Abstract
The excessive use of fertilizers in agriculture is mainly due to the recognized plant requirements for soluble phosphorus. This problem has limited the implementation of sustainable agriculture. A viable alternative is to use phosphate solubilizing soil microorganisms. This work aimed to isolate inorganic phosphorus-solubilizing bacteria from the soils of agroecosystems, to select and identify, based on sequencing and phylogenetic analysis of the 16S rRNA gene, the bacterium with the highest capacity for in vitro solubilization of inorganic phosphate. Additionally, we aimed to determine its primary phosphate solubilizing mechanisms and to evaluate its effect on Habanero pepper seedlings growth. A total of 21 bacterial strains were isolated by their activity on Pikovskaya agar. Of these, strain ITCB-09 exhibited the highest ability to solubilize inorganic phosphate (865.98 µg/mL) through the production of organic acids. This strain produced extracellular polymeric substances and siderophores that have ecological implications for phosphate solubilization. 16S rRNA gene sequence analysis revealed that strain ITCB-09 belongs to the genus Enterobacter. Enterobacter sp. ITCB-09, especially when immobilized in beads, had a positive effect on Capsicum chinense Jacq. seedling growth, indicating its potential as a biofertilizer.
1. Introduction
Phosphorus is present in soils as inorganic (with aluminum, calcium, iron, manganese) and, to a lesser extent, as organic compounds (organic matter) [1]. Plants do not absorb insoluble (inorganic) phosphorus, and is the main limiting factor for plant growth [2].
The mechanisms of microorganism-mediated phosphate solubilization and mineralization in soils, as well as their importance for plant growth, have been widely researched [3]. Bacterial solubilization of inorganic phosphate has been mainly associated with the release of low molecular weight organic acids, such as citric, oxalic, malic and gluconic, which chelate phosphate-bound cations through their hydroxyl and carboxyl groups, converting them into bioavailable forms [4,5]. On the other hand, mineralization involves enzymatic degradation of organic phosphorus releasing soluble forms [6,7,8], being phosphatases (phytases) that commonly carry out this activity in bacteria and fungi [9,10].
Another less reported mechanism is the production of siderophores, low molecular weight secondary metabolites produced by bacteria under iron deficiency. Bacteria can chelate iron from iron phosphate, leaving phosphorus available for plants [11]. A fourth mechanism is the production of extracellular polymeric substances (EPS), mainly composed of polysaccharides, proteins, lipids, and DNA [12]. In soils, microbial EPS can improve particle aggregation and benefit plants by maintaining environmental moisture and trapping nutrients [13]. Some studies have described that bacteria can synthesize EPS in soils through the change in homeostasis during phosphate solubilization [14].
A biofertilizer can be composed of a range of microorganisms, each with a specific role, from promoting plant growth by providing different nutrients to protect against pathogens [15]. Among the main bacterial genera that have reported biofertilizer potential are Rhizobium, Azotobacter, Azospirillum, Pseudomonas, Bacillus, Enterobacter, Klebsiella, Variovorax, and Serratia [16,17,18]. An important feature of the genus Enterobacter, for example, is its ability to solubilize inorganic phosphate, mainly by the production of low molecular weight organic acids. Other activities, such as nitrogen fixation and biological control, have been identified in this genus [19,20,21]. Therefore, Enterobacter is considered the most important group of plant growth-promoting bacteria or biofertilizers [22].
Although the study of beneficial microorganisms for agriculture began more than 40 years ago [23], there is a need for microorganisms with higher capacity as biofertilizers, biocontrol agents, bioremediators, and plant growth promoters. Phosphorus-solubilizing bacteria with various mechanisms of action are potential biofertilizer candidates. The objectives of this research were to isolate inorganic phosphorus-solubilizing bacteria from the soils of agroecosystems, select and identify, based on sequencing and phylogenetic analysis of the 16S rRNA gene, the isolate with the highest capacity for in vitro solubilization of inorganic phosphate. Finally, determine its main phosphate solubilization mechanisms and evaluate its effect on the growth of Habanero pepper seedlings.
2. Materials and Methods
2.1. Isolation of Inorganic Phosphate Solubilizing Bacteria from Agroecosystems Soils
Bacteria were isolated from the rhizosphere of Capsicum chinense crops at the Instituto Tecnológico de Chiná, located in the village of Chiná, State of Campeche, Mexico. Ten soil samples were aseptically collected at random, placed in sterile plastic bags, and immediately transported to the laboratory. Approximately 1 g of soil from each sample was carefully transferred and vortexed in Erlenmeyer flasks containing 100 mL of 0.9% NaCl. The serial dilution spread-plate technique was used to enumerate living bacteria [8,24]. Duplicate dilutions (10−2 to 10−5) were prepared in the same buffer and inoculated on Pikovskaya agar (PA) [25]. All plates were incubated at 28 ± 2 °C for ten days and checked daily. Based on the fact that the bacterial colonies with clear halos indicated solubilizing activity of the phosphate (dissolution tricalcium phosphate in the medium [8]), these were subcultured on trypticase soy agar (TSA, BD Difco™, Franklin Lakes, NJ, USA).
2.2. Determination of Phosphate-Solubilizing Activity and Selection of Strain ITCB-09
The phosphate solubilizing capacity of 21 bacterial strains showing clear zones on PA was determined by pH measurement and ion chromatography. Isolates were inoculated in duplicate into 10 mL of Pikovskaya broth (PB) and incubated for 15 days at 28 ± 2 °C. The pH was then measured with a potentiometer to detect acidification by organic acid formation [24]. Cultures were centrifuged at 4000 rpm for 30 min, and supernatants were diluted (1:30) with deionized water and clarified by passing them through a 0.45 μm syringe, prior to analysis of soluble phosphate by ion chromatography. A phosphate calibration curve (1 to 20 μg/mL) was generated from dilutions of a standard solution of NH4H2PO4. Both solutions and standards were injected (10 μL) and analyzed on an 883 Basic IC plus ion chromatograph (Metrohm, Herisau, Switzerland), equipped with a Metrosep A Supp 7 column (0.4 × 25 cm) and a conductivity detector. The mobile phase was sodium carbonate (5 mM) with a flow rate of 0.4 mL/min, and the regeneration solution was 100 mM sulfuric acid. The integration, calibration, and analysis of results were carried out using MagIC Net 2.3 software (Metrohm, Herisau, Switzerland). All chromatographic analyses were performed in duplicate.
2.3. 16S rRNA Gene Sequence Analysis
Isolate ITCB-09 was identified by partial sequencing of 16S rRNA gene fragments amplified by the polymerase chain reaction (PCR). Total DNA was prepared using a commercial kit (UltraClean® Microbial DNA Isolation Kit MO BIO, QIAGEN Inc., Germantown, MD, USA) following the manufacturers’ instructions. The 16S rRNA gene was amplified using primers 27f (5′-GAGTTTGATCCTGGCTCA-3′) and 1385R (5′-CGGTGTGTRCAAGGCCC-3′). The PCR cycle was initial denaturation at 95 °C for 2 min and 29 cycles of 95 °C for 60 s, annealing at 58 °C for 60 s, 72 °C for 70 s, and a final extension at 72 °C for 10 min. PCR products were purified, and DNA sequenced for MACROGEN (MACROGEN Inc., Seoul, Korea). GenBank database searches were performed using the BLAST program [26]. 16S rRNA sequences were aligned using CLUSTALW software version 2.1 (University College Dublin, Dublin, Ireland). Homology trees were constructed using Mega5 software [27]. The strain ITCB-09 was identified as Enterobacter sp. 99% similarity.
