The Joint Effect of Grazing Intensity and Soil Factors on Aboveground Net Primary Production in Hulunber Grasslands Meadow Steppe
Abstract
:1. Introduction
2. Materials and Methods
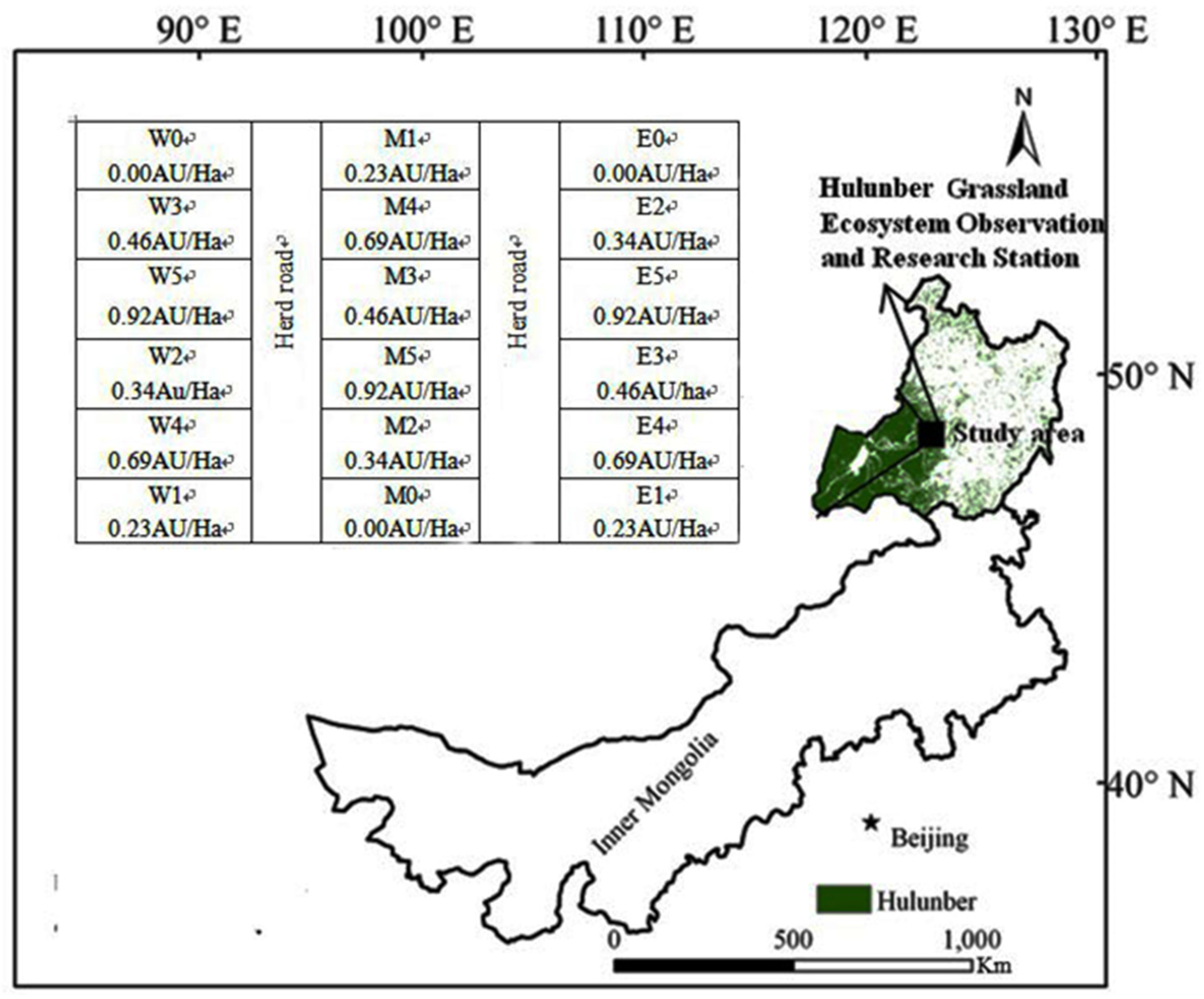
2.1. Study Area
2.2. Experimental Design and Fieldwork
2.3. Forage Dry Matter Intake (DMI) Estimated Using Grazing Cages (Cage Technique)
2.4. Grazing Intensity and ANPP
2.5. Growth Rate (GR) and Relative Growth Rate (RGR)
2.6. Soil Moisture and Temperature Measurements
2.7. C3 and C4 Plants
2.8. Soil Nutrient Contents
2.9. Statistical Analysis
3. Results
3.1. The Relationship of Climatic Conditions with Productivity Levels
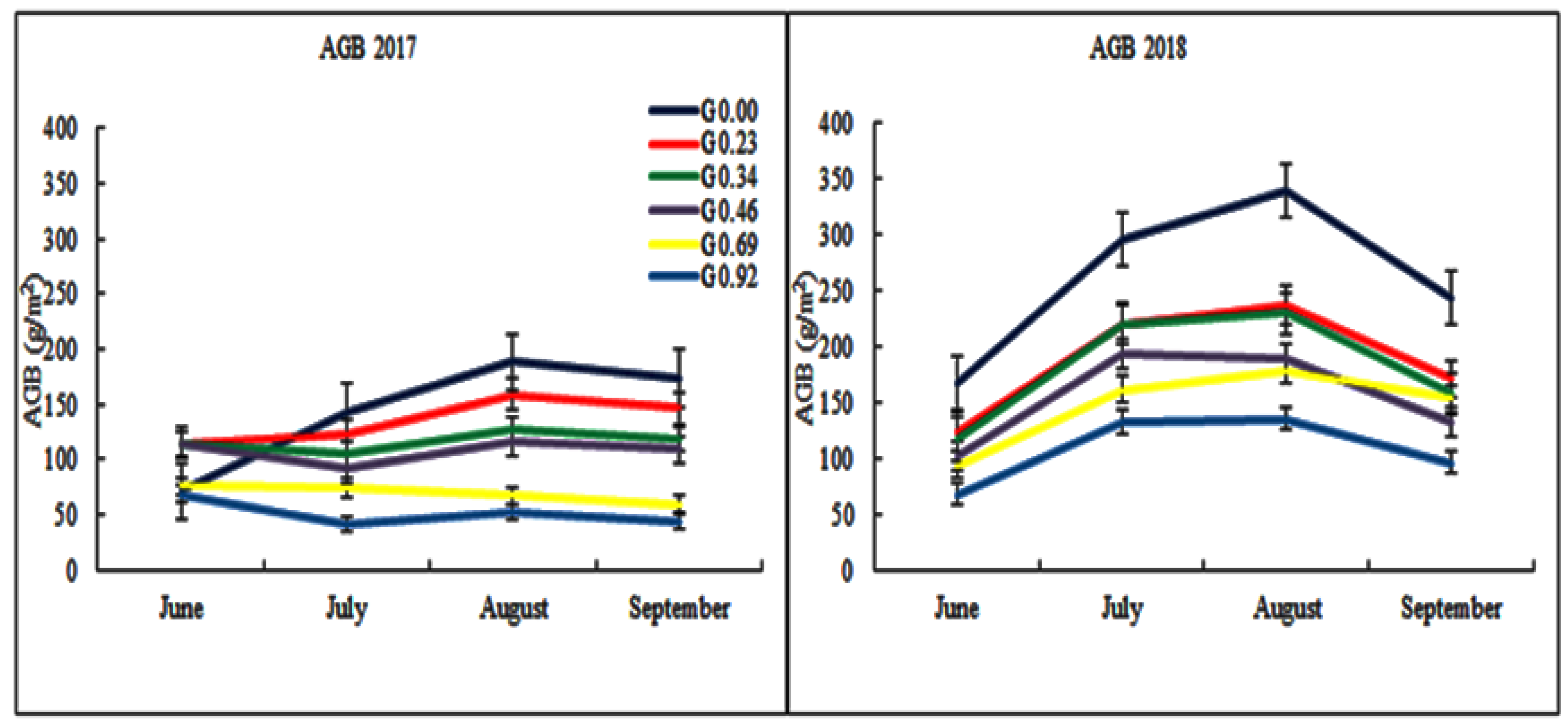
3.2. Grazing Effects on Aboveground Biomass (AGB)
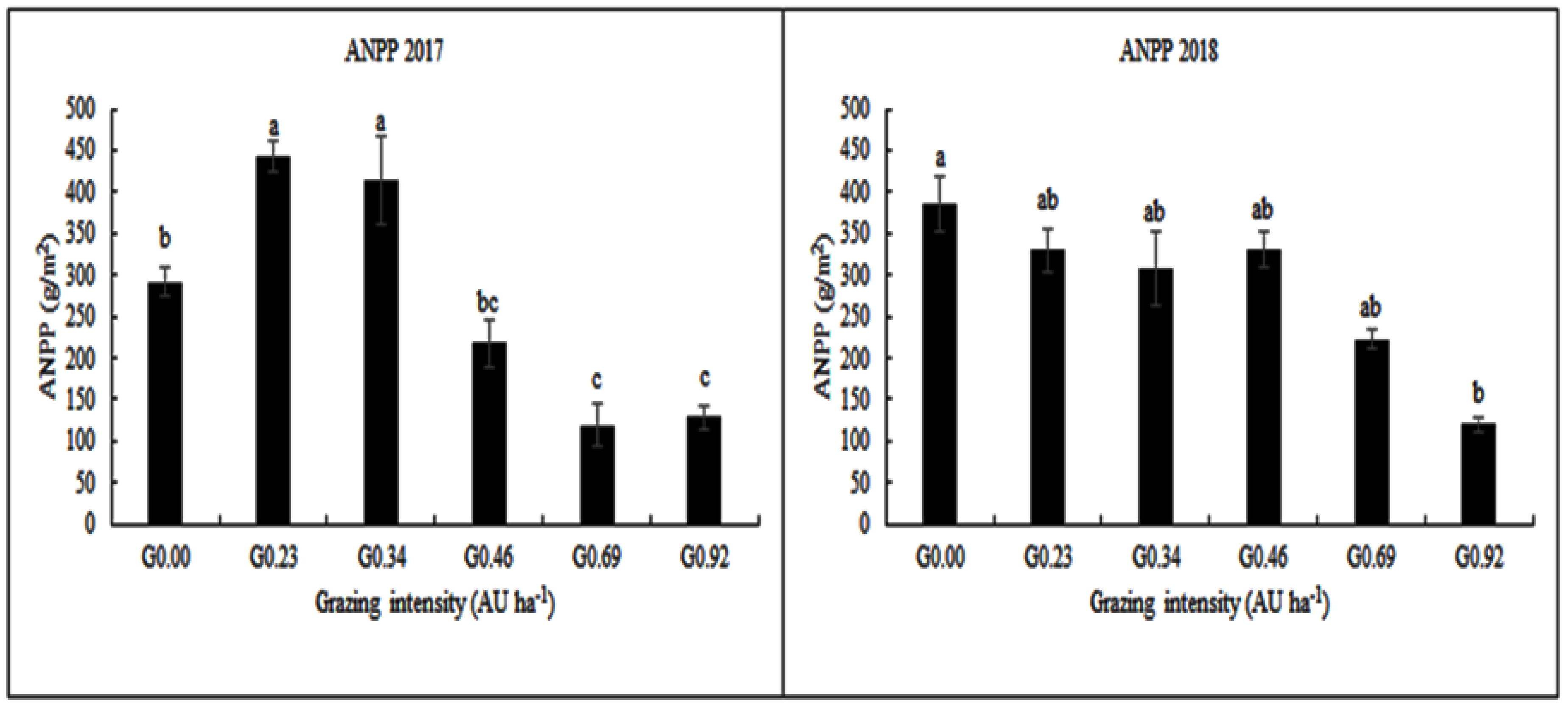
3.3. Grazing Intensity Effects on Aboveground Net Primary Production (ANPP)
3.4. Grazing Effects on the Dry Matter Intake
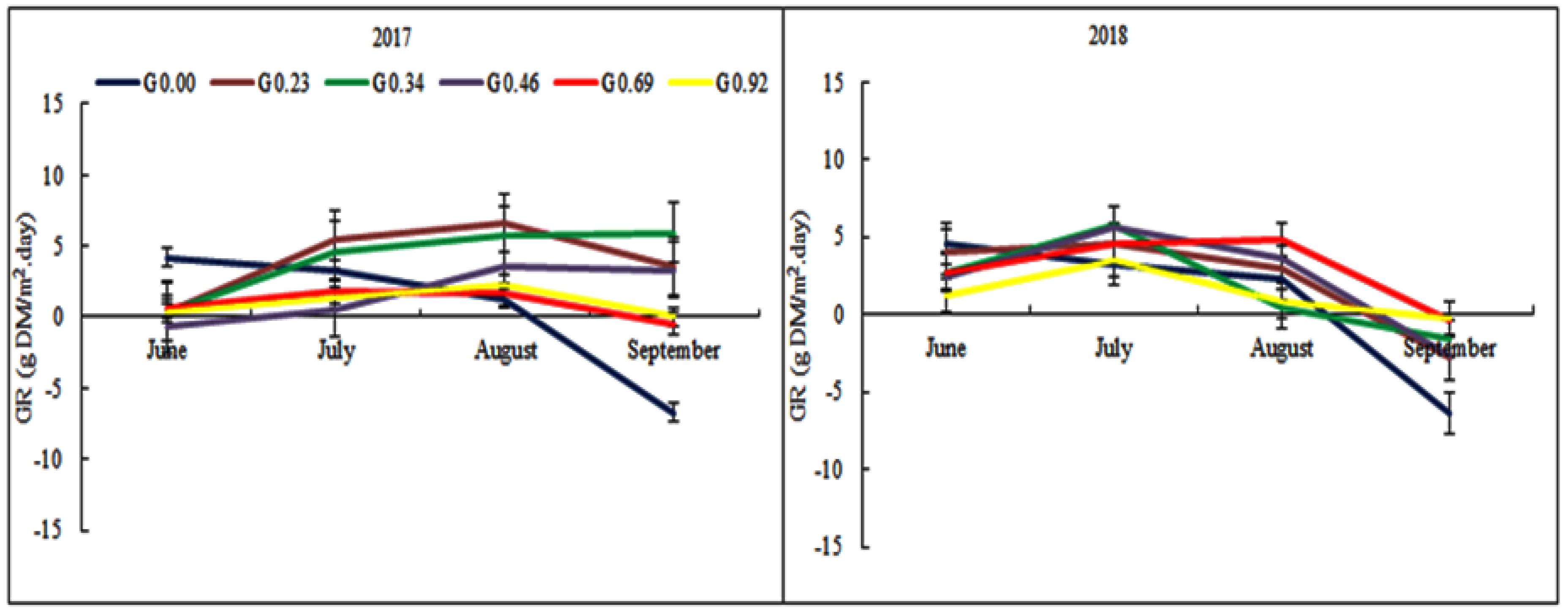
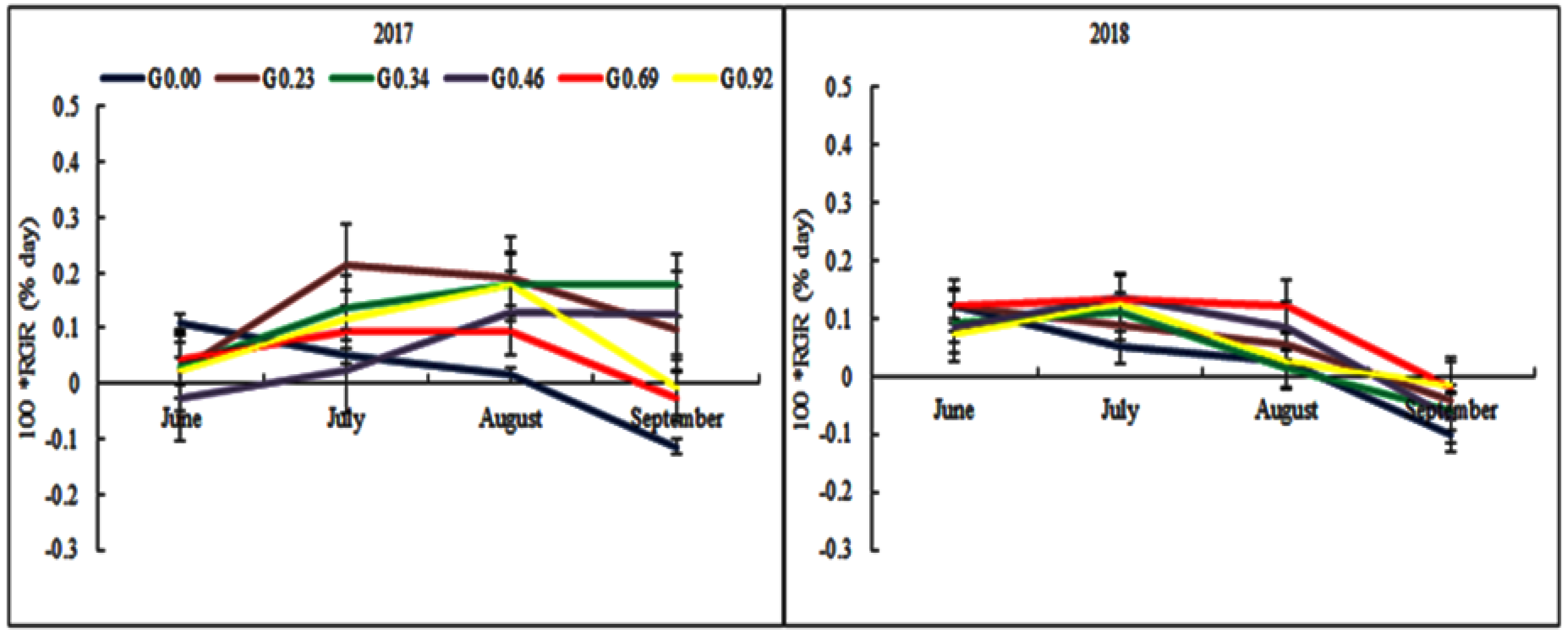
3.5. The Effect of Grazing Intensity on the Herbage Growth
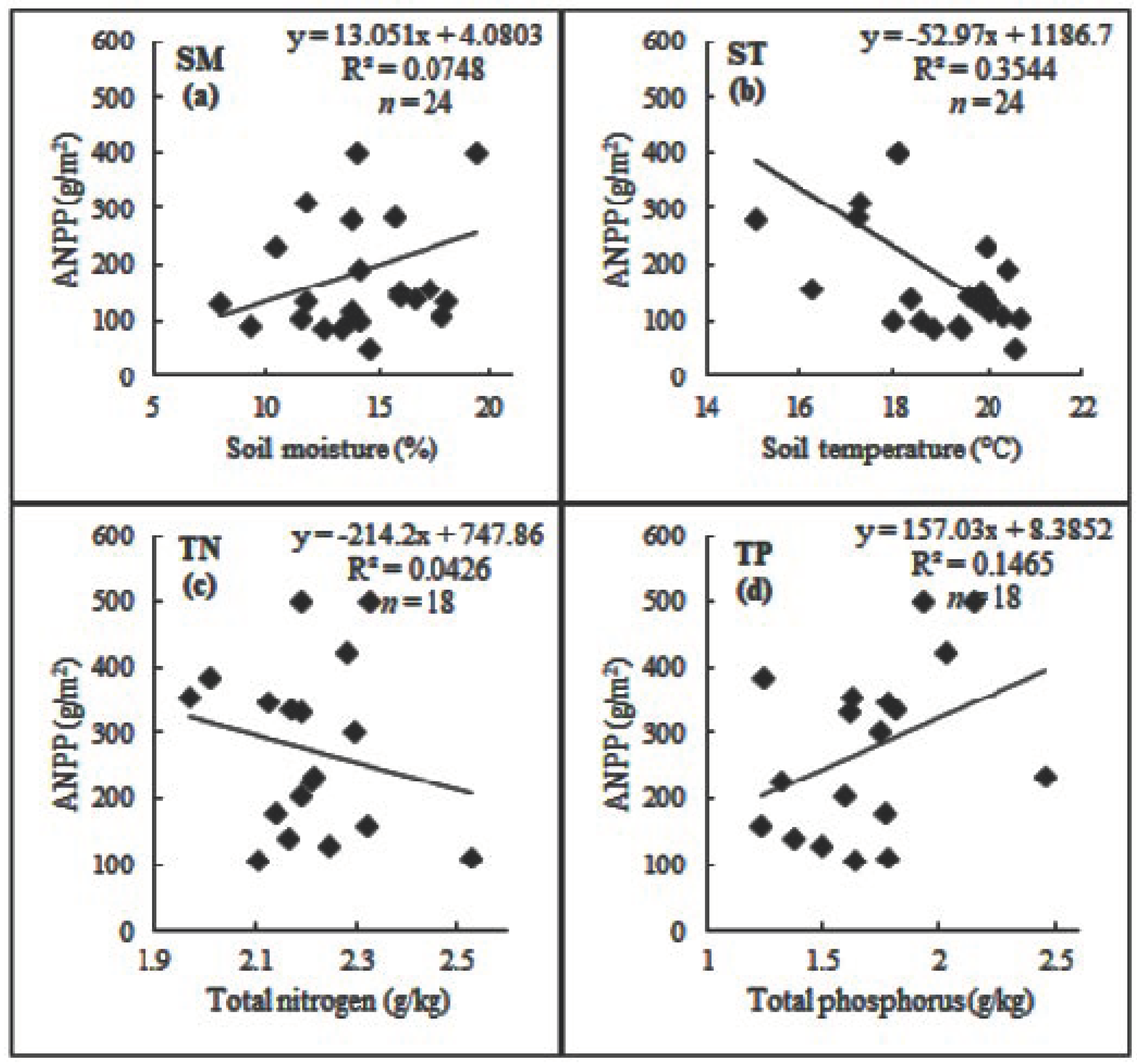
3.6. The Relationships of ANPP with Soil Moisture, Soil Temperature, and Soil Nutrient Contents
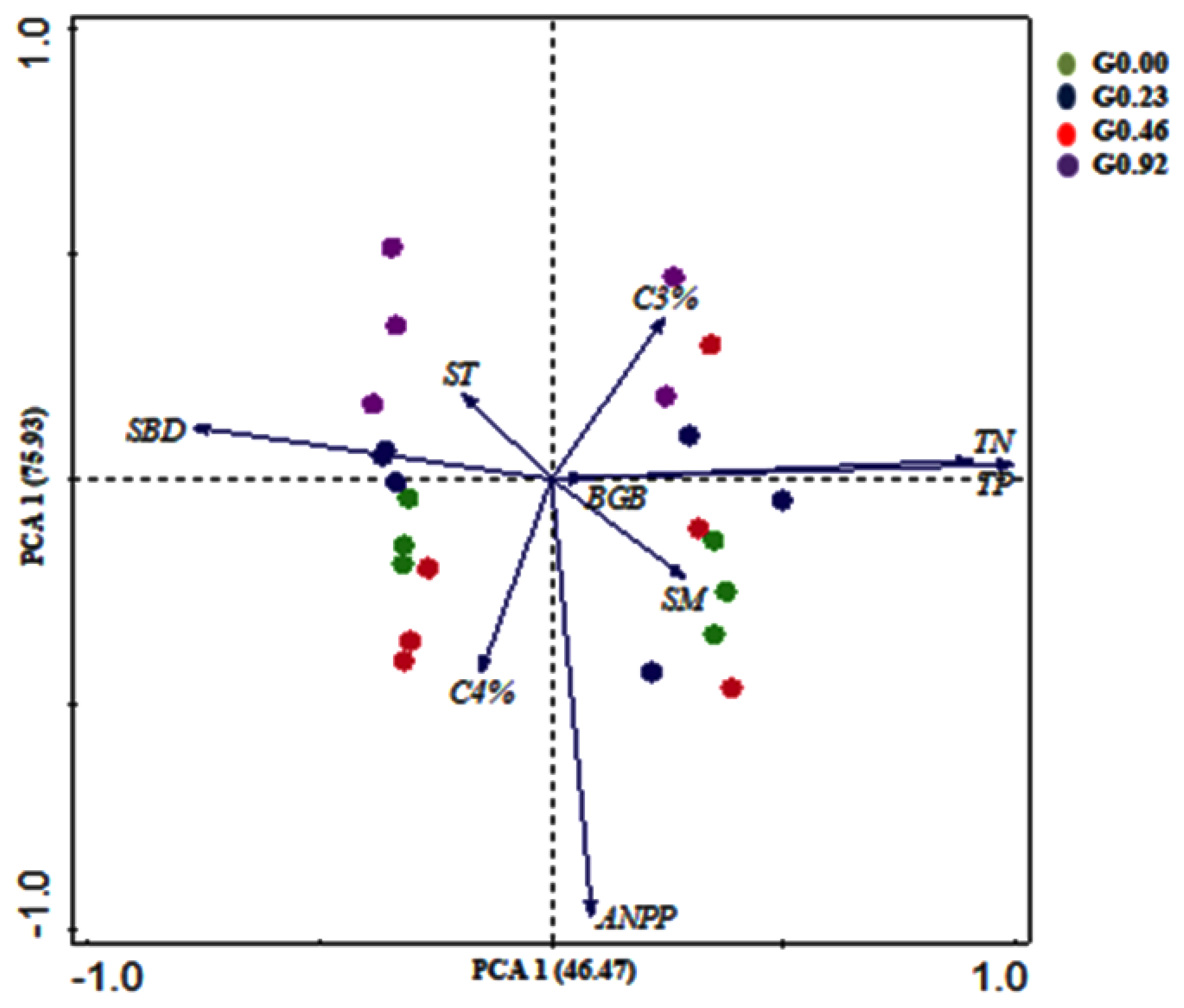
3.7. The Relationship of Plant Components and Soil Factors
4. Discussion
4.1. Grazing Effects on Aboveground Biomass (AGB)
4.2. The Effect of Grazing Intensity on Aboveground Net Primary Production (ANPP)
4.3. Grazing Effects on the Forage Dry Matter Intake
4.4. The Effect of Grazing Intensity on the Herbage Growth
4.5. Relationships of ANPP with Soil Moisture, Soil Temperature, and Soil Nutrient
4.6. Relationship of Plant Components and Soil Factors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, X.; Wang, Y.; Niu, H.; Wu, J.; Wang, S.; Schnug, E.; Rogasik, J.; Fleckenstein, J.; Tang, Y. Effect of long-term grazing on soil organic carbon content in semiarid steppes in Inner Mongolia. Ecol. Res. 2005, 20, 519–527. [Google Scholar] [CrossRef]
- Katoh, K.; Takeuchi, K.; Jiang, D.; Nan, Y.; Kou, Z. Vegetation restoration by seasonal exclosure in the Kerqin Sandy Land, Inner Mongolia. Plant Ecol. 1998, 139, 133–144. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, G.; Jia, B. Modeling SOC and NPP responses of meadow steppe to different grazing intensities in Northeast China. Ecol. Model. 2008, 217, 72–78. [Google Scholar] [CrossRef]
- Yan, R.; Xin, X.; Yan, Y.; Wang, X.; Zhang, B.; Yang, G.; Liu, S.; Deng, Y.; Li, L. Impacts of differing grazing rates on canopy structure and species composition in Hulunber meadow steppe. Rangel. Ecol. Manag. 2015, 68, 54–64. [Google Scholar] [CrossRef]
- Wang, Z.; Johnson, D.A.; Rong, Y.; Wang, K. Grazing effects on soil characteristics and vegetation of grassland in northern China. Solid Earth 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Peth, S.; Krümmelbein, J.; Horn, R.; Wang, Z.; Steffens, M.; Hoffmann, C.; Peng, X. Spatial variability of soil properties affected by grazing intensity in Inner Mongolia grassland. Ecol. Model. 2007, 205, 241–254. [Google Scholar] [CrossRef]
