Will Yellow Mealworm Become a Source of Safe Proteins for Europe?
Abstract
1. Introduction
2. Materials and Methods/Data Collection and Selection
3. Results and Discussion
3.1. Progress in Research on Yellow Mealworms in Europe
3.2. Tenebrio Molitor Development
3.3. Mealworm Physiology and Immunity
3.4. Sustainability of Mealworm Rearing
3.5. Mealworm Uses in Europe
3.5.1. Mealworms as the Sustainable Food of the Future
3.5.2. Nutrient Extraction
3.5.3. Mealworm as Feed
3.6. Safety Aspects of Mealworm as Food
3.6.1. Conservation Techniques
3.6.2. Microbial Load of Mealworm Larvae
3.6.3. Contaminants
3.7. Consumer Attitudes towards Edible Insects and Safety Concerns
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition; Food & Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Gustafsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: Global Food Losses and Food Waste-Extent, Causes and Prevention-FAO, 2013; SIK Institutet för Livsmedel och Bioteknik: Gothenburg, Sweden, 2013. [Google Scholar]
- FAO. The Future of Food and Agriculture—Trends and Challenges; Food and Agriculture Organisation: Rome, Italy, 2017. [Google Scholar]
- Alexander, P.; Brown, C.; Arneth, A.; Dias, C.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Could consumption of insects, cultured meat or imitation meat reduce global agricultural land use? Glob. Food Secur. 2017, 15, 22–32. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Eilenberg, J.; Vlak, J.M.; Nielsen-LeRoux, C.; Cappellozza, S.; Jensen, A.B. Diseases in insects produced for food and feed. J. Insects Food Feed 2015, 1, 87–102. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Mäkinen, O.E.; Rommi, K.; Heiniö, R.-L.; Holopainen-Mantila, U.; Hokkanen, S.; Hakala, T.K.; Nordlund, E. Biochemical and sensory characteristics of the cricket and mealworm fractions from supercritical carbon dioxide extraction and air classification. Eur. Food Res. Technol. 2018, 244, 19–29. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Mottet, A.; de Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Secur. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Martin, D. Edible: An Adventure into the World of Eating Insects and the Last Great Hope to Save the Planet; Houghton Mifflin Harcourt: Boston, NY, USA, 2014. [Google Scholar]
- Ramos-Elorduy, J. Energy supplied by edible insects from Mexico and their nutritional and ecological importance. Ecol. Food Nutr. 2008, 47, 280–297. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Edible insects contributing to food security? Agric. Food Secur. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J. Insects: A sustainable source of food? Ecol. Food Nutr. 1997, 36, 247–276. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as food in sub-Saharan Africa. Int. J. Trop. Insect Sci. 2003, 23, 163–185. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insects of the World, 2017; Wageningen University: Wageningen, The Netherlands, 2018. [Google Scholar]
- Jongema, Y. World List of Edible Insects; Wageningen University: Wageningen, The Netherlands, 2015; pp. 1–75. [Google Scholar]
- Parodi, A.; Leip, A.; De Boer, I.J.M.; Slegers, P.M.; Ziegler, F.; Temme, E.H.M.; Herrero, M.; Tuomisto, H.; Valin, H.; Van Middelaar, C.E. The potential of future foods for sustainable and healthy diets. Nat. Sustain. 2018, 1, 782–789. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Hoppe, C.; Roos, N.; Kaestel, P.; Stougaard, M.; Lauritzen, L.; Mølgaard, C.; Girma, T.; Friis, H. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr. Bull. 2009, 30, S343–S404. [Google Scholar] [CrossRef]
- Rana, K.J.; Siriwardena, S.; Hasan, M.R. Impact of Rising Feed Ingredient Prices on Aquafeeds and Aquaculture Production; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009. [Google Scholar]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Testa, M.; Stillo, M.; Maffei, G.; Andriolo, V.; Gardois, P.; Zotti, C.M. Ugly but tasty: A systematic review of possible human and animal health risks related to entomophagy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3747–3759. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; De Boer, I.J.M. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- Prather, C.M.; Laws, A.N. Insects as a piece of the puzzle to mitigate global problems: An opportunity for ecologists. Basic Appl. Ecol. 2018, 26, 71–81. [Google Scholar] [CrossRef]
- Premalatha, M.; Abbasi, T.; Abbasi, T.; Abbasi, S.A. Energy-efficient food production to reduce global warming and ecodegradation: The use of edible insects. Renew. Sustain. Energy Rev. 2011, 15, 4357–4360. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Reducing the global environmental impact of livestock production: The minilivestock option. J. Clean. Prod. 2016, 112, 1754–1766. [Google Scholar] [CrossRef]
- Gahukar, R.T. Entomophagy and human food security. Int. J. Trop. Insect Sci. 2011, 31, 129–144. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Sablon, L.; Geuens, M.; Brostaux, Y.; Alabi, T.; Blecker, C.; Drugmand, D.; Haubruge, É.; Francis, F. Edible insects acceptance by Belgian consumers: Promising attitude for entomophagy development. J. Sens. Stud. 2014, 29, 14–20. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Alabi, T.; Larreché, S.; Alexandra, L.; Haubruge, E.; Francis, F. Risks and valorization of insects in a food and feed context. Int. J. Entomol. 2015, 51, 215–258. [Google Scholar]
- Bednářová, M.; Borkovcová, M.; Mlček, J.; Rop, O.; Zeman, L. Edible insects-species suitable for entomophagy under condition of Czech Republic. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 587–593. [Google Scholar] [CrossRef]
- Ribeiro, N.; Abelho, M.; Costa, R. A review of the scientific literature for optimal conditions for mass rearing Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2018, 53, 434–454. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef]
- Veldkamp, T.; Van Duinkerken, G.; van Huis, A.; Lakemond, C.M.M.; Ottevanger, E.; Bosch, G.; Van Boekel, T. Insects as a Sustainable Feed Ingredient in Pig and Poultry Diets: A Feasibility Study = Insecten als Duurzame Diervoedergrondstof in Varkens-En Pluimveevoeders: Een haalbaarheidsstudie; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2012; pp. 1570–8616. [Google Scholar]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Developmental plasticity in Tenebrio molitor (Coleoptera: Tenebrionidae): Analysis of instar variation in number and development time under different diets. J. Entomol. Sci. 2010, 45, 75–90. [Google Scholar] [CrossRef]
- Pleissner, D.; Rumpold, B.A. Utilization of organic residues using heterotrophic microalgae and insects. Waste Manag. 2018, 72, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; Van Huis, A.; Van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Nat. C 2017, 72, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.; Heuzé, V.; Makkar, H.P.S. Insects in fish diets. Anim. Front. 2015, 5, 37–44. [Google Scholar] [CrossRef]
- Commission, E. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. In 2015/2283; Commission, E., Ed.; Official Journal of the European Union: Brussels, Belgium, 2015; Volume L 327/1. [Google Scholar]
- Commission, E. European Commission Regulation (EU) 2017/893 of 23 May 2017 amending Annexes I and IV o Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein. In 2017/893; Commission, E., Ed.; Official Journal of the European Union: Brussels, Belgium, 2017; Volume L 138/92. [Google Scholar]