2.4. Detection of Phosphate Solubilization Mechanisms
2.4.1. Siderophores
Siderophore production was detected on chrome azurol S (CAS) agar according to the protocol described by Louden et al. [28]. Enterobacter sp. ITCB-09 and Escherichia coli (positive control) were grown on CAS agar plates and incubated at 28 ± 2 °C for 24 h. The colonies with purplish-red or purple halos were siderophore-producing strains [29].
A culture of Enterobacter sp. ITCB-09 (48 h, 500 mL) in trypticase soy broth (TSB) was centrifuged at 6000 rpm for 15 min. The supernatant was acidified with HCl to pH 2, and three extractions with an equal volume of ethyl acetate were performed [30]. Ethyl acetate was removed under reduced pressure, and this fraction (FS) was dissolved in methanol at a final concentration of 5%. One mL of FS was mixed with 1 mL 2% FeCl3 in methanol. A FeCl3 control (1 mL of ferric chloride with 1 mL of methanol) and color control of FS (1 mL of FS with 1 mL of methanol) were also produced. All test solutions were analyzed on a UV-Vis spectrophotometer (Evolution 201, Thermo Fisher Scientific, MA, USA) at 495 nm [31].
2.4.2. Phosphatase Activity
Phosphatase activity was measured according to a modification of the protocol described by Bobadilla and Rincon [32] and Malo et al. [33]. Enterobacter sp. ITCB 09 was incubated in triplicate in 10 mL trypticase soy broth (TSB) for 48 h at 50 rpm. The biomass was separated by centrifugation at 6000 rpm for 5 min and the pellet suspended in 1 mL Tris-HCl buffer (50 mmol, pH 8.0) and vortexed. Cells were lysed by sonification (BRANSON 5510, Marshall Scientific, Hampton, NH, USA) at 40 Hz/s for 5 min and centrifuged at 6000 rpm for 5 min to remove biomass [34,35]. Twenty-five µL of the cell extract were immediately transferred to the well of a 24-well microplate and 175 µL of the p-nitrophenyl phosphate substrate (PNP) (Sigma-Aldrich, St. Louis, MO, USA) were added and incubated at 35 °C for 30 min. The enzymatic reaction was stopped by adding 50 µL of 3 M NaOH. The microplate was read in a microplate absorbance reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 405 nm. Phosphatase activity, observed as a change to a yellow color, was expressed as mmol of PNP produced per minute [34]. All measurements were performed in five replicates, and TSB was used as a negative control.
2.4.3. Production and Characterization of Extracellular Polymeric Substances (EPS)
EPS production and characterization were performed to identify the presence of proteins, carbohydrates, and uronic acids. The presence of these biomolecules may indicate that there are chemical groups (e.g., carboxylic acids, amines) that can interact with the non-soluble inorganic phosphate and convert it to a soluble form. In this regard, Enterobacter sp. ITCB-09 was cultured at 28 ± 2 °C for 48 h in TSA plates without additional glucose. EPS were produced by overnight batch shake flask fermentation, as previously reported [36]. EPS were extracted from the fermented broth, as described by Camacho-Chab et al. [37]. Finally, EPS were lyophilized, gravimetrically estimated, and kept in the dark before being characterized. UV-Visible spectrophotometric analyses (Evolution 201, Thermo Fisher Scientific, MA, USA) of total carbohydrates [38], hexuronic acids [39], and protein content [40] were performed for the characterization of the EPS of Enterobacter sp. ITCB-09. The standards used were glucose (Sigma-Aldrich, St. Louis, MO, USA), glucuronic acid (Sigma-Aldrich, St. Louis, MO, USA), and bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA).
2.5. Effect of Enterobacter sp. ITCB-09 on Habanero Pepper (Capsicum chinense Jacq.) Seedling Growth
To determine whether the bacterium Enterobacter sp. ITCB-09 had potential as a biofertilizer, a preliminary evaluation of the effect of inoculation of the bacterium on the growth of Habanero pepper seedlings was carried out as described below.
2.5.1. Cultivation of Enterobacter sp. ITCB-09 for Inoculation
Enterobacter sp. ITCB-09 was cultured at 28 ± 2 °C for 48 h on TSA and then inoculated to tubes containing 10 mL TSB supplemented with 1 g of tribasic calcium phosphate. These were incubated for 24 h on a shaking platform at 50 rpm and 28 ± 2 °C. After this time, each culture of 10 mL was added to 1 L medium in a shake flask and incubated at 110 rpm and 28 ± 2 °C for six days (end of the stationary phase). These cultures formed the inoculant. In the first test, the inoculant was sprayed directly onto the Habanero pepper seeds in the germination trays. For the second test, bacteria were immobilized in alginate beads. Previous, unpublished work [41] had shown that the bacteria remained viable after immobilization in various biopolymers, alginate being optimum for the prolonged release of the bacterium. This inoculant was prepared as follows. Sterile 3% sodium alginate was added to 500 mL of sterile deionized water, and heated to 50 °C with magnetic stirring for 5 min. Then, solution temperature was lowered to 40 °C and 500 mL of the Enterobacter sp. ITCB-09 culture carefully added, with stirring. The immobilization was performed according to the protocol of Lee and Mooney [42]. Under aseptic conditions and stirring, 1 L of 0.5 M CaCl2 solution was prepared and volumes of 1 mL of the sodium alginate/Enterobacter sp. ITCB-09 mixture were added, immediately generating the beads as a precipitate. The beads were kept in the solution for 24 h under agitation and aseptic conditions. Finally, the beads were recovered by washing with sterile deionized water to remove excess CaCl2 and were kept sterile until use.
2.5.2. Preparation of Substrates for Habanero Pepper (Capsicum chinense Jacq.) Cultivation
The substrates were those most used by Habanero pepper farmers in the region. Two substrates were selected, and two combinations established, as follows: (1) Soil (leptosol, FAO), (2) Peat Moss (Sphagnum, Premier Horticulture, Inc., Quakertown, PA, USA), (3) 50% Soil + 50% Peat Moss, and (4) Peat Moss + Fertilizer (DAP 18-46-00, Fertinova®, Atotonilco el Alto, Mexico). Nutrient concentrations in the fertilizer were 18% nitrogen, 46% phosphorus, and 0% potassium. The fertilizer application was 1 g per seed, added when the seeds were sown. The substrates were autoclaved to eradicate most of the microorganisms at the beginning of the experiment. Later, they were placed under aseptic conditions in 100-cavity polystyrene germination trays, previously disinfected with benzalkonium chloride.
2.5.3. Inoculation and Cultivation of Habanero Pepper
Habanero pepper (variety Jaguar) seeds were deposited individually in each cavity at a depth of approximately 1 cm; 40 replicate seeds were sown for each substrate. Two treatments and one control were used (Table 1). The control was not inoculated with bacteria. Treatment 1 consisted of inoculating 5 mL of Enterobacter sp. ITCB-09 culture (described above) onto each seed at the time of sowing. Treatment 2 consisted of inoculating five beads (approximately 1 g) of immobilized Enterobacter sp. ITCB-09 onto each seed at the time of sowing. All the germination trays were placed in a lighted seed germinator for 12 h at 38 ± 2 °C and were irrigated daily with 5 mL sterile water for each seed for 15 days until the emergence of the seedlings was observed. Seedlings were transferred to the greenhouse of the Technological Institute of Chiná to continue their growth until 60 days; irrigation was continued daily.