- Renzhong, W.; Ripley, E.A. Effects of grazing on aLeymus chinensisgrassland on the Songnen plain of north-eastern China. J. Arid Environ. 1997, 36, 307–318. [Google Scholar] [CrossRef]
- Schönbach, P.; Wan, H.; Gierus, M.; Bai, Y.; Müller, K.; Lin, L.; Susenbeth, A.; Taube, F. Grassland responses to grazing: Effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil 2011, 340, 103–115. [Google Scholar] [CrossRef]
- Schönbach, P.; Wan, H.; Schiborra, A.; Gierus, M.; Bai, Y.; Müller, K.; Glindemann, T.; Wang, C.; Susenbeth, A.; Taube, F. Short-term management and stocking rate effects of grazing sheep on herbage quality and productivity of Inner Mongolia steppe. Crop Pasture Sci. 2009, 60, 963–974. [Google Scholar] [CrossRef]
- Li, C.; Hao, X.; Zhao, M.; Han, G.; Willms, W.D. Influence of historic sheep grazing on vegetation and soil properties of a Desert Steppe in Inner Mongolia. Agric. Ecosyst. Environ. 2008, 128, 109–116. [Google Scholar] [CrossRef]
- Li, S.G.; Harazono, Y.; Oikawa, T.; Zhao, H.L.; He, Z.Y.; Chang, X.L. Grassland desertification by grazing and the resulting micrometeorological changes in Inner Mongolia. Agric. For. Meteorol. 2000, 102, 125–137. [Google Scholar]
- Tong, C.; Wu, J.; Yong, S.-P.; Yang, J.; Yong, W. A landscape-scale assessment of steppe degradation in the Xilin River Basin, Inner Mongolia, China. J. Arid Environ. 2004, 59, 133–149. [Google Scholar] [CrossRef]
- Bai, Y.; Han, X.; Wu, J.; Chen, Z.; Li, L. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 2004, 431, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Biondini, M.E.; Patton, B.D.; Nyren, P.E. Grazing intensity and ecosystem processes in a northern mixed-grass prairie, USA. Ecol. Appl. 1998, 8, 469–479. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Lauenroth, W.K. Quantitative effects of grazing on vegetation and soils over a global range of environments: Ecological archives M063-001. Ecol. Monogra. 1993, 63, 327–366. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y.; Jiang, S.; Ojima, D.S.; Bonham, C.D. Interannual variation in the climate and above-ground biomass of Leymus chinense steppe and Stipa grandis steppe in the Xilin river basin, Inner Mongolia, China. J. Arid Environ. 1995, 31, 283–299. [Google Scholar] [CrossRef]
- Chou, W.W.; Silver, W.L.; Jackson, R.D.; Thompson, A.W.; Allen-Diaz, B. The sensitivity of annual grassland carbon cycling to the quantity and timing of rainfall. Glob. Chang. Biol. 2008, 14, 1382–1394. [Google Scholar] [CrossRef]
- Auerswald, K.; Wittmer, M.H.O.M.; Bai, Y.; Yang, H.; Taube, F.; Susenbeth, A.; Schnyder, H. C4 abundance in an Inner Mongolia grassland system is driven by temperature–moisture interaction, not grazing pressure. Basic Appl. Ecol. 2012, 13, 67–75. [Google Scholar] [CrossRef]
- Wittmer, M.H.O.M.; Auerswald, K.; Bai, Y.; Schaeufele, R.; Schnyder, H. Changes in the abundance of C3/C4 species of Inner Mongolia grassland: Evidence from isotopic composition of soil and vegetation. Glob. Chang. Biol. 2010, 16, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Trlica, M.J.; Rittenhouse, L.R. Grazing and plant performance. Ecol. Appl. 1993, 3, 21–23. [Google Scholar] [CrossRef]