- Thévenot, A.; Rivera, J.L.; Wilfart, A.; Maillard, F.; Hassouna, M.; Senga-Kiesse, T.; Le Féon, S.; Aubin, J. Mealworm meal for animal feed: Environmental assessment and sensitivity analysis to guide future prospects. J. Clean. Prod. 2018, 170, 1260–1267. [Google Scholar] [CrossRef]
- Poelaert, C.; Beckers, Y.; Despret, X.; Portetelle, D.; Francis, F.; Bindelle, J. In vitro evaluation of fermentation characteristics of two types of insects as potential novel protein feeds for pigs. J. Anim. Sci. 2016, 94, 198–201. [Google Scholar] [CrossRef]
- Veldkamp, T.; Bosch, G. Insects: A protein-rich feed ingredient in pig and poultry diets. Anim. Front. 2015, 5, 45–50. [Google Scholar]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Bianchi, C. Effects of yellow mealworm larvae (Tenebrio molitor) inclusion in diets for female broiler chickens: Implications for animal health and gut histology. Anim. Feed Sci. Technol. 2017, 234, 253–263. [Google Scholar] [CrossRef]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Sterpone, L. Yellow mealworm larvae (Tenebrio molitor) inclusion in diets for male broiler chickens: Effects on growth performance, gut morphology, and histological findings. Poult. Sci. 2018, 97, 540–548. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Gut microbiota and mucin composition in female broiler chickens fed diets including yellow mealworm (Tenebrio molitor, L.). Animals 2019, 9, 213. [Google Scholar] [CrossRef]
- Bovera, F.; Piccolo, G.; Gasco, L.; Marono, S.; Loponte, R.; Vassalotti, G.; Mastellone, V.; Lombardi, P.; Attia, Y.A.; Nizza, A. Yellow mealworm larvae (Tenebrio molitor, L.) as a possible alternative to soybean meal in broiler diets. Br. Poult. Sci. 2015, 56, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Fontanari, L.; Rugani, R.; Regolin, L.; Vallortigara, G. Use of kind information for object individuation in young domestic chicks. Anim. Cogn. 2014, 17, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Józefiak, D.; Engberg, R.M. Insects as poultry feed. In Proceedings of the 20th European Symposium on Poultry Nutrition, Prague, Czech, 24–27 August 2015; pp. 24–27. [Google Scholar]
- Józefiak, D.; Józefiak, A.; Kierończyk, B.; Rawski, M.; Świątkiewicz, S.; Długosz, J.; Engberg, R.M. 1. Insects—A natural nutrient source for poultry—A review. Ann. Anim. Sci. 2016, 16, 297–313. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Józefiak, A.; Mazurkiewicz, J.; Świątkiewicz, S.; Siwek, M.; Bednarczyk, M.; Szumacher-Strabel, M.; Cieślak, A.; Benzertiha, A. Effects of replacing soybean oil with selected insect fats on broilers. Anim. Feed Sci. Technol. 2018, 240, 170–183. [Google Scholar] [CrossRef]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Henry, M.A.; Gasco, L.; Chatzifotis, S.; Piccolo, G. Does dietary insect meal affect the fish immune system? The case of mealworm, Tenebrio molitor on European sea bass, Dicentrarchus labrax. Dev. Comp. Immunol. 2018, 81, 204–209. [Google Scholar] [CrossRef]
- Henry, M.A.; Gai, F.; Enes, P.; Peréz-Jiménez, A.; Gasco, L. Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 83, 308–313. [Google Scholar] [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Roncarati, A.; Gasco, L.; Parisi, G.; Terova, G. Growth performance of common catfish (Ameiurus melas Raf.) fingerlings fed mealworm (Tenebrio molitor) diet. J. Insects Food Feed 2015, 1, 233–240. [Google Scholar] [CrossRef]
- Secci, G.; Moniello, G.; Gasco, L.; Bovera, F.; Parisi, G. Barbary partridge meat quality as affected by Hermetia illucens and Tenebrio molitor larva meals in feeds. Food Res. Int. 2018, 112, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Loponte, R.; Nizza, S.; Bovera, F.; De Riu, N.; Fliegerova, K.; Lombardi, P.; Vassalotti, G.; Mastellone, V.; Nizza, A.; Moniello, G. Growth performance, blood profiles and carcass traits of Barbary partridge (Alectoris barbara) fed two different insect larvae meals (Tenebrio molitor and Hermetia illucens). Res. Vet. Sci. 2017, 115, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Favreau-Peigné, A.; Calandreau, L.; Constantin, P.; Bertin, A.; Arnould, C.; Laurence, A.; Richard-Yris, M.-A.; Houdelier, C.; Lumineau, S.; Boissy, A. Unpredictable and repeated negative stimuli increased emotional reactivity in male quail. Appl. Anim. Behav. Sci. 2016, 183, 86–94. [Google Scholar] [CrossRef]
- Laurence, A.; Lumineau, S.; Calandreau, L.; Arnould, C.; Leterrier, C.; Boissy, A.; Houdelier, C. Short- and long-term effects of unpredictable repeated negative stimuli on Japanese quail’s fear of humans. PLoS ONE 2014, 9, e93259. [Google Scholar] [CrossRef]
- Whiteside, M.A.; Sage, R.; Madden, J.R. Diet complexity in early life affects survival in released pheasants by altering foraging efficiency, food choice, handling skills and gut morphology. J. Anim. Ecol. 2015, 84, 1480–1489. [Google Scholar] [CrossRef]
- Bosch, G.; Zhang, S.; Oonincx, D.G.A.B.; Hendriks, W.H. Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 2014, 3, e29. [Google Scholar] [CrossRef]
- Bosch, G.; Vervoort, J.J.M.; Hendriks, W.H. In vitro digestibility and fermentability of selected insects for dog foods. Anim. Feed Sci. Technol. 2016, 221, 174–184. [Google Scholar] [CrossRef]
- Jefimow, M.; Wojciechowski, M.S. Effect of dietary fatty acids on metabolic rate and nonshivering thermogenesis in golden hamsters. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 98–107. [Google Scholar] [CrossRef]
- Peach, W.J.; Mallord, J.W.; Orsman, C.J.; Ockendon, N.; Haines, W.G. Testing assumptions of a supplementary feeding experiment aimed at suburban House Sparrows Passer domesticus. Bird Study 2013, 60, 308–320. [Google Scholar] [CrossRef]
- Peach, W.J.; Sheehan, D.K.; Kirby, W.B. Supplementary feeding of mealworms enhances reproductive success in garden nesting House Sparrows Passer domesticus. Bird Study 2014, 61, 378–385. [Google Scholar] [CrossRef]
- Peach, W.J.; Mallord, J.W.; Ockendon, N.; Orsman, C.J.; Haines, W.G. Invertebrate prey availability limits reproductive success but not breeding population size in suburban House Sparrows Passer domesticus. IBIS 2015, 157, 601–613. [Google Scholar] [CrossRef]
- Roswag, A.; Becker, N.I.; Encarnação, J.A. Inter-and intraspecific comparisons of retention time in insectivorous bat species (V espertilionidae). J. Zool. 2012, 288, 85–92. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.-H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef]
- Adámková, A.; Adámek, M.; Mlček, J.; Borkovcová, M.; Bednářová, M.; Kouřimská, L.; Skácel, J.; Vítová, E. Welfare of the mealworm (Tenebrio molitor) breeding with regard to nutrition value and food safety. Potravin. Slovak J. Food Sci. 2017, 11, 460–465. [Google Scholar] [CrossRef]
- Goptar, I.A.; Semashko, T.A.; Danilenko, S.A.; Lysogorskaya, E.N.; Oksenoit, E.S.; Zhuzhikov, D.P.; Belozersky, M.A.; Dunaevsky, Y.E.; Oppert, B.; Filippova, I.Y. Cysteine digestive peptidases function as post-glutamine cleaving enzymes in tenebrionid stored-product pests. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 161, 148–154. [Google Scholar] [CrossRef]
- Tereshchenkova, V.F.; Goptar, I.A.; Kulemzina, I.A.; Zhuzhikov, D.P.; Serebryakova, M.V.; Belozersky, M.A.; Dunaevsky, Y.E.; Oppert, B.; Filippova, I.Y.; Elpidina, E.N. Dipeptidyl peptidase 4—An important digestive peptidase in Tenebrio molitor larvae. Insect Biochem. Mol. Biol. 2016, 76, 38–48. [Google Scholar] [CrossRef]
- Monceau, K.; Dechaume-Moncharmont, F.X.; Moreau, J.; Lucas, C.; Capoduro, R.; Motreuil, S.; Moret, Y. Personality, immune response and reproductive success: An appraisal of the pace-of-life syndrome hypothesis. J. Anim. Ecol. 2017, 86, 932–942. [Google Scholar] [CrossRef]