Table 1.
Treatments and substrates used for the inoculation of Enterobacter sp. ITCB-09 on Habanero pepper (Capsicum chinense Jacq.).
2.6. Data Analysis
The data of soluble phosphate by ion chromatography were analyzed using one-way ANOVA in inter-group comparisons and the Tukey test to determine different groups using program R 3.24 (Vienna, Austria) [43]. The level of significance was taken as p < 0.01. On the other hand, seedling emergence times were noted. After emergence and every 10 days until 60 days, heights were determined with the help of a Vernier scale, measuring from the stem emergence point to the upper end, and the leaves were counted. For statistical analysis of these results, a mixed linear model was used, with treatments and substrates (Table 1) considered fixed factors and time and measurement intervals as the random factors. The Type III sum of squares was selected because, during the experiment, the design became unbalanced [44], since not all the seedlings germinated. Significance values for paired comparisons (e.g., between treatments) were obtained using Bonferroni’s correction (p < 0.001); this was done with the lme function and the REML method was applied in program R 3.24 [43].
3. Results
3.1. Isolation of Inorganic Phosphate Solubilizing Bacteria
A total of 21 bacterial strains with inorganic phosphate solubilizing activity (presence of a halo around the bacterial colony on PA medium) were isolated from the rhizosphere of Capsicum chinense agroecosystems. All isolates were characterized according to the colony and cell morphology (Table 2).

Table 2.
Bacterial strains with demonstrated inorganic phosphate solubilizing activity isolate from Capsicum chinense Jacq. agroecosystems.
3.2. Determination of Phosphate-Solubilizing Ability and Selection of the Strain ITCB-09
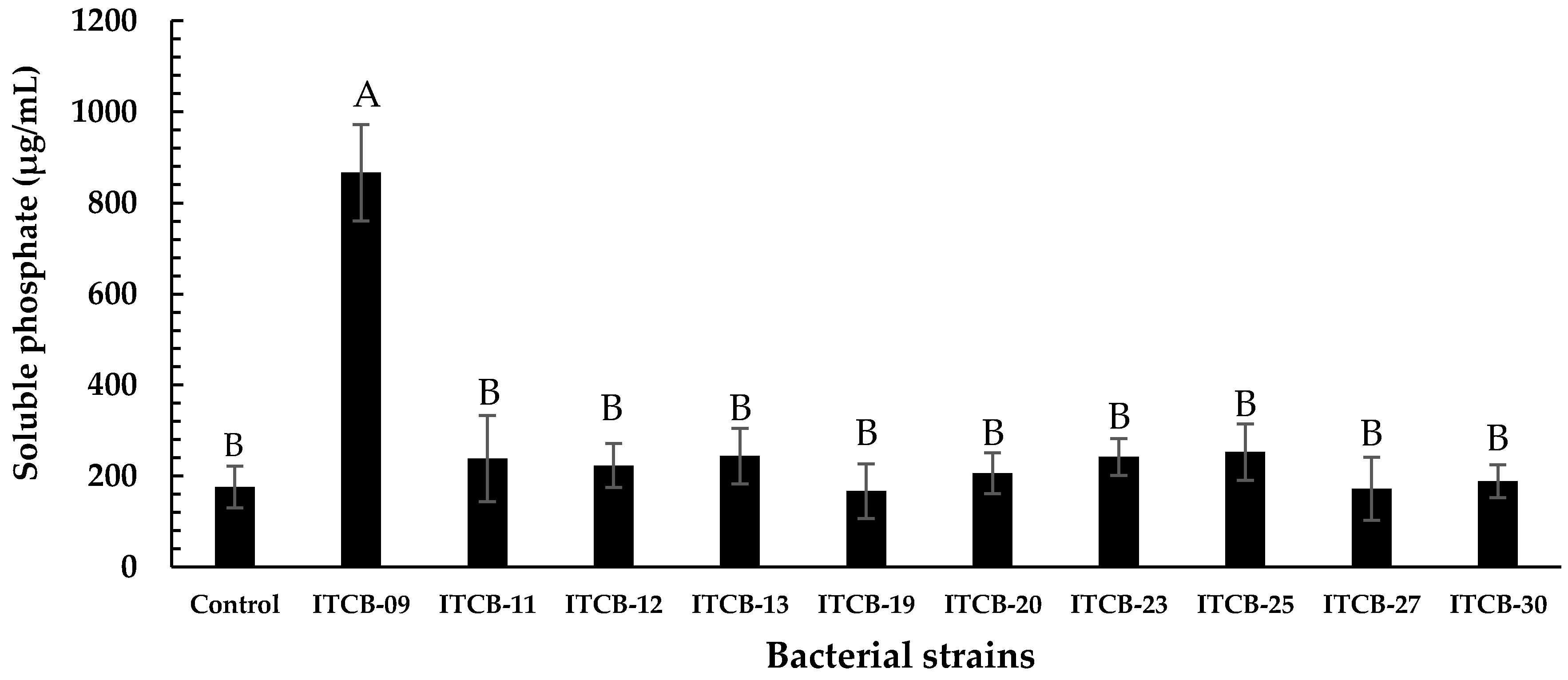
Only ten isolates produced low pH values (4.9 to 4.2) in Pikovskaya broth after 15 days of growth. Ion chromatography showed that strain ITCB-09 had the highest phosphate solubilization rate (865.98 ± 105.69 µg/mL); this was significantly different from the other isolates (p < 0.01 by the Tukey test). The remaining paired comparisons were not significantly different from one another (Figure 1). Thus, ITCB-09 was selected for identification by molecular biology and assessment of other phosphate solubilization mechanisms.
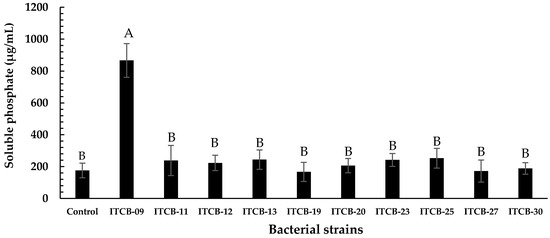
Figure 1.
Phosphate solubilization rate of bacterial strains after 15 days on Pikovskaya broth. Values were the average of at least two determinations. The different letters on the bars represent significant differences (p < 0.01) based on Tukey’s comparisons.