- Wang, R.Z. Responses of Leymus chinensis (Poaceae) to long-term grazing disturbance in the Songnen grasslands of north-eastern China. Grass Forage Sci. 2004, 59, 191–195. [Google Scholar] [CrossRef]
- Yu, M.; Ellis, J.E.; Epstein, H.E. Regional analysis of climate, primary production, and livestock density in Inner Mongolia. J. Environ. Qual. 2004, 33, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.J.; Milchunas, D.G.; Frank, D.A. How can net primary productivity be measured in grazing ecosystems? Ecology 1996, 77, 974–977. [Google Scholar] [CrossRef]
- Rickart, E.A.; Bienek, K.G.; Rowe, R.J. Impact of livestock grazing on plant and small mammal communities in the Ruby Mountains, northeastern Nevada. West. N. Am. Nat. 2013, 73, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, X.; Wang, H.; Huang, Q. Comprehensive analysis of grazing intensity impacts soil organic carbon: A case study in typical steppe of Inner Mongolia, China. Appl. Soil Ecol. 2018, 129, 1–12. [Google Scholar] [CrossRef]
- Yong-Zhong, S.; Yu-Lin, L.; Jian-Yuan, C.; Wen-Zhi, Z. Influences of continuous grazing and livestock exclusion on soil properties in a degraded sandy grassland, Inner Mongolia, northern China. Catena 2005, 59, 267–278. [Google Scholar] [CrossRef]
- Guo, Y.; Du, Q.; Li, G.; Ni, Y.; Zhang, Z.; Ren, W.; Hou, X. Soil phosphorus fractions and arbuscular mycorrhizal fungi diversity following long-term grazing exclusion on semi-arid steppes in Inner Mongolia. Geoderma 2016, 269, 79–90. [Google Scholar] [CrossRef]
- Lu, X.; Yan, Y.; Sun, J.; Zhang, X.; Chen, Y.; Wang, X.; Cheng, G. Carbon, nitrogen, and phosphorus storage in alpine grassland ecosystems of Tibet: Effects of grazing exclusion. Ecol. Evol. 2015, 5, 4492–4504. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ding, K.; Li, Z. Comparison of soil carbon and nitrogen stocks at grazing-excluded and yak grazed alpine meadow sites in Qinghai–Tibetan Plateau, China. Ecol. Eng. 2016, 87, 203–211. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Cao, Y. Impact of historic grazing on steppe soils on the northern Tibetan Plateau. Plant Soil 2012, 354, 173–183. [Google Scholar] [CrossRef]
- Ma, L.; Yuan, F.; Liang, H.; Rong, Y. The effects of grazing management strategies on the vegetation, diet quality, intake and performance of free grazing sheep. Livest. Sci. 2014, 161, 185–192. [Google Scholar] [CrossRef]
- Patton, B.D.; Dong, X.; Nyren, P.E.; Nyren, A. Effects of grazing intensity, precipitation, and temperature on forage production. Rangel. Ecol. Manag. 2007, 60, 656–665. [Google Scholar] [CrossRef]
- De Mazancourt, C.; Loreau, M.; Abbadie, L. Grazing optimization and nutrient cycling: When do herbivores enhance plant production? Ecology 1998, 79, 2242–2252. [Google Scholar] [CrossRef]
- Hilbert, D.W.; Swift, D.M.; Detling, J.K.; Dyer, M.I. Relative growth rates and the grazing optimization hypothesis. Oecologia 1981, 51, 14–18. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.J. Grazing as an optimization process: Grass-ungulate relationships in the Serengeti. Am. Nat. 1979, 113, 691–703. [Google Scholar] [CrossRef]