- Dhinaut, J.; Balourdet, A.; Teixeira, M.; Chogne, M.; Moret, Y. A dietary carotenoid reduces immunopathology and enhances longevity through an immune depressive effect in an insect model. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Dobson, A.J.; Johnston, P.R.; Vilcinskas, A.; Rolff, J. Identification of immunological expressed sequence tags in the mealworm beetle Tenebrio molitor. J. Insect Physiol. 2012, 58, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Krams, I.A.; Krama, T.; Moore, F.R.; Kivleniece, I.; Kuusik, A.; Freeberg, T.M.; Mänd, R.; Rantala, M.J.; Daukšte, J.; Mänd, M. Male mealworm beetles increase resting metabolic rate under terminal investment. J. Evol. Biol. 2014, 27, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Prokkola, J.; Roff, D.; Kärkkäinen, T.; Krams, I.; Rantala, M.J. Genetic and phenotypic relationships between immune defense, melanism and life-history traits at different temperatures and sexes in Tenebrio molitor. Heredity 2013, 111, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Berggreen, I.E.; Offenberg, J.; Calis, M.; Heckmann, L.H. Impact of density, reproduction period and age on fecundity of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). J. Insects Food Feed 2018, 4, 43–50. [Google Scholar] [CrossRef]
- Pölkki, M.; Krams, I.; Kangassalo, K.; Rantala, M.J. Inbreeding affects sexual signalling in males but not females of Tenebrio molitor. Biol. Lett. 2012, 8, 423–425. [Google Scholar] [CrossRef]
- Iwan, D.; Kamiński, M.J.; Raś, M. The last breath: A μCT-based method for investigating the tracheal system in Hexapoda. Arthropod Struct. Dev. 2015, 44, 218–227. [Google Scholar] [CrossRef]
- Raś, M.; Iwan, D.; Kamiński, M.J. The tracheal system in post-embryonic development of holometabolous insects: A case study using the mealworm beetle. J. Anat. 2018, 232, 997–1015. [Google Scholar] [CrossRef]
- Krams, I.; Kivleniece, I.; Kuusik, A.; Krama, T.; Mänd, R.; Rantala, M.J.; Znotiņa, S.; Freeberg, T.M.; Mänd, M. Predation promotes survival of beetles with lower resting metabolic rates. Entomol. Exp. Appl. 2013, 148, 94–103. [Google Scholar] [CrossRef]
- Krams, I.; Kivleniece, I.; Kuusik, A.; Krama, T.; Freeberg, T.M.; Mänd, R.; Vrublevska, J.; Rantala, M.J.; Mänd, M. Predation selects for low resting metabolic rate and consistent individual differences in anti-predator behavior in a beetle. Acta Ethologica 2013, 16, 163–172. [Google Scholar] [CrossRef]
- Krams, I.; Kivleniece, I.; Kuusik, A.; Krama, T.; Freeberg, T.M.; Mänd, R.; Sivacova, L.; Rantala, M.J.; Mänd, M. High repeatability of anti-predator responses and resting metabolic rate in a beetle. J. Insect Behav. 2014, 27, 57–66. [Google Scholar] [CrossRef]
- Krams, I.A.; Krama, T.; Moore, F.R.; Rantala, M.J.; Mänd, R.; Mierauskas, P.; Mänd, M. Resource availability as a proxy for terminal investment in a beetle. Oecologia 2015, 178, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.L.; Holman, L. Terminal investment in multiple sexual signals: Immune-challenged males produce more attractive pheromones. Funct. Ecol. 2012, 26, 20–28. [Google Scholar] [CrossRef]
- Bogolyubov, D.S.; Kiselyov, A.M.; Shabelnikov, S.V.; Parfenov, V.N. Polyadenylated RNA and mRNA export factors in extrachromosomal nuclear domains of vitellogenic oocytes in the yellow mealworm Tenebrio molitor. Cell Tissue Biol. 2012, 6, 412–422. [Google Scholar] [CrossRef]
- Dhinaut, J.; Chogne, M.; Moret, Y. Trans-generational immune priming in the mealworm beetle protects eggs through pathogen-dependent mechanisms imposing no immediate fitness cost for the offspring. Dev. Comp. Immunol. 2018, 79, 105–112. [Google Scholar] [CrossRef]
- Dhinaut, J.; Chogne, M.; Moret, Y. Immune priming specificity within and across generations reveals the range of pathogens affecting evolution of immunity in an insect. J. Anim. Ecol. 2018, 87, 448–463. [Google Scholar] [CrossRef]
- Johnston, P.R.; Makarova, O.; Rolff, J. Inducible defenses stay up late: Temporal patterns of immune gene expression in Tenebrio molitor. G3 Genes Genomes Genet. 2014, 4, 947–955. [Google Scholar] [CrossRef]
- Makarova, O.; Rodriguez-Rojas, A.; Eravci, M.; Weise, C.; Dobson, A.; Johnston, P.; Rolff, J. Antimicrobial defence and persistent infection in insects revisited. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150296. [Google Scholar] [CrossRef]
- Maistrou, S.; Paris, V.; Jensen, A.B.; Rolff, J.; Meyling, N.V.; Zanchi, C. A constitutively expressed antifungal peptide protects Tenebrio molitor during a natural infection by the entomopathogenic fungus Beauveria bassiana. Dev. Comp. Immunol. 2018, 86, 26–33. [Google Scholar] [CrossRef]
- Krams, I.; Daukste, J.; Kivleniece, I.; Krama, T.; Rantala, M.J. Previous encapsulation response enhances within individual protection against fungal parasite in the mealworm beetle Tenebrio molitor. Insect Sci. 2013, 20, 771–777. [Google Scholar] [CrossRef]
- Jacobs, C.G.C.; Gallagher, J.D.; Evison, S.E.F.; Heckel, D.G.; Vilcinskas, A.; Vogel, H. Endogenous egg immune defenses in the yellow mealworm beetle (Tenebrio molitor). Dev. Comp. Immunol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Daukšte, J.; Kivleniece, I.; Krama, T.; Rantala, M.J.; Krams, I. Senescence in immune priming and attractiveness in a beetle. J. Evol. Biol. 2012, 25, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Kangassalo, K.; Kosonen, K.; Pölkki, M.; Sorvari, J.; Krams, I.; Rantala, M.J. Immune challenge has a negative effect on cuticular darkness in the mealworm beetle, Tenebrio molitor. Ann. Zool. Fenn. 2016, 53, 255–262. [Google Scholar] [CrossRef]
- Joensuu, K.; Silvenius, F. Production of mealworms for human consumption in Finland: A preliminary life cycle assessment. J. Insects Food Feed 2017, 3, 211–216. [Google Scholar] [CrossRef]
- Miglietta, P.P.; De Leo, F.; Ruberti, M.; Massari, S. Mealworms for food: A water footprint perspective. Water 2015, 7, 6190–6203. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J. Anthropo-entomophagy: Cultures, evolution and sustainability. Entomol. Res. 2009, 39, 271–288. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J.; Camenzuli, L.; Belluco, S.; Meijer, N.; Ricci, A. Food safety issues related to uses of insects for feeds and foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1172–1183. [Google Scholar] [CrossRef]
- Jonas-Levi, A.; Martinez, J.-J.I. The high level of protein content reported in insects for food and feed is overestimated. J. Food Compos. Anal. 2017, 62, 184–188. [Google Scholar] [CrossRef]
- Heinz, G.; Hautzinger, P. Meat Processing Technology for Small to Medium Scale Producers; FAO: Bangkok, Thailand, 2007. [Google Scholar]
- Smil, V. Eating meat: Evolution, patterns, and consequences. Popul. Dev. Rev. 2002, 28, 599–639. [Google Scholar] [CrossRef]
- Kenya, F.G. Kenya Food Composition Tables 2018; FAO: Nairobi, Kenya, 2018; p. 254. [Google Scholar]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jedras, M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 2013, 4, 287–291. [Google Scholar]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In vitro crude protein digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef]
- Kulma, M.; Plachý, V.; Kouřimská, L.; Vrabec, V.; Bubová, T.; Adámková, A.; Hučko, B. Nutritional value of three Blattodea species used as feed for animals. J. Anim. Feed Sci. 2016, 25, 354–360. [Google Scholar] [CrossRef]