3.3. Identification of Bacterial Strain ITCB-09
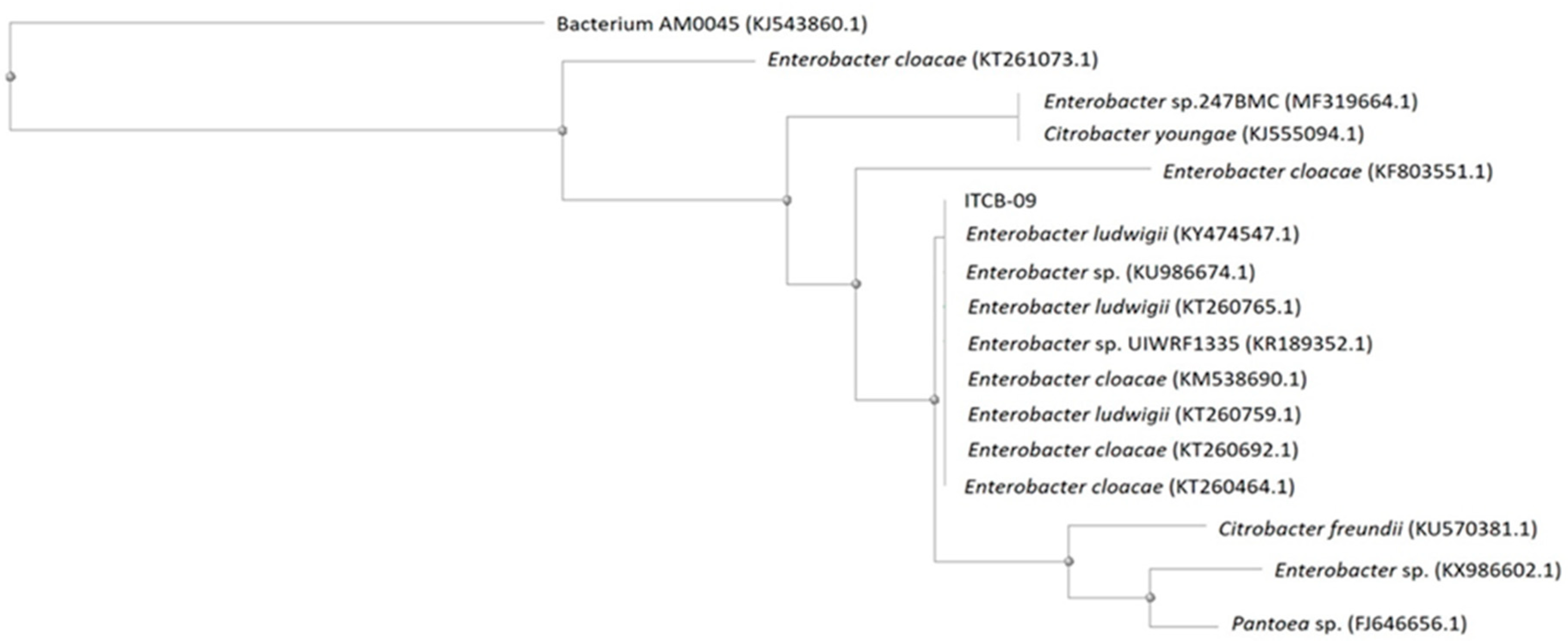
The partial sequencing of the 16S rRNA gene indicated that strain ITCB-09 had a close relationship (above 99% similarity) with species belonging to the genus Enterobacter (Figure 2). The similarity matrix and evolutionary history were inferred using the Neighbor-Joining method [45], which showed multiple alignments of the ITCB-09 sequence with other Enterobacter isolates obtained from the GenBank database. The phylogenetic tree constructed showed these relationships, 100% homology being shown with E. ludwigii and E. cloacae. A large number of species in this genus have been isolated from the rhizosphere in plant crops [19,20,21] and it is recognized for the large number of species that have plant growth-promoting activity [46,47,48].
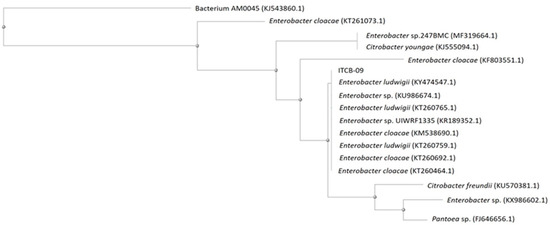
Figure 2.
Neighbor-joining dendrogram constructed from the 16S rDNA region of isolate ITCB-09 isolate and other bacterial taxa with high nucleotide similarity from the GenBank database. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distance method and are in the units of the number of base differences per site.
3.4. Mechanisms of Phosphate Solubilization of Enterobacter sp. ITCB-09
3.4.1. Siderophores
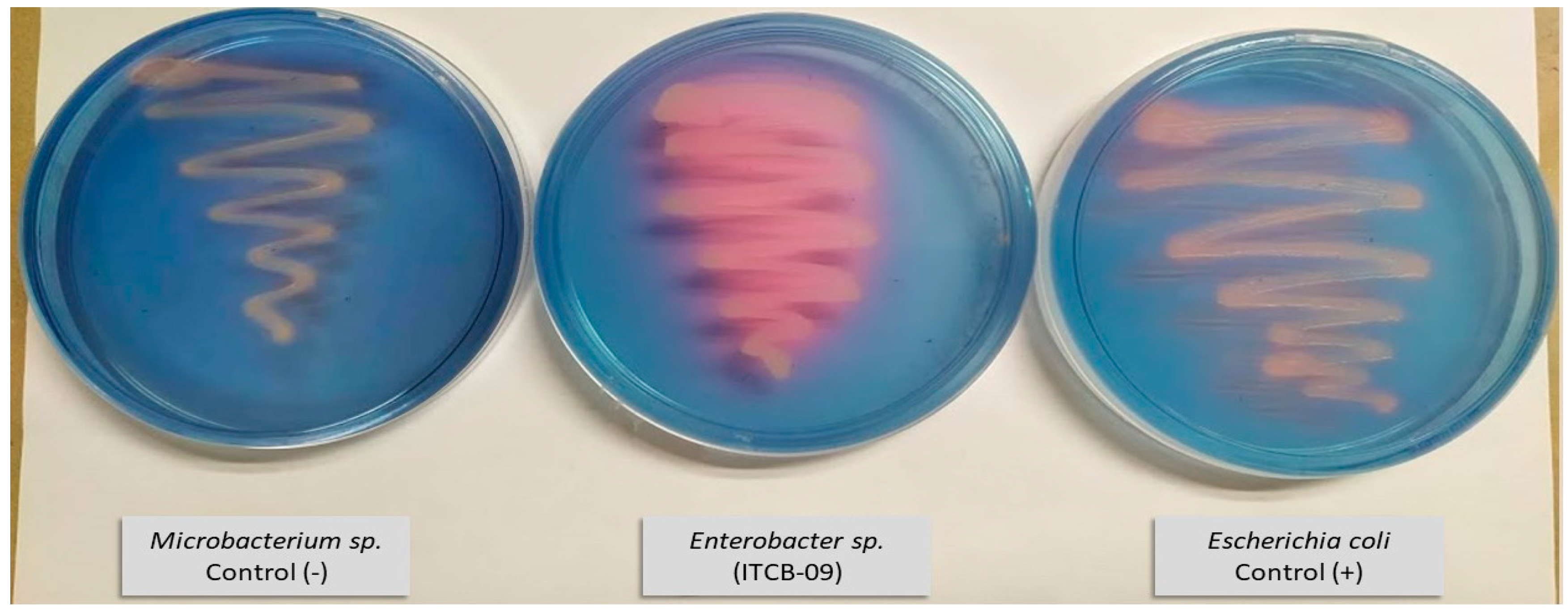
The production of siderophores was investigated qualitatively in Enterobacter sp. ITCB-09 and E. coli by plating on CAS agar (Figure 3). A color change from blue to purplish-red was observed around the colonies of both bacteria, indicating the production of siderophores.
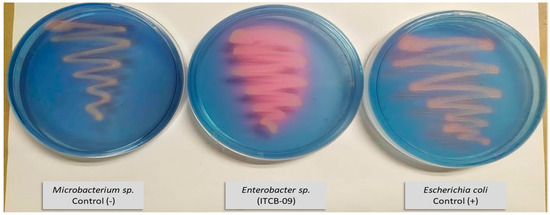
Figure 3.
Culture on CAS medium of E. coli and Enterobacter sp. ITCB-09.
In the FeCl3 test, subtracting the readings of the two controls (FS and FeCl3) showed that there is an increase in absorbance in the test solution of 1.347 units (Table 3), which corresponds to the complexes generated between catechol siderophores and iron [31].