- Mannetje, L.T. Measurement of Grassland Vegetation and Anmal Production; CABI Publishing: Wallingford, UK, 1978. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Rockville, MD, USA, 1990; Volume 1. [Google Scholar]
- Frank, C. Facility location. In Algorithms for Sensor and Ad Hoc Networks; Springer: Berlin, Germany, 2007; pp. 131–159. [Google Scholar]
- McNaughton, S.J. Ecology of a grazing ecosystem: The Serengeti. Ecol. Monogra. 1985, 55, 259–294. [Google Scholar] [CrossRef]
- Fisher, R.A. Some remarks on the methods formulated in a recent article on “The quantitative analysis of plant growth”. Ann. Appl. Biol. 1921, 7, 367–372. [Google Scholar] [CrossRef] [Green Version]
- D’Odorico, P.; Caylor, K.; Okin, G.S.; Scanlon, T.M. On soil moisture-vegetation feedbacks and their possible effects on the dynamics of dryland ecosystems. J. Geophys. Res. Biogeosci. 2007, 112. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zhang, S.-Y.; Peng, H.-Y.; Hu, X.; Ma, Y.-J. Soil water and temperature dynamics in shrub-encroached grasslands and climatic implications: Results from Inner Mongolia steppe ecosystem of north China. Agric. For. Meteorol. 2013, 171, 20–30. [Google Scholar] [CrossRef]
- Yan, Y.; Yan, R.; Chen, J.; Xin, X.; Eldridge, D.J.; Shao, C.; Wang, X.; Lv, S.; Jin, D.; Chen, J. Grazing modulates soil temperature and moisture in a Eurasian steppe. Agric. For. Meteorol. 2018, 262, 157–165. [Google Scholar] [CrossRef]
- Bao, S.D. Agro-Chemical Analysis of Soil; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Statistical Analysis System Institute. SAS Users’ Guide (9.2 Version); Statistical Analysis System Institute: Cary, NC, USA, 2007. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5). 2002. Available online: www.canoco.com (accessed on 18 June 2020).
- Ni, J. Estimating net primary productivity of grasslands from field biomass measurements in temperate northern China. Plant Ecol. 2004, 174, 217–234. [Google Scholar] [CrossRef]
- Schönbach, P.; Wan, H.; Gierus, M.; Loges, R.; Müller, K.; Lin, L.; Susenbeth, A.; Taube, F. Effects of grazing and precipitation on herbage production, herbage nutritive value and performance of sheep in continental steppe. Grass Forage Sci. 2012, 67, 535–545. [Google Scholar] [CrossRef]
- Boone, R.B.; Wang, G. Cattle dynamics in African grazing systems under variable climates. J. Arid Environ. 2007, 70, 495–513. [Google Scholar] [CrossRef]
- Ho, P. Rangeland degradation in north China revisited? A preliminary statistical analysis to validate non-equilibrium range ecology. J. Dev. Stud. 2001, 37, 99–133. [Google Scholar] [CrossRef]
- Briske, D.D.; Fuhlendorf, S.D.; Smeins, F.E. Vegetation dynamics on rangelands: A critique of the current paradigms. J. Appl. Ecol. 2003, 40, 601–614. [Google Scholar] [CrossRef]
- Fernandez-Gimenez, M.E.; Allen-Diaz, B. Testing a non-equilibrium model of rangeland vegetation dynamics in Mongolia. J. Appl. Ecol. 1999, 36, 871–885. [Google Scholar] [CrossRef]
- Vetter, S. Rangelands at equilibrium and non-equilibrium: Recent developments in the debate. J. Arid Environ. 2005, 62, 321–341. [Google Scholar] [CrossRef]