- Lenaerts, S.; Van Der Borght, M.; Callens, A.; Van Campenhout, L. Suitability of microwave drying for mealworms (Tenebrio molitor) as alternative to freeze drying: Impact on nutritional quality and colour. Food Chem. 2018, 254, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Caparros Megido, R.; Poelaert, C.; Ernens, M.; Liotta, M.; Blecker, C.; Danthine, S.; Tyteca, E.; Haubruge, É.; Alabi, T.; Bindelle, J. Effect of household cooking techniques on the microbiological load and the nutritional quality of mealworms (Tenebrio molitor L. 1758). Food Res. Int. 2018, 106, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Vazquez-Gutierrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow mealworm protein for food purposes-extraction and functional properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef]
- Bednářová, M.; Borkovcová, M.; Komprda, T. Purine derivate content and amino acid profile in larval stages of three edible insects. J. Sci. Food Agric. 2014, 94, 71–76. [Google Scholar] [CrossRef]
- Adámková, A.; Kouřimská, L.; Borkovcová, M.; Kulma, M.; Mlček, J. Nutritional values of edible Coleoptera (Tenebrio molitor, Zophobas morio and Alphitobius diaperinus) reared in the Czech Republic. Potravinarstvo 2016, 10, 663–671. [Google Scholar]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia Pacific Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Francardi, V.; Cito, A.; Fusi, S.; Botta, M.; Dreassi, E. Linseed to increase N-3 fatty acids in Tenebrio molitor (Coleoptera Tenebrionidae). Redia 2017, 100, 73–76. [Google Scholar]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.F.; van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Sabolová, M.; Adámková, A.; Kouřimská, L.; Chrpová, D.; Pánek, J. Minor lipophilic compounds in edible insects. Potravinarstvo 2016, 10, 400–406. [Google Scholar] [CrossRef]
- Simon, E.; Baranyai, E.; Braun, M.; Fábián, I.; Tóthmérész, B. Elemental concentration in mealworm beetle (Tenebrio molitor L.) during metamorphosis. Biol. Trace Elem. Res. 2013, 154, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Latunde-Dada, G.O.; Yang, W.; Vera Aviles, M. In vitro iron availability from insects and sirloin beef. J. Agric. Food Chem. 2016, 64, 8420–8424. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.; Van Keulen, P.; Finke, M.D.; Baines, F.M.; Vermeulen, M.; Bosch, G. Evidence of vitamin D synthesis in insects exposed to UVb light. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Purschke, B.; Sanchez, Y.D.M.; Jäger, H. Centrifugal fractionation of mealworm larvae (Tenebrio molitor, L.) for protein recovery and concentration. LWT 2018, 89, 224–228. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Yi, L.; Van Boekel, M.; Lakemond, C.M.M. Extracting Tenebrio molitor protein while preventing browning: Effect of pH and NaCl on protein yield. J. Insects Food Feed 2017, 3, 21–31. [Google Scholar] [CrossRef]
- Gould, J.; Wolf, B. Interfacial and emulsifying properties of mealworm protein at the oil/water interface. Food Hydrocoll. 2018, 77, 57–65. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- da Silva, F.K.P.; Brück, D.W.; Brück, W.M. Isolation of proteolytic bacteria from mealworm (Tenebrio molitor) exoskeletons to produce chitinous material. FEMS Microbiol. Lett. 2017, 364, 1–6. [Google Scholar] [CrossRef]
- Purschke, B.; Stegmann, T.; Schreiner, M.; Jäger, H. Pilot-scale supercritical CO2 extraction of edible insect oil from Tenebrio molitor L. larvae—Influence of extraction conditions on kinetics, defatting performance and compositional properties. Eur. J. Lipid Sci. Technol. 2017, 119, 1600134. [Google Scholar] [CrossRef]
- Biasato, I.; De Marco, M.; Rotolo, L.; Renna, M.; Lussiana, C.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Costa, P.; Gai, F. Effects of dietary Tenebrio molitor meal inclusion in free-range chickens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Gasco, L.; Dabbou, S.; Trocino, A.; Xiccato, G.; Capucchio, M.T.; Biasato, I.; Dezzutto, D.; Birolo, M.; Meneguz, M.; Schiavone, A. Effect of dietary supplementation with insect fats on growth performance, digestive efficiency and health of rabbits. J. Anim. Sci. Biotechnol. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Gasco, L.; Dabbou, S.; Gai, F.; Brugiapaglia, A.; Schiavone, A.; Birolo, M.; Xiccato, G.; Trocino, A. Quality and consumer acceptance of meat from rabbits fed diets in which soybean oil is replaced with black soldier fly and yellow mealworm fats. Animals 2019, 9, 629. [Google Scholar] [CrossRef]
- Pichova, K.; Nordgreen, J.; Leterrier, C.; Kostal, L.; Moe, R.O. The effects of food-related environmental complexity on litter directed behaviour, fear and exploration of novel stimuli in young broiler chickens. Appl. Anim. Behav. Sci. 2016, 174, 83–89. [Google Scholar] [CrossRef]
- Seehuus, B.; Mendl, M.; Keeling, L.J.; Blokhuis, H. Disrupting motivational sequences in chicks: Are there affective consequences? Appl. Anim. Behav. Sci. 2013, 148, 85–92. [Google Scholar] [CrossRef]
- Iaconisi, V.; Bonelli, A.; Pupino, R.; Gai, F.; Parisi, G. Mealworm as dietary protein source for rainbow trout: Body and fillet quality traits. Aquaculture 2018, 484, 197–204. [Google Scholar] [CrossRef]
- Pasmans, F.; Janssens, G.P.J.; Sparreboom, M.; Jiang, J.P.; Nishikawa, K. Reproduction, development, and growth response to captive diets in the Shangcheng stout salamander, Pachyhynobius shangchengensis (Amphibia, Urodela, Hynobiidae). Asian Herpetol. Res. 2012, 3, 192–197. [Google Scholar]
- Gregorovičová, M.; Černíková, A. Reactions of green lizards (Lacerta viridis) to major repellent compounds secreted by Graphosoma lineatum (Heteroptera: Pentatomidae). Zoology 2015, 118, 176–182. [Google Scholar] [CrossRef]
- Gregorovičová, M.; Černíková, A. Reactions of leopard geckos (Eublepharis macularius) to defensive secretion of Graphosoma lineatum (Heteroptera Pentatomidae): An experimental approach. Ethol. Ecol. Evol. 2016, 28, 367–384. [Google Scholar] [CrossRef]
- Gimmel, A.; Kempf, H.; Öfner, S.; Müller, D.; Liesegang, A. Cholelithiasis in adult bearded dragons: Retrospective study of nine adult bearded dragons (Pogona vitticeps) with cholelithiasis between 2013 and 2015 in southern Germany. J. Anim. Physiol. Anim. Nutr. 2017, 101, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Adamová-Ježová, D.; Hospodková, E.; Fuchsová, L.; Štys, P.; Exnerová, A. Through experience to boldness? Deactivation of neophobia towards novel and aposematic prey in three European species of tits (Paridae). Behav. Process. 2016, 131, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Turini, A.; Veselý, P.; Fuchs, R. Five species of passerine bird differ in their ability to detect Batesian mimics. Biol. J. Linn. Soc. 2016, 117, 832–841. [Google Scholar] [CrossRef]
- Parois, S.; Calandreau, L.; Kraimi, N.; Gabriel, I.; Leterrier, C. The influence of a probiotic supplementation on memory in quail suggests a role of gut microbiota on cognitive abilities in birds. Behav. Brain Res. 2017, 331, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Budenz, T.; Denzinger, A.; Schnitzler, H.-U. Reduction of emission level in approach signals of greater mouse-eared bats (Myotis myotis): No evidence for a closed loop control system for intensity compensation. PLoS ONE 2018, 13, 1–20. [Google Scholar] [CrossRef]
- Bańbura, J.; Babura, M.; Gldalski, M.; Kaliński, A.; Marciniak, B.; Markowski, M.; Michalski, M.; Nadolski, J.; Skwarska, J.; Wawrzyniak, J. Consequences of experimental changes in the rearing conditions of Blue Tit Cyanistes caeruleus and Great Tit Parus major nestlings. Acta Ornithol. 2013, 48, 129–139. [Google Scholar] [CrossRef]
- Belivanov, Y.K.; Hambäck, P.A. The time scale of isotope signals in spiders: Molting the remains of a previous diet. Entomol. Exp. Appl. 2015, 156, 271–278. [Google Scholar] [CrossRef]