Table 3.
Absorbances of test solutions at 495 nm.
3.4.2. Phosphatase Activity
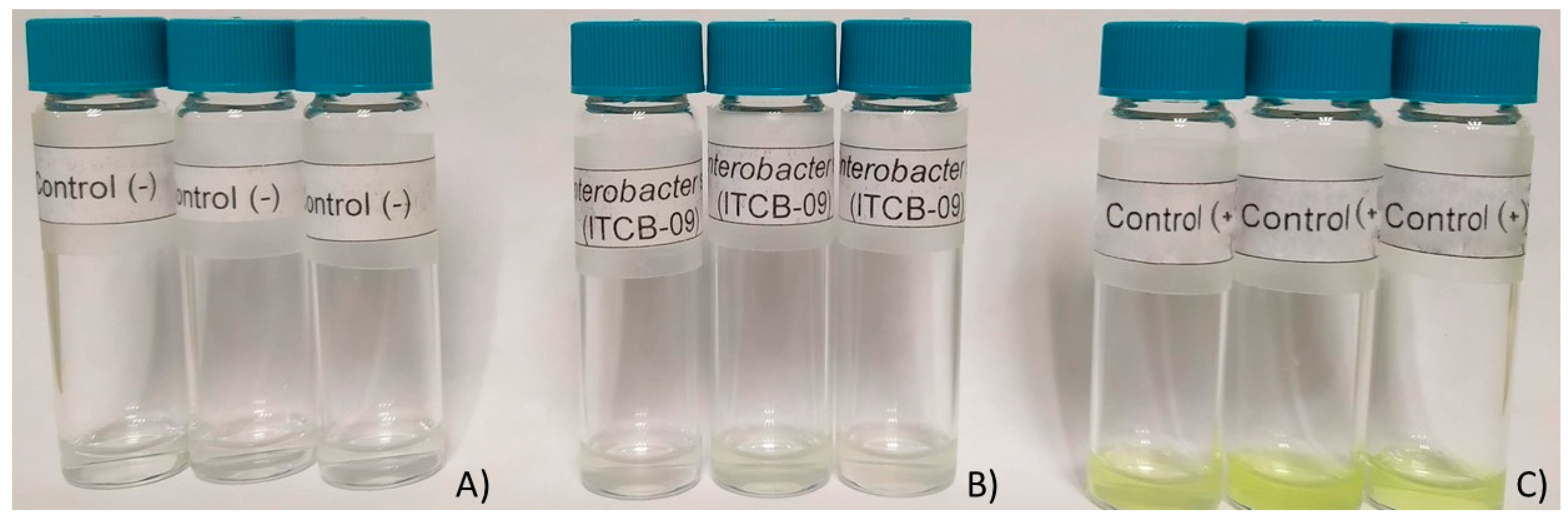
The results of the qualitative determination of phosphatase activity showed that Enterobacter sp. ITCB-09 does not synthesize enzymes of this type. No color change was observed (Figure 4).
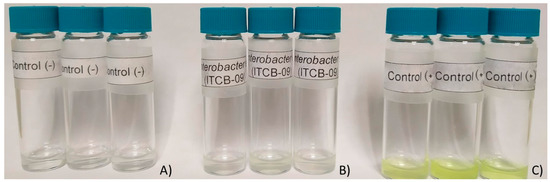
Figure 4.
Results of phosphatase activity assay. (A) Negative control; (B) Enterobacter sp. ITCB-09; (C) E. coli.
3.4.3. EPS
Enterobacter sp. ITCB-09 yielded 500 mg/L EPS under the laboratory conditions described. Spectrophotometry confirmed that this EPS is mainly composed of carbohydrates and proteins (Table 4). Hexuronic acids were also present. These compounds may interact with insoluble inorganic phosphate and convert it to a soluble form, via their ionizable functional groups such as carboxyl, carbonyl, or amino [49].

Table 4.
Carbohydrate and protein percentage of extracellular polymeric substances (EPS) synthetized by Enterobacter sp. ITCB-09.
3.5. Effect of Enterobacter sp. ITCB-09 on the Growth of Habanero Pepper (Capsicum chinense Jacq.) Seedlings
Most of the plants, including the control, germinated between 10 and 15 days (Table 5). The number of seedlings that germinated was maintained throughout the experiment (60 days).

Table 5.
Germination percentage of seedlings Capsicum chinense Jacq. after 15 days.
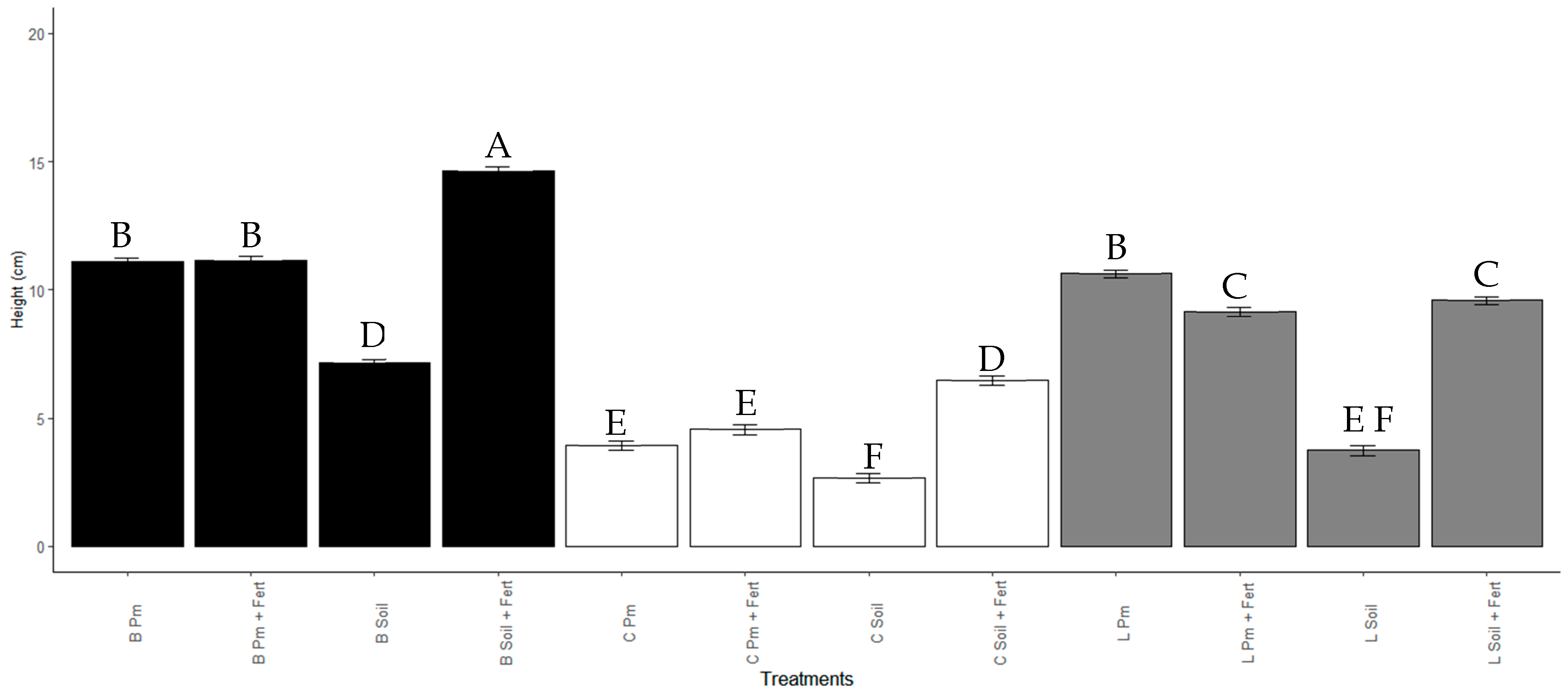
After 60 days, the increase in the height of the seedlings was significantly higher (Mean 14.66 ± 3.47 cm, p < 0.001) for the treatment with Enterobacter sp. ITCB-09 in beads in Soil + Pm substrate (Figure 5 and Figure 6). All treatments with Enterobacter sp. ITCB-09 immobilized with the different substrates showed a significant effect (p < 0.001) on plant growth concerning the control. Treatment with Enterobacter sp. ITCB-09 in liquid also had a more significant effect than the control, except for L soil treatment (Figure 6).