- Wessels, K.J.; Prince, S.D.; Carroll, M.; Malherbe, J. Relevance of rangeland degradation in semiarid northeastern South Africa to the nonequilibrium theory. Ecol. Appl. 2007, 17, 815–827. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, Y.; Zhao, X.; Xie, G.; Zhang, T. Grassland recovery by protection from grazing in a semi-arid sandy region of northern China. N. Z. J. Agric. Res. 2005, 48, 277–284. [Google Scholar] [CrossRef]
- Ganjurjav, H.; Duan, M.-J.; Wan, Y.-F.; Zhang, W.-N.; Gao, Q.-Z.; Li, Y.; Jiangcun, W.-Z.; Danjiu, L.-B.; Guo, H.-B. Effects of grazing by large herbivores on plant diversity and productivity of semi-arid alpine steppe on the Qinghai-Tibetan Plateau. Rangel. J. 2015, 37, 389–397. [Google Scholar] [CrossRef]
- Hope, G. The sensitivity of the high mountain ecosystems of New Guinea to climatic change and anthropogenic impact. Arct. Antarct. Alp. Res. 2014, 46, 777–786. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, S.; Lavorel, S.; Landsberg, J.; Forbes, T.D.A. Disturbance response in vegetation-towards a global perspective on functional traits. J. Veg. Sci. 1999, 10, 621–630. [Google Scholar] [CrossRef]
- Altesor, A.; Oesterheld, M.; Leoni, E.; Lezama, F.; Rodríguez, C. Effect of grazing on community structure and productivity of a Uruguayan grassland. Plant Ecol. 2005, 179, 83–91. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogra. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Scimone, M.; Rook, A.J.; Garel, J.-P.; Sahin, N. Effects of livestock breed and grazing intensity on grazing systems: 3. Effects on diversity of vegetation. Grass Forage Sci. 2007, 62, 172–184. [Google Scholar] [CrossRef]
- Hou, F.J.; Yang, Z.Y. Effects of grazing of livestock on grassland. Acta Ecol. Sin. 2006, 26, 244–264. [Google Scholar]
- Butler, D.M.; Franklin, D.H.; Cabrera, M.L.; Risse, L.M.; Radcliffe, D.E.; West, L.T.; Gaskin, J.W. Assessment of the Georgia Phosphorus Index on farm at the field scale for grassland management. J. Soil Water Conserv. 2010, 65, 200–210. [Google Scholar] [CrossRef]
- Hendricks, T.; Franklin, D.; Dahal, S.; Hancock, D.; Stewart, L.; Cabrera, M.; Hawkins, G. Soil carbon and bulk density distribution within 10 Southern Piedmont grazing systems. J. Soil Water Conserv. 2019, 74, 323–333. [Google Scholar] [CrossRef]
- Tesk, C.R.M.; Cavalli, J.; Pina, D.S.; Pereira, D.H.; Pedreira, C.G.S.; Jank, L.; Sollenberger, L.E.; Pedreira, B.C. Herbage responses of Tamani and Quênia guineagrasses to grazing intensity. Agron. J. 2020. [Google Scholar] [CrossRef]
- Briske, D.D. Grazing optimization: A plea for a balanced perspective. Ecol. Appl. 1993, 3, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Knapp, A.K.; Hoover, D.L.; Blair, J.M.; Buis, G.; Burkepile, D.E.; Chamberlain, A.; Collins, S.L.; Fynn, R.W.S.; Kirkman, K.P.; Smith, M.D. A test of two mechanisms proposed to optimize grassland aboveground primary productivity in response to grazing. J. Plant Ecol. 2012, 5, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Wu, J.; Xing, Q.; Pan, Q.; Huang, J.; Yang, D.; Han, X. Primary production and rain use efficiency across a precipitation gradient on the Mongolia plateau. Ecology 2008, 89, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.L.; Seastedt, T.R.; Dyer, M.I. Maximization of aboveground grassland production: The role of defoliation frequency, intensity, and history. Ecol. Appl. 1993, 3, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Leriche, H.; Le Roux, X.; Desnoyers, F.; Benest, D.; Simioni, G.; Abbadie, L. Grass response to clipping in an African savanna: Testing the grazing optimization hypothesis. Ecol. Appl. 2003, 13, 1346–1354. [Google Scholar] [CrossRef]