- Burgess, M.D.; Woolcock, D.; Hales, R.B.; Waite, R.; Hales, A.J. Captive husbandry and socialization of the red-billed chough (P yrrhocorax pyrrhocorax). Zoo Biol. 2012, 31, 725–735. [Google Scholar] [CrossRef]
- Jones, R.S.; Davis, S.C.; Speed, M.P. Defence cheats can degrade protection of chemically defended prey. Ethology 2013, 119, 52–57. [Google Scholar] [CrossRef]
- Barnett, C.A.; Skelhorn, J.; Bateson, M.; Rowe, C. Educated predators make strategic decisions to eat defended prey according to their toxin content. Behav. Ecol. 2012, 23, 418–424. [Google Scholar] [CrossRef]
- Bloxham, L.; Bateson, M.; Bedford, T.; Brilot, B.; Nettle, D. The memory of hunger: Developmental plasticity of dietary selectivity in the European starling, Sturnus vulgaris. Anim. Behav. 2014, 91, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, M.; Halpin, C.G.; Rowe, C. Ambient temperature influences birds’ decisions to eat toxic prey. Anim. Behav. 2013, 86, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Carle, T.; Rowe, C. Avian predators change their foraging strategy on defended prey when undefended prey are hard to find. Anim. Behav. 2014, 93, 97–103. [Google Scholar] [CrossRef]
- Halpin, C.G.; Skelhorn, J.; Rowe, C. Predators’ decisions to eat defended prey depend on the size of undefended prey. Anim. Behav. 2013, 85, 1315–1321. [Google Scholar] [CrossRef]
- Smith, K.E.; Halpin, C.G.; Rowe, C. Body size matters for aposematic prey during predator aversion learning. Behav. Process. 2014, 109, 173–179. [Google Scholar] [CrossRef]
- Motte, C.; Rios, A.; Lefebvre, T.; Do, H.; Henry, M.; Jintasataporn, O. Replacing fish meal with defatted insect meal (Yellow Mealworm Tenebrio molitor) improves the growth and immunity of pacific white shrimp (Litopenaeus vannamei). Animals 2019, 9, 258. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Pawełczyk, P.; Różyńska, J.; Golusik, J.; Mikołajczak, Z.; Józefiak, D. Do insects smell attractive to dogs? A comparison of dog reactions to insects and commercial feed aromas—A preliminary study. Ann. Anim. Sci. 2018, 18, 795–800. [Google Scholar] [CrossRef]
- Osimani, A.; Cardinali, F.; Aquilanti, L.; Garofalo, C.; Roncolini, A.; Milanović, V.; Pasquini, M.; Tavoletti, S.; Clementi, F. Occurrence of transferable antibiotic resistances in commercialized ready-to-eat mealworms (Tenebrio molitor L.). Int. J. Food Microbiol. 2017, 263, 38–46. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Ruschioni, S.; Riolo, P.; Isidoro, N.; Loreto, N.; Galarini, R. Distribution of transferable antibiotic resistance genes in laboratory-reared edible mealworms (Tenebrio molitor L.). Front. Microbiol. 2018, 9, 2702. [Google Scholar] [CrossRef]
- Milanović, V.; Osimani, A.; Roncolini, A.; Garofalo, C.; Aquilanti, L.; Pasquini, M.; Tavoletti, S.; Vignaroli, C.; Canonico, L.; Ciani, M. Investigation of the dominant microbiota in ready-to-eat grasshoppers and mealworms and quantification of carbapenem resistance genes by qPCR. Front. Microbiol. 2018, 9, 3036. [Google Scholar] [CrossRef]
- Barre, A.; Velazquez, E.; Delplanque, A.; Caze-Subra, S.; Bienvenu, F.; Bienvenu, J.; Benoist, H.; Rougé, P. Cross-reacting allergens of edible insects. Rev. Fr. D Allergol. 2016, 56, 522–532. [Google Scholar] [CrossRef]
- Broekman, H.; Knulst, A.; den Hartog Jager, S.; Monteleone, F.; Gaspari, M.; De Jong, G.; Houben, G.; Verhoeckx, K. Effect of thermal processing on mealworm allergenicity. Mol. Nutr. Food Res. 2015, 59, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Broekman, H.; Verhoeckx, K.C.; den Hartog Jager, C.F.; Kruizinga, A.G.; Pronk-Kleinjan, M.; Remington, B.C.; Bruijnzeel-Koomen, C.A.; Houben, G.F.; Knulst, A.C. Majority of shrimp-allergic patients are allergic to mealworm. J. Allergy Clin. Immunol. 2016, 137, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Broekman, H.C.H.P.; Knulst, A.C.; de Jong, G.; Gaspari, M.; den Hartog Jager, C.F.; Houben, G.F.; Verhoeckx, K.C.M. Is mealworm or shrimp allergy indicative for food allergy to insects? Mol. Nutr. Food Res. 2017, 61, 1601061. [Google Scholar] [CrossRef] [PubMed]
- Broekman, H.C.H.P.; Knulst, A.C.; den Hartog Jager, C.F.; van Bilsen, J.H.M.; Raymakers, F.M.L.; Kruizinga, A.G.; Gaspari, M.; Gabriele, C.; Bruijnzeel-Koomen, C.A.F.M.; Houben, G.F. Primary respiratory and food allergy to mealworm. J. Allergy Clin. Immunol. 2017, 140, 600–603. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Bastiaan-Net, S.; de Jong, N.W.; Wichers, H.J. Influence of processing and in vitro digestion on the allergic cross-reactivity of three mealworm species. Food Chem. 2016, 196, 1075–1083. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; Van Broekhoven, S.; Broekman, H.; Den Hartog Jager, C.F.; Gaspari, M.; de Jong, G.A.H.; Wichers, H.; Van Hoffen, E.; Houben, G.F.; Knulst, A.C. Are house dust mite or shellfish allergic patients at risk when consuming food containing mealworm proteins. Toxicol. Lett. 2013, 221, S119–S120. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; van Broekhoven, S.; den Hartog-Jager, C.F.; Gaspari, M.; de Jong, G.A.H.; Wichers, H.J.; van Hoffen, E.; Houben, G.F.; Knulst, A.C. House dust mite (Der p 10) and crustacean allergic patients may react to food containing Yellow mealworm proteins. Food Chem. Toxicol. 2014, 65, 364–373. [Google Scholar] [CrossRef]
- Nebbia, S.; Lamberti, C.; Giorgis, V.; Giuffrida, M.G.; Manfredi, M.; Marengo, E.; Pessione, E.; Schiavone, A.; Boita, M.; Brussino, L. The cockroach allergen-like protein is involved in primary respiratory and food allergy to yellow mealworm (Tenebrio molitor). Clin. Exp. Allergy 2019, 49, 1379–1382. [Google Scholar] [CrossRef]
- Bednarska, A.J.; Świątek, Z. Subcellular partitioning of cadmium and zinc in mealworm beetle (Tenebrio molitor) larvae exposed to metal-contaminated flour. Ecotoxicol. Environ. Saf. 2016, 133, 82–89. [Google Scholar] [CrossRef]
- Mlček, J.; Adamek, M.; Adámková, A.; Borkovcová, M.; Bednářová, M.; Skácel, J. Detection of selected heavy metals and micronutrients in edible insect and their dependency on the feed using XRF spectrometry. Potravin. Slovak J. Food Sci. 2017, 11, 725–730. [Google Scholar]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Van der Fels-Klerx, H.J.; Camenzuli, L.; Van der Lee, M.K.; Oonincx, D. Uptake of cadmium, lead and arsenic by Tenebrio molitor and Hermetia illucens from contaminated substrates. PLoS ONE 2016, 11, e0166186. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Boukouvala, M.C.; Mavroforos, M.E.; Kontodimas, D.C. Efficacy of alpha-cypermethrin and thiamethoxam against Trogoderma granarium Everts (Coleoptera: Dermestidae) and Tenebrio molitor L. (Coleoptera: Tenebrionidae) on concrete. J. Stored Prod. Res. 2015, 62, 101–107. [Google Scholar] [CrossRef]
- Houbraken, M.; Spranghers, T.; De Clercq, P.; Cooreman-Algoed, M.; Couchement, T.; De Clercq, G.; Verbeke, S.; Spanoghe, P. Pesticide contamination of Tenebrio molitor (Coleoptera: Tenebrionidae) for human consumption. Food Chem. 2016, 201, 264–269. [Google Scholar] [CrossRef]
- Maliszewska, J.; TĘGowska, E. Is there a relationship between insect metabolic rate and mortality of mealworms Tenebrio molitor L. after insecticide exposure? J. Cent. Eur. Agric. 2016, 17, 685–694. [Google Scholar] [CrossRef]
- Mora, C.A.; Halter, J.G.; Adler, C.; Hund, A.; Anders, H.; Yu, K.; Stark, W.J. Application of the Prunus spp. cyanide seed defense system onto wheat: Reduced insect feeding and field growth tests. J. Agric. Food Chem. 2016, 64, 3501–3507. [Google Scholar] [CrossRef]