Figure 5.
The response of Capsicum chinense Jacq. plants after 60 days of greenhouse cultivation.
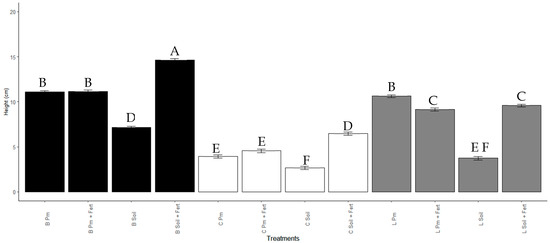
Figure 6.
Height of Habanero pepper seedlings with or without inoculation with Enterobacter sp. ITCB-09 after 60 days of cultivation. The different letters on the bars indicate significant differences (p < 0.001) based on Bonferroni comparisons; whiskers represent the standard errors. Black bars indicate the treatments with pearls (Enterobacter sp. ITCB-09); white bars indicate the controls; gray bars indicate the treatments with liquid (Enterobacter sp. ITCB-09).
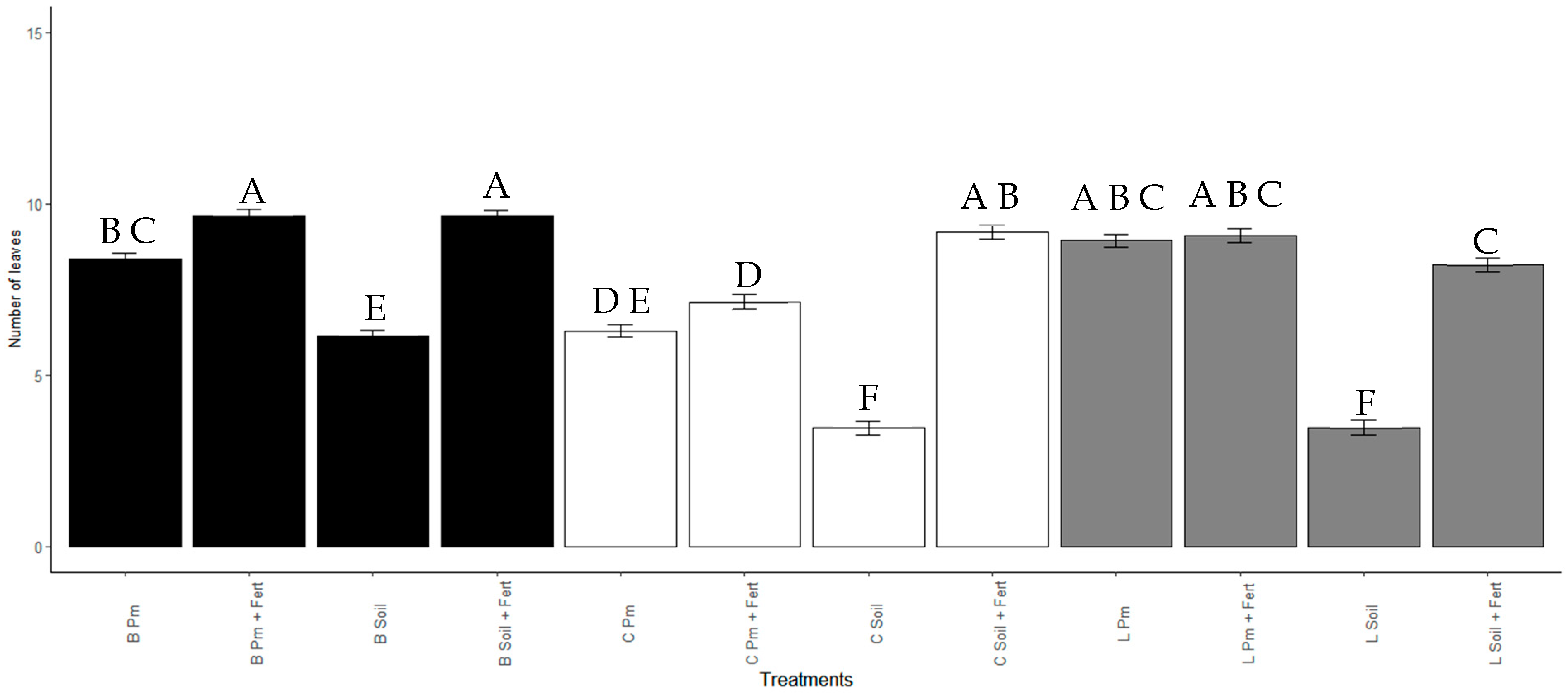
The number of leaves after 60 days was significantly higher (p < 0.001) in the plants treated with Enterobacter sp. ITCB-09 beads in the substrates Soil + Pm (9.6 ± 2.2) and Pm + Fert (9.6 ± 3.7). Treatments with Enterobacter sp. ITCB-09 in Liquid/Soil (3.5 ± 2.8) and Control/Soil (3.5 ± 2.6) produced the lowest number of leaves. Treatments and control with the soil substrate (B Soil = 6.1 ± 3; C Soil = 3.5 ± 2.6, L Soil = 3.5 ± 2.8) produced the lowest number of leaves concerning the other substrates used in their respective treatments (Figure 7).
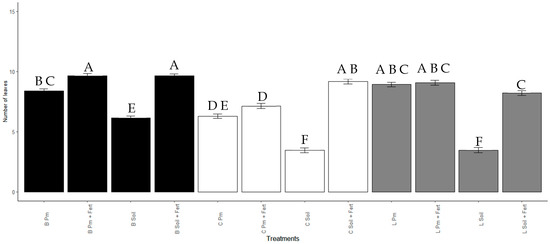
Figure 7.
Number of leaves of Habanero pepper seedlings with or without inoculation with Enterobacter sp. ITCB-09 after 60 days of cultivation. The different letters on the bars indicate significant differences (p < 0.001) based on Bonferroni comparisons; whiskers represent the standard errors. Black bars indicate the treatments with pearls (Enterobacter sp. ITCB-09); white bars indicate the controls; gray bars indicate the treatments with liquid (Enterobacter sp. ITCB-09).
4. Discussion
Phosphorus is a key nutrient in plant growth and is found abundantly in soils and water, but not in the form required by plants. As a result, agriculture has overused phosphorus-rich fertilizers. The role of bacteria is vital in the biogeochemical processes involving phosphorus. Most studies have focused on the production of organic acids or phosphatase by bacterial cells. However, understanding all the microbial mechanisms involved could help to establish better strategies for sustainable agriculture.
