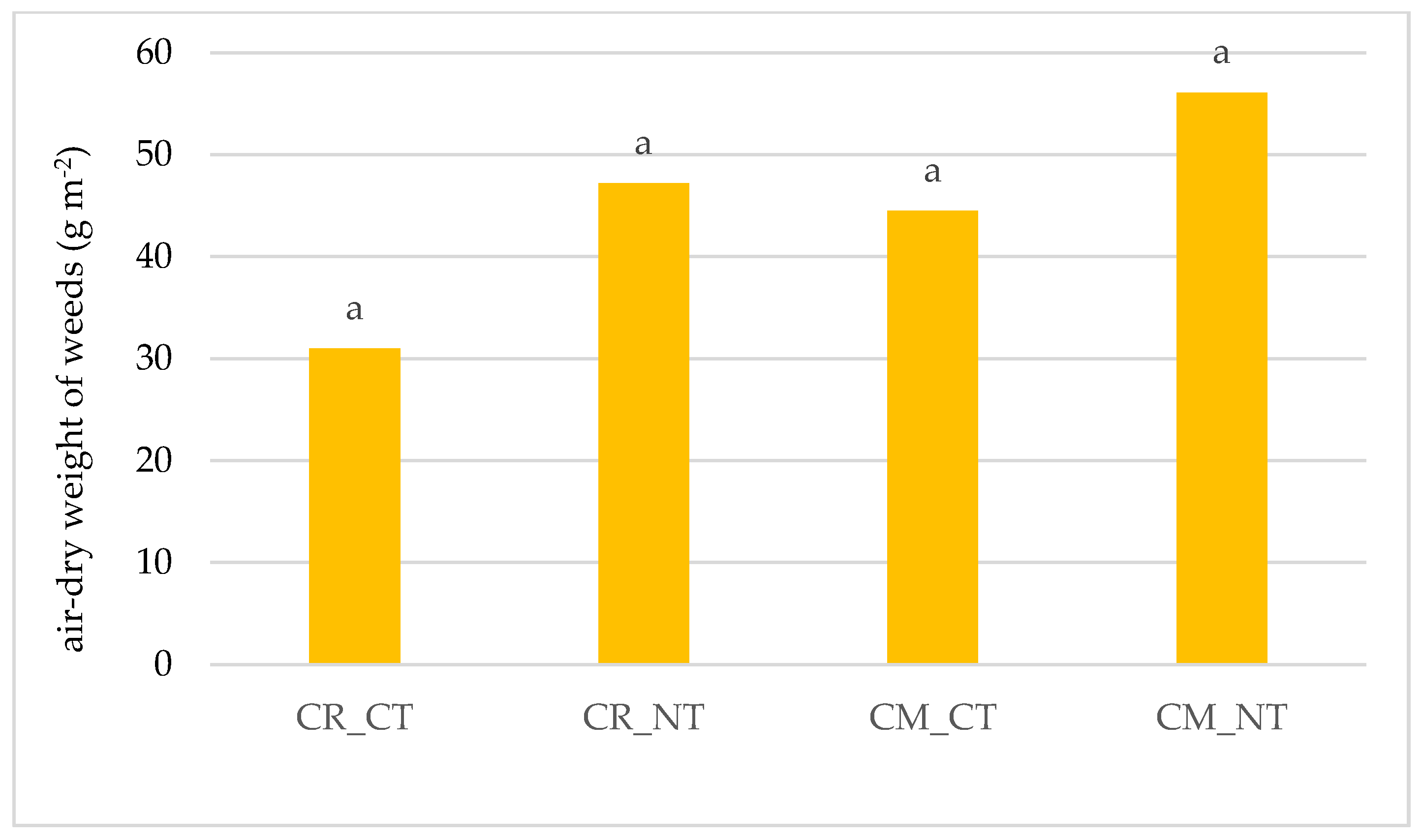







| CT | NT | CT | NT | CT | NT | ||
|---|---|---|---|---|---|---|---|
| CM | CM CT | CM NT | CM CT | CM NT | CM CT | CM NT | *soybean 2014–2017 |
| CR | CR CT | CR NT | CR CT | CR NT | CR CT | CR NT | *soybean 2015 |
| CR CT | CR NT | CR CT | CR NT | CR CT | CR NT | *soybean 2016 | |
| CR CT | CR NT | CR CT | CR NT | CR CT | CR NT | *soybean 2017 | |
| CR CT | CR NT | CR CT | CR NT | CR CT | CR NT | *soybean 2014 | |
| replicates I | replicates II | replicates III | |||||
| Species | Cropping System | Tillage System | ||
|---|---|---|---|---|
| CR | CM | CT | NT | |
| I. Short-lived | ||||
| Amaranthus retroflexus L. | 3.4a | 7.3b | 4.5a | 6.1a |
| Anagallis arvensis L. | 0.1a | 0.1a | 0.1a | 0.1a |
| Anchusa arvensis (L.) M. Bieb. | 0.3a | - | - | 0.3a |
| Avena fatua L. | 1.5a | 2.8b | 1.4a | 2.9b |
| Capsella bursa-pastoris (L.) Medik. | 0.8a | 1.2a | 1.2a | 0.9a |
| Chenopodium album L. | 0.8a | 3.6b | 2.2a | 2.2a |
| Echinochloa crus-galli (L.) P. Beauv. | 4.2a | 2.5a | 3.1a | 3.7a |
| Euphorbia helioscopia L. | - | 0.2a | 0.1a | 0.2a |
| Fallopia convolvulus (L.) Á. Löve | 0.6a | 0.3a | 0.4a | 0.6a |
| Galium aparine L. | 0.8a | 0.2a | 0.4a | 0.6a |
| Lamium amplexicaule L. | 0.1a | 0.2a | 0.2a | 0.1a |
| Matricaria maritima ssp. inodora (L.) Dostál | 0.5a | 1.2a | 0.6a | 1.2a |
| Melandrium album (Mill.) Garcke | 0.2a | 0.2a | 0.1a | 0.2a |
| Polygonum aviculare L. | 1.6a | 1.8a | 1.9a | 1.4a |
| Solanum nigrum L. Emend. Mill. | 0.4a | 2.0b | 0.5a | 1.9b |
| Sonchus asper (L.) Hill. | 0.6a | 0.5a | 0.4a | 0.6a |
| Stellaria media (L.) Vill. | 0.3a | 0.4a | 0.3a | 0.4a |
| Thlaspi arvense L. | - | 0.1a | 0.1a | - |
| Veronica persica Poir. | 0.1a | - | - | 0.1a |
| Viola arvensis Murr. | 0.3a | - | 0.2a | 0.1a |
| II. Perennial | ||||
| Cirsium arvense (L.) Scop. | 0.3a | 1.0a | 0.5a | 0.7a |
| Convolvulus arvensis L. | 0.1a | - | - | 0.1a |
| Elymus repens (L.) Gould | 0.1a | 3.4b | 0.8a | 2.7a |
| Plantago major L. | - | 0.2a | 0.1a | 0.1a |
| Sonchus arvensis L. | 0.2a | 0.1a | 0.1a | 0.2a |
| Number of weed species | 22a | 21a | 22a | 24a |
| Species | CR_CT | CR_NT | CM_CT | CM_NT |
|---|---|---|---|---|
| I. Short-lived | ||||
| Amaranthus retroflexus L. | 3.2a | 3.6a | 5.9a | 8.7a |
| Anagallis arvensis L. | 0.1a | 0.1a | 0.1a | 0.1a |