- Semmartin, M.; Oesterheld, M. Effects of grazing pattern and nitrogen availability on primary productivity. Oecologia 2001, 126, 225–230. [Google Scholar] [CrossRef]
- Ferraro, D.O.; Oesterheld, M. Effect of defoliation on grass growth: A quantitative review. Oikos 2002, 98, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Glindemann, T.; Wang, C.; Tas, B.M.; Schiborra, A.; Gierus, M.; Taube, F.; Susenbeth, A. Impact of grazing intensity on herbage intake, composition, and digestibility and on live weight gain of sheep on the Inner Mongolian steppe. Livest. Sci. 2009, 124, 142–147. [Google Scholar] [CrossRef]
- Ackerman, C.J.; Purvis, H.T.; Horn, G.W.; Paisley, S.I.; Reuter, R.R.; Bodine, T.N. Performance of light vs heavy steers grazing Plains Old World bluestem at three stocking rates. J. Anim. Sci. 2001, 79, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Animut, G.; Goetsch, A.L.; Aiken, G.E.; Puchala, R.; Detweiler, G.; Krehbiel, C.R.; Merkel, R.C.; Sahlu, T.; Dawson, L.J.; Johnson, Z.B. Performance and forage selectivity of sheep and goats co-grazing grass/forb pastures at three stocking rates. Small Rumi. Res. 2005, 59, 203–215. [Google Scholar] [CrossRef]
- Derner, J.D.; Raynor, E.J.; Reeves, J.L.; Augustine, D.J.; Milchunas, D.G. Climatic and management determinants of large herbivore production in semiarid grassland. Agric. Ecosyst. Environ. 2020, 290, 106761. [Google Scholar] [CrossRef]
- Zoby, J.L.F.; Holmes, W. The influence of size of animal and stocking rate on the herbage intake and grazing behaviour of cattle. J. Agric. Sci. 1983, 100, 139–148. [Google Scholar] [CrossRef]
- Langlands, J.P.; Bennett, I.L. Stocking intensity and pastoral production: II. Herbage intake of Merino sheep grazed at different stocking rates. J. Agric. Sci. 1973, 81, 205–209. [Google Scholar] [CrossRef]
- Allison, C.D. Factors affecting forage intake by range ruminants: A review. J. Range Manag. 1985, 38, 305–311. [Google Scholar] [CrossRef]
- Noy-Meir, I. Compensating growth of grazed plants and its relevance to the use of rangelands. Ecol. Appl. 1993, 3, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Varnamkhasti, A.S.; Milchunas, D.G.; Lauenroth, W.K.; Goetz, H. Production and rain use efficiency in short-grass steppe: Grazing history, defoliation and water resource. J. Veg. Sci. 1995, 6, 787–796. [Google Scholar] [CrossRef]
- Van Staalduinen, M.A.; Anten, N.P.R. Differences in the compensatory growth of two co-occurring grass species in relation to water availability. Oecologia 2005, 146, 190–199. [Google Scholar] [CrossRef]
- Christie, K.M.; Smith, A.P.; Rawnsley, R.P.; Harrison, M.T.; Eckard, R.J. Simulated seasonal responses of grazed dairy pastures to nitrogen fertilizer in SE Australia: N loss and recovery. Agric. Syst. 2020, 182, 102847. [Google Scholar] [CrossRef]