In this study, 21 strains with phosphate-solubilizing activity detected in Pikovskaya agar were isolated from Capsicum chinense agroecosystems. A similar isolation project was reported by Oliveira et al. [8], who isolated 36 strains from maize crops. Other studies have reported different isolation strategies, mainly resulting in more than 20 microbial strains from different culture media [24,25,50].
Acidification of liquid culture medium is a characteristic that may indirectly indicate phosphate-solubilizing activity [24,51]. However, ion chromatography is a more accurate and quantitative method [52]. In the present study, ion chromatography showed that strain ITCB-09 had the highest solubilization rate (865.98 ± 105.69 µg/mL) of the isolates. These values are higher than those reported by Chen et al. [53], who found solubilization values of 519.7 µg/mL in a strain of Arthrobacter. Yi and Ge [14] highlighted the phosphate solubilization potential of a strain of Enterobacter sp. with a solubilization rate of 632.6 µg/mL, also lower than our isolate.
In a similar study, Teng et al. [20] isolated and characterized phosphate-solubilizing bacteria from the rhizosphere of three plants, identifying three strains of the genus Enterobacter with this ability. These strains caused acidification of the liquid culture medium, and there was a correlation between the decrease in pH and the phosphate solubilization rate, which suggested that the principal mechanism of phosphate solubilization by these strains was the production of organic acids.
In addition to the production of organic acids, the second most reported mechanism is the synthesis of phosphatases [54]. Phosphate solubilizing activity by the action of these enzymes was, however, ruled out in Enterobacter sp. ITCB-09. Although the biosynthesis of phosphatases occurs in the solubilization of organic phosphorus (mineralization), the evaluation of enzymatic activity is widely reported because the roots of plants can also produce this type of enzyme and not the microorganism [11].
The 16S rRNA gene sequence analysis established that strain ITCB-09 belongs to the genus Enterobacter. This genus has been widely reported in the literature for the biofertilization potential of some of its species [22]. The solubilization of inorganic phosphate, biological nitrogen fixation, and protection against phytopathogens have been the most economically interesting activities of this genus [19,20,21].
Enterobacter sp. ITCB-09 showed to synthesize siderophores, an important property of phosphorus-solubilizing bacteria [55]. However, most studies associate these metabolites with iron deficiency in plants and not with phosphorus solubilization. Today, there is an increasing number of studies highlighting the potential of siderophores as biological control agents for plant pathogens [56,57]. Mineral dissolution predominates over the exchange of ligands with the anions of organic acids as a phosphate solubilization mechanism [58], and therefore a potential role of siderophores in improving phosphate availability in the rhizosphere may be anticipated. Bendaha and Belaouni [22] studied the effect of the bacterium Enterobacter ludwigii EB4B as a growth promoter in tomato plants. They isolated two strains of Enterobacter ludwigii that formed transparent halos on the Pikovskaya culture medium, demonstrating inorganic phosphate solubilizing activity, and that changed CAS medium from blue to purplish red, indicating siderophore production. Inoculation of Enterobacter ludwigii EB4B onto tomato seeds improved root growth by at least 300% compared to the control. Thus, strains of the genus Enterobacter with the ability to solubilize inorganic phosphate and produce siderophores can be considered bio-sustainable alternatives for the improvement of plant growth in agriculture.
There is little information on the role of EPS in phosphate solubilization. This study shows that Enterobacter sp. ITCB-09 produces a biopolymer of glycoprotein nature, which suggests functional groups that may have calcium chelating activity [36,49]. Recently, Costa et al. [13] suggested the ecological role of bacterial EPS in the aggregation of soil particles, highlighting that EPS are directly involved in the formation of organo-mineral associations in the soil, affecting the composition of immobile and mobile organic matter, as well as the reactivity of minerals. Mahmood et al. [59] stated that EPS-producing bacteria could promote plant growth since EPS production in the rhizosphere can increase water availability and nutrients such as phosphorus and potassium, as well as helping plants tolerate salinity. Microbial EPS are an important factor for soil structure, health, and fertility.
The results reported here showed a positive effect on growth (height and number of leaves) of Habanero pepper seedlings for substrates inoculated with alginate beads containing Enterobacter sp. ITCB-09. Pan et al. [60] similarly observed that Enterobacter ludwigii inoculated on tomato seeds promoted their growth and improved their foliar protein properties and the elongation of their roots and stem. Borham et al. [61] evaluated the effect of a strain of Enterobacter on wheat crops, inoculating it with or without fertilizers. The bacterium showed to increase stem elongation and chlorophyll production in the crop, and also an improvement in fertilizer utilization. These two examples establish that the genus Enterobacter bacteria can be an ecologically friendly alternative to promote plant growth and improve the performance of fertilizers and substrates. In the current research, the sap content, chlorophyll, and leaf area of the Habanero pepper seedlings was not determined, but the difference between the color and leaf area of the seedlings inoculated or not with Enterobacter sp. ITCB-09, Soil 50%, and Peat Moss 50% can be seen in Figure 5, indicating the efficacy of the treatment.
In conclusion, the present work has demonstrated that Enterobacter sp. ITCB-09 can solubilize inorganic phosphate in higher concentrations than those reported in other bacteria. The predominant mechanism of solubilization is the production of organic acids. The strain produces siderophores and extracellular polymeric substances, which are biomolecules that could contribute to plant growth, control of pathogens, or soil health and fertility. Enterobacter sp. ITCB-09, mainly in the immobilized form, promoted the growth of Habanero pepper seedlings. This bacterium thus has biofertilization potential; further studies should confirm this potential and its future commercial application.
Author Contributions
All authors contributed on this research. J.C.C.-C. and G.E.M.-A. designed the study and wrote the manuscript; A.O.C.-R. and K.I.C.-R. provided funding acquisition and project administration; M.J.C.-B., R.N.A.-R., B.O.O.-M., R.E.C.S., B.D.-C. and R.E.T.-C. contributed to methodology and analysis of data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by TECNOLÓGICO NACIONAL DE MÉXICO, grant number 6422.18-P.