- Spochacz, M.; Chowański, S.; Szymczak, M.; Lelario, F.; Bufo, S.A.; Adamski, Z. Sublethal effects of solanum nigrum fruit extract and its pure glycoalkaloids on the physiology of Tenebrio molitor (Mealworm). Toxins 2018, 10, 504. [Google Scholar] [CrossRef]
- Bosch, G.; Van Der Fels-Klerx, H.J.; Rijk, T.C.d.; Oonincx, D.G.A.B. Aflatoxin B1 tolerance and accumulation in black soldier fly larvae (Hermetia illucens) and yellow mealworms (Tenebrio molitor). Toxins 2017, 9, 185. [Google Scholar] [CrossRef]
- Edgington, S.; Thompson, E.; Moore, D.; Hughes, K.A.; Bridge, P. Investigating the insecticidal potential of Geomyces (Myxotrichaceae: Helotiales) and Mortierella (Mortierellacea: Mortierellales) isolated from Antarctica. SpringerPlus 2014, 3, 289. [Google Scholar] [CrossRef]
- Novikov, V.V.; Sheiman, I.M.; Yablokova, E.V.; Fesenko, E.E. The modulating effect of weak combined magnetic fields on the duration of the stages of metamorphosis of the Tenebrio molitor mealworm beetle. Biophysics 2014, 59, 940–943. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Gutierrez, J.M.; De Rijk, T.C.; De Nijs, W.C.M.; Van Loon, J.J.A. Degradation and excretion of the Fusarium toxin deoxynivalenol by an edible insect, the Yellow mealworm (Tenebrio molitor L.). World Mycotoxin J. 2017, 10, 163–169. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of processed edible insect products—Results of a preliminary survey. Int. J. Food Microbiol. 2017, 243, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Azzollini, D.; Derossi, A.; Severini, C. Understanding the drying kinetic and hygroscopic behaviour of larvae of yellow mealworm (Tenebrio molitor) and the effects on their quality. J. Insects Food Feed 2016, 2, 233–243. [Google Scholar] [CrossRef]
- Purschke, B.; Brüggen, H.; Scheibelberger, R.; Jäger, H. Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.). Eur. Food Res. Technol. 2018, 244, 269–280. [Google Scholar] [CrossRef]
- Kröncke, N.; Böschen, V.; Woyzichovski, J.; Demtröder, S.; Benning, R. Comparison of suitable drying processes for mealworms (Tenebrio molitor). Innov. Food Sci. Emerg. Technol. 2018, 50, 20–25. [Google Scholar] [CrossRef]
- Kröncke, N.; Grebenteuch, S.; Keil, C.; Demtröder, S.; Kroh, L.; Thünemann, A.F.; Benning, R.; Haase, H. Effect of different drying methods on nutrient quality of the yellow mealworm (Tenebrio molitor L.). Insects 2019, 10, 84. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Desmedt, S.; Blecker, C.; Béra, F.; Haubruge, É.; Alabi, T.; Francis, F. Microbiological load of edible insects found in Belgium. Insects 2017, 8, 12. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Lenaerts, S.; Callens, A.; Van Campenhout, L. Effect of blanching followed by refrigerated storage or industrial microwave drying on the microbial load of yellow mealworm larvae (Tenebrio molitor). Food Control 2017, 71, 311–314. [Google Scholar] [CrossRef]
- Adámek, M.; Mlček, J.; Adámková, A.; Suchánková, J.; Janalíková, M.; Borkovcová, M.; Bednářová, M. Effect of different storage conditions on the microbiological characteristics of insect. Potravin. Slovak J. Food Sci. 2018, 12, 248–253. [Google Scholar] [CrossRef]
- Borremans, A.; Lenaerts, S.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Marination and fermentation of yellow mealworm larvae (Tenebrio molitor). Food Control 2018, 92, 47–52. [Google Scholar] [CrossRef]
- An, B.; Sam, C.; Dries, V.; Ruben, S.; Christel, V.; Mik, V.D.B.; Bart, L.; Leen, V.C. Comparison of Six Commercial Meat Starter Cultures for the Fermentation of Yellow Mealworm (Tenebrio molitor) Paste. Microorganisms 2019, 7, 540. [Google Scholar] [CrossRef] [PubMed]
- Melis, R.; Braca, A.; Mulas, G.; Sanna, R.; Spada, S.; Serra, G.; Fadda, M.L.; Roggio, T.; Uzzau, S.; Anedda, R. Effect of freezing and drying processes on the molecular traits of edible yellow mealworm. Innov. Food Sci. Emerg. Technol. 2018, 48, 138–149. [Google Scholar] [CrossRef]
- Poelaert, C.; Francis, F.; Alabi, T.; Megido, R.C.; Crahay, B.; Bindelle, J.; Beckers, Y. Protein value of two insects, subjected to various heat treatments, using growing rats and the protein digestibility-corrected amino acid score. J. Insects Food Feed 2018, 4, 77–87. [Google Scholar] [CrossRef]
- De Smet, J.; Lenaerts, S.; Borremans, A.; Scholliers, J.; Van Der Borght, M.; Van Campenhout, L. Stability assessment and laboratory scale fermentation of pastes produced on a pilot scale from mealworms (Tenebrio molitor). LWT 2019, 102, 113–121. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Pasquini, M.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N. The bacterial biota of laboratory-reared edible mealworms (Tenebrio molitor L.): From feed to frass. Int. J. Food Microbiol. 2018, 272, 49–60. [Google Scholar] [CrossRef]
- Garofalo, C.; Osimani, A.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Clementi, F. The microbiota of marketed processed edible insects as revealed by high-throughput sequencing. Food Microbiol. 2017, 62, 15–22. [Google Scholar] [CrossRef]
- Klunder, H.C.; Wolkers-Rooijackers, J.; Korpela, J.M.; Nout, M.J.R. Microbiological aspects of processing and storage of edible insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Froehling, A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Cold atmospheric pressure plasma processing of insect flour from Tenebrio molitor: Impact on microbial load and quality attributes in comparison to dry heat treatment. Innov. Food Sci. Emerg. Technol. 2016, 36, 277–286. [Google Scholar] [CrossRef]
- Fasolato, L.; Cardazzo, B.; Carraro, L.; Fontana, F.; Novelli, E.; Balzan, S. Edible processed insects from e-commerce: Food safety with a focus on the Bacillus cereus group. Food Microbiol. 2018, 76, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Fröhling, A.; Reineke, K.; Knorr, D.; Boguslawski, S.; Ehlbeck, J.; Schlüter, O. Comparison of volumetric and surface decontamination techniques for innovative processing of mealworm larvae (Tenebrio molitor). Innov. Food Sci. Emerg. Technol. 2014, 26, 232–241. [Google Scholar] [CrossRef]
- Stoops, J.; Crauwels, S.; Waud, M.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial community assessment of mealworm larvae (Tenebrio molitor) and grasshoppers (Locusta migratoria migratorioides) sold for human consumption. Food Microbiol. 2016, 53, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Stoops, J.; Vandeweyer, D.; Crauwels, S.; Verreth, C.; Boeckx, H.; Van Der Borght, M.; Claes, J.; Lievens, B.; Van Campenhout, L. Minced meat-like products from mealworm larvae (Tenebrio molitor and Alphitobius diaperinus): Microbial dynamics during production and storage. Innov. Food Sci. Emerg. Technol. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Metagenetic analysis of the bacterial communities of edible insects from diverse production cycles at industrial rearing companies. Int. J. Food Microbiol. 2017, 261, 11–18. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Microbial counts of mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus and Gryllodes sigillatus) from different rearing companies and different production batches. Int. J. Food Microbiol. 2017, 242, 13–18. [Google Scholar] [CrossRef]
- Wynants, E.; Crauwels, S.; Lievens, B.; Luca, S.; Claes, J.; Borremans, A.; Bruyninckx, L.; Van Campenhout, L. Effect of post-harvest starvation and rinsing on the microbial numbers and the bacterial community composition of mealworm larvae (Tenebrio molitor). Innov. Food Sci. Emerg. Technol. 2017, 42, 8–15. [Google Scholar] [CrossRef]