- Dahal, S.; Franklin, D.; Subedi, A.; Cabrera, M.; Hancock, D.; Mahmud, K.; Ney, L.; Park, C.; Mishra, D. Strategic Grazing in Beef-Pastures for Improved Soil Health and Reduced Runoff-Nitrate-A Step towards Sustainability. Sustainability 2020, 12, 558. [Google Scholar] [CrossRef] [Green Version]
- Volenec, J.J.; Ourry, A.; Joern, B.C. A role for nitrogen reserves in forage regrowth and stress tolerance. Physiol. Plant. 1996, 97, 185–193. [Google Scholar] [CrossRef]
- Berdanier, A.B.; Klein, J.A. Growing season length and soil moisture interactively constrain high elevation aboveground net primary production. Ecosystems 2011, 14, 963–974. [Google Scholar] [CrossRef]
- Sherry, R.A.; Weng, E.; Arnone III, J.A.; Johnson, D.W.; Schimel, D.S.; Verburg, P.S.; Wallace, L.L.; Luo, Y. Lagged effects of experimental warming and doubled precipitation on annual and seasonal aboveground biomass production in a tallgrass prairie. Glob. Chang. Biol. 2008, 14, 2923–2936. [Google Scholar] [CrossRef]
- Zhang, F.; Quan, Q.; Song, B.; Sun, J.; Chen, Y.; Zhou, Q.; Niu, S. Net primary productivity and its partitioning in response to precipitation gradient in an alpine meadow. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fay, P.A.; Prober, S.M.; Harpole, W.S.; Knops, J.M.H.; Bakker, J.D.; Borer, E.T.; Lind, E.M.; MacDougall, A.S.; Seabloom, E.W.; Wragg, P.D. Grassland productivity limited by multiple nutrients. Nat. Plants 2015, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nippert, J.B.; Knapp, A.K.; Briggs, J.M. Intra-annual rainfall variability and grassland productivity: Can the past predict the future? Plant Ecol. 2006, 184, 65–74. [Google Scholar] [CrossRef]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A. Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Eltahir, E.A.B. A soil moisture-rainfall feedback mechanism: 1. Theory and observations. Water Resour. Res. 1998, 34, 765–776. [Google Scholar] [CrossRef]
- Prevéy, J.S.; Seastedt, T.R. Seasonality of precipitation interacts with exotic species to alter composition and phenology of a semi-arid grassland. J. Ecol. 2014, 102, 1549–1561. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, G.W.; Li, W.P. Meta-analysis of relationships between environmental factors and aboveground biomass in the alpine grassland on the Tibetan Plateau. Biogeosciences 2013, 10, 1707–1715. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, J.; Liu, M.; Zeng, T.; Tsunekawa, A.; Mubarak, A.A.; Zhou, H. Precipitation-use efficiency may explain net primary productivity allocation under different precipitation conditions across global grassland ecosystems. Glob. Ecol. Conserv. 2019, 20, e00713. [Google Scholar] [CrossRef]
- Xu, Z.; Ren, H.; Cai, J.; Wang, R.; Li, M.-H.; Wan, S.; Han, X.; Lewis, B.J.; Jiang, Y. Effects of experimentally-enhanced precipitation and nitrogen on resistance, recovery and resilience of a semi-arid grassland after drought. Oecologia 2014, 176, 1187–1197. [Google Scholar] [CrossRef]