| Anchusa arvensis (L.) M. Bieb. | - | 0.7a | - | - |
| Avena fatua L. | 1.2a | 1.8a | 1.5a | 4.1a |
| Capsella bursa-pastoris (L.) Medik. | 1.0a | 0.6a | 1.3a | 1.2a |
| Chenopodium album L. | 0.6a | 1.0a | 3.8a | 3.3a |
| Echinochloa crus-galli (L.) P. Beauv. | 3.6a | 4.8a | 2.5a | 2.5a |
| Euphorbia helioscopia L. | - | - | 0.1a | 0.3a |
| Fallopia convolvulus (L.) Á. Löve | 0.5a | 0.7a | 0.2a | 0.4a |
| Galium aparine L. | 0.5a | 1.1a | 0.2a | 0.2a |
| Lamium amplexicaule L. | - | 0.2a | 0.5a | - |
| Matricaria maritima ssp. inodora (L.) Dostál | 0.4a | 0.6a | 0.8a | 1.7a |
| Melandrium album (Mill.) Garcke | 0.1a | 0.2a | 0.2a | 0.2a |
| Polygonum aviculare L. | 1.8a | 1.3a | 2.0a | 1.5a |
| Solanum nigrum L. Emend. Mill. | 0.5a | 0.4a | 0.5a | 3.4b |
| Sonchus asper (L.) Hill. | 0.4a | 0.7a | 0.5a | 0.6a |
| Stellaria media (L.) Vill. | 0.3a | 0.4a | 0.4a | 0.4a |
| Thlaspi arvense L. | - | - | 0.2a | - |
| Veronica persica Poir. | - | 0.2a | - | - |
| Viola arvensis Murr. | 0.4a | 0.2a | - | - |
| II. Perennial | ||||
| Cirsium arvense (L.) Scop. | 0.1a | 0.5a | 1.0a | 1.0a |
| Convolvulus arvensis L. | - | 0.2a | - | - |
| Elymus repens (L.) Gould | - | 0.2a | 1.7a | 5.1a |
| Plantago major L. | - | - | 0.1a | 0.2a |
| Sonchus arvensis L. | - | 0.3a | 0.1a | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawęda, D.; Haliniarz, M.; Bronowicka-Mielniczuk, U.; Łukasz, J. Weed Infestation and Health of the Soybean Crop Depending on Cropping System and Tillage System. Agriculture 2020, 10, 208. https://doi.org/10.3390/agriculture10060208
Gawęda D, Haliniarz M, Bronowicka-Mielniczuk U, Łukasz J. Weed Infestation and Health of the Soybean Crop Depending on Cropping System and Tillage System. Agriculture. 2020; 10(6):208. https://doi.org/10.3390/agriculture10060208
Chicago/Turabian StyleGawęda, Dorota, Małgorzata Haliniarz, Urszula Bronowicka-Mielniczuk, and Justyna Łukasz. 2020. "Weed Infestation and Health of the Soybean Crop Depending on Cropping System and Tillage System" Agriculture 10, no. 6: 208. https://doi.org/10.3390/agriculture10060208
APA StyleGawęda, D., Haliniarz, M., Bronowicka-Mielniczuk, U., & Łukasz, J. (2020). Weed Infestation and Health of the Soybean Crop Depending on Cropping System and Tillage System. Agriculture, 10(6), 208. https://doi.org/10.3390/agriculture10060208