- Czarniewska, E.; Rosiński, G.; Gabała, E.; Kuczer, M. The natural insect peptide Neb-colloostatin induces ovarian atresia and apoptosis in the mealworm Tenebrio molitor. BMC Dev. Biol. 2014, 14, 4. [Google Scholar] [CrossRef][Green Version]
- Czarniewska, E.; Urbański, A.; Chowański, S.; Kuczer, M. The long-term immunological effects of alloferon and its analogues in the mealworm Tenebrio molitor. Insect Sci. 2018, 25, 429–438. [Google Scholar] [CrossRef]
- Walkowiak-Nowicka, K.; Nowicki, G.; Kuczer, M.; Rosiński, G. New activity of yamamarin, an insect pentapeptide, on immune system of mealworm, Tenebrio molitor. Bull. Entomol. Res. 2018, 108, 351–359. [Google Scholar] [CrossRef]
- Niermans, K.; Woyzichovski, J.; Kröncke, N.; Benning, R.; Maul, R. Feeding study for the mycotoxin zearalenone in yellow mealworm (Tenebrio molitor) larvae—Investigation of biological impact and metabolic conversion. Mycotoxin Res. 2019, 35, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, N.T.; Grootaert, P.; Ahlfeld, B.; Klein, G. Practical key to identify entire edible insects sold as foodstuff or feedstuff in central Europe. J. Food Saf. Food Qual. Arch. Lebensm. 2016, 67, 4–11. [Google Scholar]
- Tan, H.S.G.; Verbaan, Y.T.; Stieger, M. How will better products improve the sensory-liking and willingness to buy insect-based foods? Food Res. Int. 2017, 92, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.S.G.; van den Berg, E.; Stieger, M. The influence of product preparation, familiarity and individual traits on the consumer acceptance of insects as food. Food Qual. Prefer. 2016, 52, 222–231. [Google Scholar] [CrossRef]
- Tan, H.S.G.; Fischer, A.R.H.; van Trijp, H.C.M.; Stieger, M. Tasty but nasty? Exploring the role of sensory-liking and food appropriateness in the willingness to eat unusual novel foods like insects. Food Qual. Prefer. 2016, 48, 293–302. [Google Scholar] [CrossRef]
- Tan, H.S.G.; Tibboel, C.J.; Stieger, M. Why do unusual novel foods like insects lack sensory appeal? Investigating the underlying sensory perceptions. Food Qual. Prefer. 2017, 60, 48–58. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Gierts, C.; Blecker, C.; Brostaux, Y.; Haubruge, É.; Alabi, T.; Francis, F. Consumer acceptance of insect-based alternative meat products in Western countries. Food Qual. Prefer. 2016, 52, 237–243. [Google Scholar] [CrossRef]
- Beaumont, P.; Courtois, J.; Van der Brempt, X.; Tollenaere, S. Food-induced anaphylaxis to Tenebrio molitor and allergens implicated. Rev. Française d’Allergologie 2019, 59, 389–393. [Google Scholar] [CrossRef]
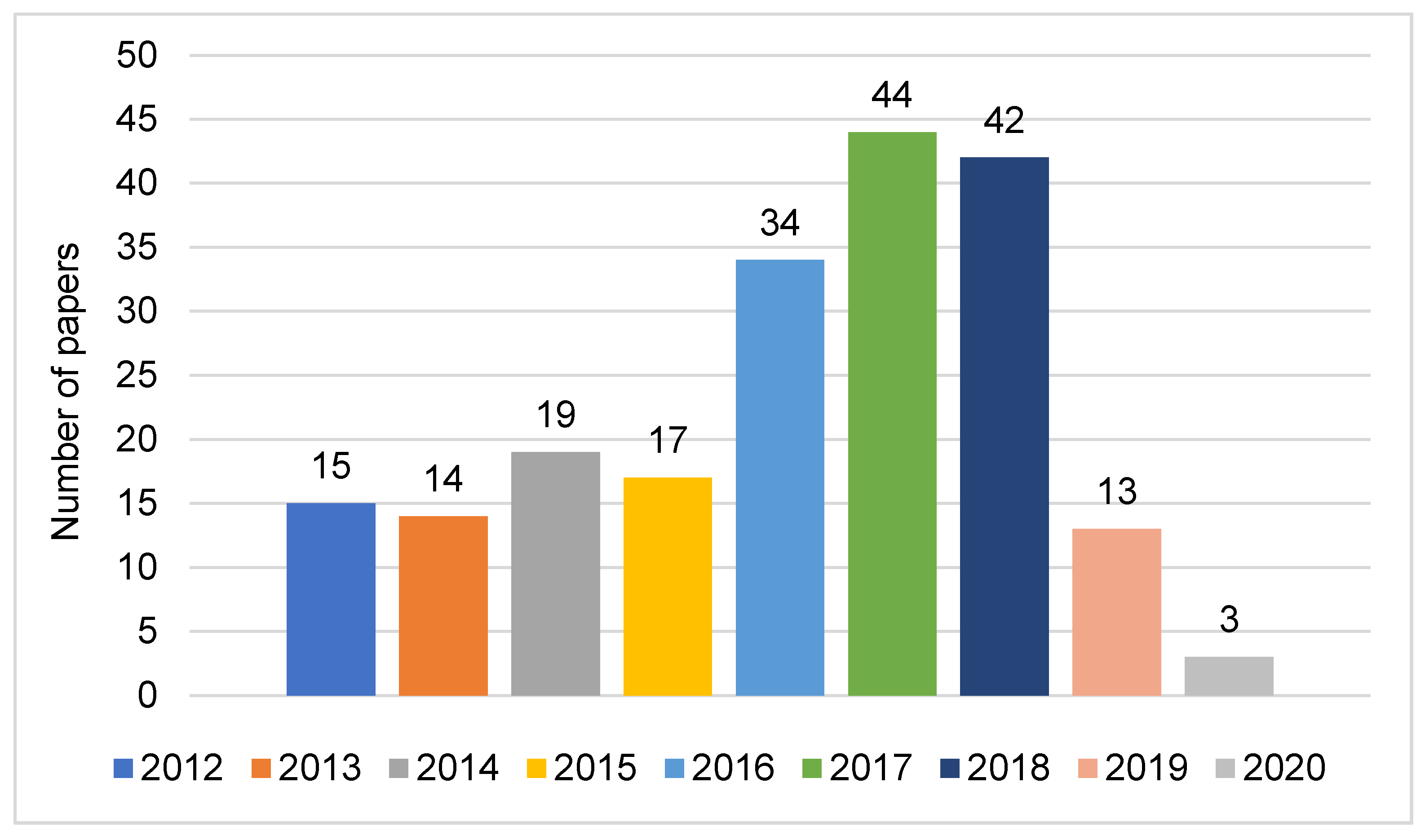


| Research Topic | Topic |
|---|---|
| Mealworm development | Review of the optimal rearing conditions [35]. Rearing of mealworms for use in various by-products (beer brewing, baking/cookies, potato processing and bioethanol production) [40]. Possibility of modifying the fat and fatty acid composition of mealworm by feeding different substrates (ground oat flour, corn flour, wheat flour, chickpea flour, bread and beer yeasts) [76]. Different temperature rates influence larval metabolism, growth rate, efficiency and macronutrient composition [76]. The influence of processing temperature on the fat content of mealworm [77] An analysis of the mealworm digestive system (role of peptidases in digestion) [78]. Isolation and characterization of proline-specific serine peptidase from the anterior midgut of mealworm larvae [79]. |
| Physiology | Use of mealworm beetle as a model species (reproductive characteristics, survival and three components of innate immunity) [80]. Mealworm beetle as a biological model for analysing the effect of lifetime dietary supplementation with astaxanthin (antioxidant) when exposed to early life inflammation [81]. Immune responses of mealworm beetle to the microbial activity of Staphylococcus aureus [82,83]. Correlation between cuticle melanism, immune defence and life-history traits [84]. Impacts of adult density, reproduction period and age on the fecundity of mealworm beetles [85]. Influence of inbreeding on the attractiveness of sexual signalling in TM beetle [86]. The applicability of X-ray microcomputed tomography (μCT) based methods for investigating the insect tracheal system at different stages of development [87,88]. Analysis of hiding behaviour and anti-predator responses of TM beetle exposed to a predator [89,90,91]. |
| Immunity | Testing the mealworm beetle terminal investment hypothesis [92,93]. Correlations between melanism, immune defence by beetle traits at different temperatures and in different sexes [84]. Morphofunctional organization of extrachromosomal nuclear structures in insects [94]. Trans-generational immune priming [95,96]. RNAseq analysis of the temporal dynamics of insect immune responses (TM was the model insect) [97]. Antimicrobial/antifungal immune responses [98,99,100]. Endogenous egg and beetle immune responses [101]. Senescence in immune priming and attractiveness of beetles [102]. Influence of immune challenge [93,103]. |
| Source | Water (%) | Protein (%) | Fat (%) | Ash (%) | Feed Conversion Ratio (kg Feed per kg of Live Weight) | References |
|---|---|---|---|---|---|---|
| Beef (lean) | 75.0 | 22.3 | 1.8 | 1.2 | 10.0 | [109,110] |
| Veal (lean) | 76.4 | 21.3 | 0.8 | 1.2 | - | [109] |
| Pork (lean) | 75.1 | 22.8 | 1.2 | 1.0 | 5.4 | [109,110] |
| Lamb, raw (unspecified part) | 60 | 22.7 | 1 | - | [111] | |
| Goat, lean, raw | 74.6 | 19.5 | 4.3 | 1 | - | [111] |
| Chicken | 75.0 | 22.8 | 0.9 | 1.2 | 2.5 | [109,110] |
| Fish (cod fillet, raw) | 81.6 | 17 | 0.6 | 1.2 | 1.5 (for carp) | [110,111] |
| Mealworm (fresh larvae) | 56.27 | 17.92 | 21.93 | 1.55 | 3.4–6.1 | [74,112] |
| Mealworm (powdered larvae) | 2.43 | 44.72 | 42.48 | 3.69 |
| Life Stage/Processed Form | Protein Content (%) | Fat Content (%) | Ash Content (%) | Dry Matter Content (%) | Comments | References |
|---|---|---|---|---|---|---|
| Raw mealworms | 43 | 40.9 | 3.4 | 85 | Dry matter basis | [116] |
| Mealworm larvae | 52.35 | 18.23 | 4.74 | Dry matter basis | [24] | |
| Fresh larvae | 17.92 | 21.93 | 1.55 | Dry matter basis (different types of diet) | [74] | |
| Fresh larvae | 44.1–53.6 | 22.6–34.5 | 30.2–41.5 | Dry matter basis (different types of diet) | [74] | |
| Freeze-dried larvae | 51.5 | 32.9 | 4.9 | 96.1 | Dry matter basis. Larvae were not starved before the experiment | [117] |
| Fresh larvae | 63.93 | n.d. | 4.37 | 37.55 | Mean value of 3 repetitions | [115] |
| Fried * mealworms | 43 | 64.9 | 2.2 | 63.5 | Dry matter basis | [116] |
| Fatty Acid | C:D | % of Total Fatty Acids |
|---|---|---|
| SFAs | ||
| Lauric acid | C12:0 | 0.3–0.38 |
| Myristic acid | C14:0 | 3.19–5.5 |
| Palmitic acid | C16:0 | 15.5–21.33 |
| Stearic acid | C18:0 | 2.72–7.92 |
| Arachidic acid | C20:0 | 0.16 |
| MUFAs and PUFAs | ||
| Palmitoleic acid | C16:1 | 1.4–2.88 |
| Oleic | C18:1 | 35.83–49.5 |
| Linoleic acid | C18:2 | 16.3–25.4 |
| Linolenic acid | C18:3 | 0.2–0.8 |
| Livestock/Product | References |
|---|---|
| livestock feed | [8,41,45] |
| pigs | [46,47] |
| rabbits | [136,137] |
| poultry | [47,48,49,50,51,52,54,55,135,138,139] |
| fish | [42,56,57,58,59,61,140] |
| reptiles, amphibians | [141,142,143,144] |
| other animals | [62,63]—Barbary partridge; [145]—tit bird; [146]—passerine birds; [65]—Japanese quail; [64,73,147]—bats; [68,148]—dog; [149]—passerine birds; [69]—golden hamsters; [150]—spiders; [151]—red billed chough; [152]—European robin (Erithacus rubecula), great tit (Parus major), European black bird (Turdus merula); [153,154,155,156,157,158]—European starlings (Sturnus vulgaris); [70,71,72]—house sparrows; [66]—pheasants; [67,68]—cats and dogs |