- Kang, M.; Dai, C.; Ji, W.; Jiang, Y.; Yuan, Z.; Chen, H.Y.H. Biomass and its allocation in relation to temperature, precipitation, and soil nutrients in Inner Mongolia grasslands, China. PLoS ONE 2013, 8, e69561. [Google Scholar] [CrossRef] [PubMed]
- Shaver, G.R.; Giblin, A.E.; Nadelhoffer, K.J.; Thieler, K.K.; Downs, M.R.; Laundre, J.A.; Rastetter, E.B. Carbon turnover in Alaskan tundra soils: Effects of organic matter quality, temperature, moisture and fertilizer. J. Ecol. 2006, 94, 740–753. [Google Scholar] [CrossRef] [Green Version]
- Del Grosso, S.; Parton, W.; Stohlgren, T.; Zheng, D.; Bachelet, D.; Prince, S.; Hibbard, K.; Olson, R. Global potential net primary production predicted from vegetation class, precipitation, and temperature. Ecology 2008, 89, 2117–2126. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Clark, C.M.; Naeem, S.; Pan, Q.; Huang, J.; Zhang, L.; Han, X. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Chang. Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Fang, Y.; Xun, F.; Bai, W.; Zhang, W.; Li, L. Long-term nitrogen addition leads to loss of species richness due to litter accumulation and soil acidification in a temperate steppe. PLoS ONE 2012, 7, e47369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, J.; Isbell, F.; Lü, X.; Han, X. Productivity depends more on the rate than the frequency of N addition in a temperate grassland. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Carnicer, J.; Sardans, J.; Stefanescu, C.; Ubach, A.; Bartrons, M.; Asensio, D.; Penuelas, J. Global biodiversity, stoichiometry and ecosystem function responses to human-induced C-N-P imbalances. J. Plant Physiol. 2015, 172, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.B.; Badgley, G.; Blyth, E. Global nutrient limitation in terrestrial vegetation. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Harpole, W.S.; Ngai, J.T.; Cleland, E.E.; Seabloom, E.W.; Borer, E.T.; Bracken, M.E.S.; Elser, J.J.; Gruner, D.S.; Hillebrand, H.; Shurin, J.B. Nutrient co-limitation of primary producer communities. Ecol. Lett. 2011, 14, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol. Lett. 2005, 8, 23–29. [Google Scholar] [CrossRef]


| AGB | ANPP | GR | RGR | |
|---|---|---|---|---|
| Grazing | 0.000 | 0.431 | 0.089 | 0.005 |
| Year | 0.000 | 0.005 | 0.058 | 0.016 |
| Grazing * Year | 0.499 | 0.188 | 0.004 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.I.; Hou, L.; Yan, R.; Xin, X.; Zainelabdeen, Y.M. The Joint Effect of Grazing Intensity and Soil Factors on Aboveground Net Primary Production in Hulunber Grasslands Meadow Steppe. Agriculture 2020, 10, 263. https://doi.org/10.3390/agriculture10070263
Ahmed AI, Hou L, Yan R, Xin X, Zainelabdeen YM. The Joint Effect of Grazing Intensity and Soil Factors on Aboveground Net Primary Production in Hulunber Grasslands Meadow Steppe. Agriculture. 2020; 10(7):263. https://doi.org/10.3390/agriculture10070263
Chicago/Turabian StyleAhmed, Ahmed Ibrahim, Lulu Hou, Ruirui Yan, Xiaoping Xin, and Yousif Mohamed Zainelabdeen. 2020. "The Joint Effect of Grazing Intensity and Soil Factors on Aboveground Net Primary Production in Hulunber Grasslands Meadow Steppe" Agriculture 10, no. 7: 263. https://doi.org/10.3390/agriculture10070263