| Topic | References |
|---|---|
| Antibiotic resistance | [82,161,162,163] |
| Allergies | [107,164,165,166,167,168,169,170,171,172] |
| Heavy metals | [77,173,174,175,176] |
| Pesticides | [175,177,178,179,180,181] |
| Hazardous substances, chemicals, toxins, mycotoxins and other compounds | [175,182,183,184,185] |
| Samples | Microbial Species | References |
|---|---|---|
| Insect samples analysed by the Mendel University in Brno. Year of insect breeding: 2012 (killed by freezing), 2015 (killed by boiling water, dried at 103 °C for 12 h, homogenised and stored at room temperature until analysis in January 2017) and 2016 (killed by freezing). | Freshly killed: total microbial counts (2.2 × 108 cfu g−1), enterobacteria (1.9108 cfu g−1), Lactic acid bacteria (7.2 × 107 cfu g−1), yeasts and moulds (8.9 × 103 107 cfu g−1). Frozen: total microbial count (TMC) (3.4 × 107 cfu g−1), enterobacteria (4.2 × 106 cfu g−1), lactic acid bacteria (2.4 × 105 107 cfu g−1), Yeasts and Moulds (3.3 × 103 cfu g−1). Dried: TMC (max. 6.6 × 103 cfu g−1), enterobacteria (˂10 cfu g−1), lactic acid bacteria (up to 3.8 × 103 cfu g−1), yeasts and moulds (up to 1.7 × 104 cfu g−1). | [193] |
| Fully grown, non-starved live larvae from a breeder in Belgium | Enterobacteriaceae, bacterial endospores, lactic acid bacteria, sulphite reducing clostridia. | [194] |
| Flour prepared from T. molitor that were purchased from a local breeder (Germany) | 7.72 cfu g−1 DM: microbial load at the beginning of processing with cold atmospheric pressure plasma. | [203] |
| Live mealworms were bought from a local company (Sixlegs SA, Belgium). Freeze-dried mealworms were also supplied by a local company (BugsInMugs, Belgium) | Total aerobic count (TAC) in untreated freshly killed mealworms (8.58 log cfu g−1) and freeze-dried mealworms (4.47 log cfu g−1), yeast and mould counts (4.70 log cfu g−1). Blanching (1 min) and sterilisation reduced TAC by around 50% in freshly killed mealworm and decreased yeast and mould counts by less than 1 log cfu g−1. | [191] |
| Fresh mealworms reared by the Laboratory of Functional Entomology (Liège University, Belgium) | Total aerobic count. | [116] |
| Pre-packed, shelf-stable insects purchased online from various commercial suppliers (10 samples) | Total aerobic spore count Log10 (˂5 cfu g−1), Bacillus cereus Kocuria rhizofila, Macrococcus spp., and other. | [204] |
| Whole dried mealworm larvae from a company located in The Netherlands. | Enterobacteriaceae, total mesophilic aerobes, lactic acid bacteria, Clostriudium perfrimgens spores, yeasts, Moulds (˂2.00 log cfu g−1 of all enumerated microbiological parameters). No Salmonella spp., and Listeria monocytogenes in 25 g of samples. | [201] |
| Insect samples (reared in Europe or Asia) were obtained from Germany and the Netherlands between 2015 and 2016. Different processing methods were analysed | Aerobic bacterial count: Enterobacteriaceae (1/3 samples), E. coli (3/3 samples), coagulase-positive staphylococci (3/3 samples) (mealworms were dried, powdered and cooked). Dried mealworms were colonised by Listeria ivanivii, Penicillium spp., Mucor spp. | [186] |
| Live mealworm larvae supplied by Van de Ven, the Netherlands, were processed with the use of different methods and stored for different periods of time | Enterobacteriaceae, Bacterial spores. | [202] |
| 30 samples were purchased online from European (Belgium and the Netherlands) and Asian suppliers | Staphilococcus spp., Exiguobacterium sp., Eikenella corrodens, Eikenella sp., Bacillus sp. were identified in more than 82% of the samples. | [163] |
| Samples of processed (dried) edible insects were purchased online from two suppliers in the Netherlands and one supplier in Belgium. | Enterobacteriaceae (˂log 1 cfu g−1), total mesophilic aerobes (up to log 4.8 cfu g−1), sulphite-reducing clostridia (up to log 4 cfu g−1), Staphylococcus aureus (˂log 1 cfu g−1), Bacillus cereus (˂log 1 cfu g−1), lactic acid bacteria (up to log 2.8 cfu g−1), yeasts (up to log 2.4 cfu g−1—in samples from one Dutch supplier; ˂log 1 cfu g−1 in the remaining samples), moulds (up to log 2.3 cfu g−1—in samples from one Dutch supplier; ˂log 1 cfu g−1 in the remaining samples). | [199] |
| Second generation, last instar mealworms purchased from a local pet store (Italy). Larval frass was also analysed. | Enterobacteriaceae (Xenorhabdus spp., Enterobacter spp. and Pantoea spp.), Lactic acid bacteria (Lactococcus garviae, Enterococcus: E. faecium, E. gallinarum, E. mundtii), mesophilic aerobes, spore-forming bacteria. Psychrobacillus spp., Serratia spp., Erwinia spp., Aeromonas spp., Burkholderia spp., Klebsiella spp. and other. | [200] |
| Live mealworm larvae were purchased from Futtertier-Shop.de (Germany) | Microbial surface load. | [205] |
| Samples were obtained from a supplier of organic insects (Belgium) | Enterobacteriaceae, lactic acid bacteria, bacterial endospores, yeasts and moulds. (Propionibacterium sp., Lactobacillus sp., Streptococcus sp., Haemophilus sp., Enterobacteriaceae bacterium, Pseudomonas sp., Staphylococcus sp., Acidovarax sp., Varibaculum sp., Clostridium sp. and other. | [206] |
| Samples were obtained from a supplier of organic insects (Belgium). The insects were used to prepare a minced meat-like product | Enterobacteriaceae, lactic acid bacteria, yeasts and moulds. (Serratia sp., Erwinia sp., Rickettsiealla sp., Spiroplasma sp., Pseudomonas sp., Enterobacter sp., Hafnia sp./Citrobacter sp., Propionibacterium sp.). | [207] |
| Samples were purchased from Belgian and Dutch suppliers | Spiroplasma sp., Erwinia oleae, Eneterobacteriaceae sp., Buttiauxella agrestis, Pseudomonas deceptionensis, Lactococcus sp., Citrobacter koseri, Brevibacillus sp., Enterococcus sp., Clostridia sp. | [208] |
| Samples were purchased from four suppliers of edible insects in Belgium and the Netherlands, and were prepared according to the method described by Stoops et al. (2016). | Lactic acid bacteria, Enterobacteriaceae, aerobic bacterial endospores, psychrotrophic aerobic counts, Yeasts and moulds. | [209] |
| Last instar larvae were purchased from an industrial rearing company in Belgium | Lactic acid bacteria, Enterobacteriaceae, yeasts and moulds, aerobic bacterial endospores. | [192] |
| Mealworm larvae were supplied by an industrial rearing company in Belgium | Enterobacteriaceae, aerobic psychotropic count, yeasts and moulds, aerobic bacterial endospores Erwinia sp., Gammaproteobacteria sp., Lactococcus sp., Enterococcus sp., Cronobacter sp., Enterobacteriaceae sp., Weissella sp., Pseudomonas sp., Staphylococcus sp., Lactobacillus sp., Pseudomonas sp., Pediococcus sp., and other. | [210] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Czachorowski, S.; Peni, D. Will Yellow Mealworm Become a Source of Safe Proteins for Europe? Agriculture 2020, 10, 233. https://doi.org/10.3390/agriculture10060233
Bordiean A, Krzyżaniak M, Stolarski MJ, Czachorowski S, Peni D. Will Yellow Mealworm Become a Source of Safe Proteins for Europe? Agriculture. 2020; 10(6):233. https://doi.org/10.3390/agriculture10060233
Chicago/Turabian StyleBordiean, Anna, Michał Krzyżaniak, Mariusz J. Stolarski, Stanisław Czachorowski, and Dumitru Peni. 2020. "Will Yellow Mealworm Become a Source of Safe Proteins for Europe?" Agriculture 10, no. 6: 233. https://doi.org/10.3390/agriculture10060233
APA StyleBordiean, A., Krzyżaniak, M., Stolarski, M. J., Czachorowski, S., & Peni, D. (2020). Will Yellow Mealworm Become a Source of Safe Proteins for Europe? Agriculture, 10(6), 233. https://doi.org/10.3390/agriculture10060233