Acknowledgments
The authors are grateful to Christine Gaylarde for help with English editing. We also thank comments and suggestions from anonymous reviewers helped to improve our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Liu, X.; Hao, T.; Chen, S. Colonization and maize growth promotion induced by phosphate solubilizing bacterial isolates. Int. J. Mol. Sci. 2017, 18, 1253. [Google Scholar]
- Mpanga, I.K.; Nkebiwe, P.M.; Kuhkmann, M.; Cozzolino, V.; Piccolo, A.; Geistlinger, J.; Berger, N.; Ludewig, U.; Neumann, G. The form of N supply determines plant growth promotion by P-solubilizing microorganisms in maize. Microorganisms 2019, 7, 38. [Google Scholar] [CrossRef]
- Khan, S.; Zaidi, A.; Ahmad, E. Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms. In Phosphate Solubilizing Microorganisms. Principles and Application of Microphos Technology, 1st ed.; Khan, S., Zaidi, A., Musarrat, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 31–62. [Google Scholar]
- Goldstein, A.H. Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by gram negative bacteria. Biol. Agric. Hortic 1995, 12, 185–193. [Google Scholar] [CrossRef]
- Kim, K.Y.; McDonald, G.A.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Oliveira-Rita, J.; Gama-Rodrigues, A.; Gama-Rodrigues, E.; Costa-Zaia, F.; Duarte-Nunes, D. Mineralization of organic phosphorus in soil size fractions under different vegetation covers in the north of Rio de Janeiro. Rev. Bras. Cienc Solo 2013, 37, 1207–1215. [Google Scholar] [CrossRef][Green Version]
- Oliveira, C.; Alves, V.; Marriel, I.; Gomes, E.; Scotti, M.; Carneiro, N.; Sá, N. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol. Biochem. 2009, 41, 1782–1787. [Google Scholar] [CrossRef]
- Aseri, G.K.; Jain, N.; Tarafdar, J.C. Hydrolysis of organic phosphate forms by phosphatases and phytase producing fungi of arid and semi-arid soils of India. Am.-Eurasian JAES 2009, 5, 564–570. [Google Scholar]
- Maougal, R.T.; Brauman, A.; Plassard, C.; Abadie, J.; Djekoun, A.; Drevon, J.J. Bacterial capacities to mineralize phytate increase in the rhizosphere of nodulated common bean (Phaseolus vulgaris) under P deficiency. Eur. J. Soil Biol. 2014, 62, 8–14. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)—Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef]
- Costa, O.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, W.; Ge, Y. Exopolysaccharide: A novel important factor in the microbial dissolution of tricalcium phosphate. World J. Microbiol. Biotechnol. 2008, 24, 1059–1065. [Google Scholar] [CrossRef]
- Chaudhary, I.; Neeraj, A.; Siddiqui, M.; Singh, V. Nutrient Management Technologies and the Role of Organic Matrix-Based Slow-Release Biofertilizers for Agricultural Sustainability: A Review. Agric. Rev. 2020, 41, 1–13. [Google Scholar] [CrossRef]
- Glick, B. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Sarikhani, M.; Aliasgharzad, N.; Khoshru, B. P Solubilizing Potential of Some Plant Growth Promoting Bacteria Used as Ingredient in Phosphatic Biofertilizers with Emphasis on Growth Promotion of Zea mays L. Geomicrobiol. J. 2020, 37, 327–335. [Google Scholar] [CrossRef]
- Ribaudo, C.; Zaballa, J.; Golluscio, R. Effect of the Phosphorus-Solubilizing Bacterium Enterobacter ludwigii on Barley Growth Promotion. Am. Sci. Res. J. Eng. Technol. Sci. 2020, 63, 144–157. [Google Scholar]
- Lee, K.; Adhikari, A.; Kang, S.; You, Y.; Joo, G.; Kim, J.; Lee, I. Isolation and characterization of the high silicate and phosphate solubilizing novel strain Enterobacter ludwigii GAK2 that promotes growth in rice plants. Agronomy 2019, 9, 144. [Google Scholar] [CrossRef]
- Teng, Z.; Chen, Z.; Zhang, Q.; Yao, Y.; Song, M.; Li, M. Isolation and characterization of phosphate solubilizing bacteria from rhizosphere soils of the Yeyahu Wetland in Beijing, China. Environ. Sci. Pollut. Res. 2019, 26, 33976–33987. [Google Scholar] [CrossRef]
- Widdig, M.; Schleuss, P.; Weig, A.; Guhr, A.; Biederman, L.; Borer, E.; Spohn, M. Nitrogen and phosphorus additions alter the abundance of phosphorus-solubilizing bacteria and phosphatase activity in grassland soils. Front. Environ. Sci. 2019, 7, 185–200. [Google Scholar] [CrossRef]
- Bendaha, M.E.; Belaouni, H. Effect of the endophytic plant growth promoting Enterobacter ludwigii EB4B on tomato growth. Hell. Plant. Prot. J. 2020, 13, 54–65. [Google Scholar] [CrossRef]
- Vyas, R.; Panpatte, D.; Jhala, Y.; Shelat, H. Wonders of microbes in agriculture for productivity and sustainability. In Microorganisms for Green Revolution, 1st ed.; Kumar, N., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1, pp. 1–24. [Google Scholar]
- Keneni, A.; Assefa, F.; Prabu, P.C. Isolation of phosphate solubilizing bacteria from the rhizosphere of faba bean of Ethiopia and their abilities on solubilizing insoluble phosphates. J. Agric. Sci. Technol. 2010, 12, 79–89. [Google Scholar]
- Singh, N.K.; Patel, D.B.; Chaudhari, S.R.; Morad, B.G.; Rabari, S.M. Characterization of phosphate-solubilizing isolates of Bacillus from cumin rhizosphere. IJTA 2016, 34, 1469–1480. [Google Scholar]
- National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov (accessed on 1 June 2020).
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA 5: Molecular evolutionary genetics analysis using maximun likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef]
- Shin, S.H.; Lim, Y.; Lee, S.E.; Yang, N.W.; Rhee, J.H. CAS agar diffusion assay for the measurement of siderophores in biological fluids. J. Microbiol. Meth. 2001, 44, 89–95. [Google Scholar] [CrossRef]
- Schiessl, K.T.; Janssen, E.M.; Kraemer, S.M.; McNeill, K.; Ackermann, M. Magnitude and mechanism of siderophore-mediated competition at low iron solubility in the Pseudomonas aeruginosa pyochelin system. Front. Microbiol. 2017, 8, 1964. [Google Scholar] [CrossRef]
- Dave, B.P.; Anshuman, K.; Hajela, P. Siderophores of halophilic archaea and their chemical characterization. Indian J. Exp. Biol. 2006, 44, 340–344. [Google Scholar]
- Bobadilla, C.; Rincón, S. Aislamiento y Producción de Bacterias Fosfato Solubilizadoras a Partir de Compost Obtenido de Residuos de Plaza. Bachelor’s Thesis, Facultad de Ciencias de la Universidad Javeriana, Bogotá, Colombia, June 2008. [Google Scholar]
- Malo, M.S.; Moaven, O.; Muhammad, N.; Biswas, B.; Alam, S.N.; Economopoulos, K.P.; Mohamed, M.M.R. Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Metcalf, W.W. A new activity for an old enzyme: Escherichia coli bacterial alkaline phosphatase is a phosphite-dependent hydrogenase. PNAS 2004, 101, 7919–7924. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Uma-Devi, K.; Gopishankar, Y.; Shivaji, S. Thermolabile alkaline phosphatase from Sphingobacterium antarticus a psychrotrophic bacterium from Antarctica. Polar Biol 1995, 15, 215–219. [Google Scholar] [CrossRef]
- Ortega-Morales, B.O.; Santiago-García, J.L.; Chan-Bacab, M.J.; Moppert, X.; Miranda-Tello, E.; Fardeau, M.L.; Guezennec, J. Characterization of extracellular polymers synthesized by tropical intertidal biofilm bacteria. J. Appl. Microbiol. 2007, 102, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Chab, J.C.; Guézennec, J.; Chan-Bacab, M.J.; Ríos-Leal, E.; Sinquin, C.; Munñiz-Salazar, R.; Ortega-Morales, B.O. Emulsifying activity and stability of a non-toxic bioemulsifier synthesized by Microbacterium sp. MC3B-10. Int. J. Mol. Sci. 2013, 14, 18959–18972. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 15, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, H.; Farr, A.; Randall, R. Protein measurement with the folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Colli-Camal, G. Efecto de la Cepa Solubilizadora de Fosfato ITCB-09 en el Crecimiento de Plántulas de Chile Habanero (Capsicum chinense) var. Jaguar. Bachelor’s Thesis, Instituto Tecnológico de Chiná, Chiná, Mexico, September 2017. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org (accessed on 1 June 2020).
- Fabián, D.; Guadarrama, P.; Hernadez-Cuevas, L.; Ramos-Zapata, J.A. Arbuscular mycorrhizal fungi in a coastal wetland in Yucatan, Mexico. Bot. Sci. 2018, 96, 24–34. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Shoebitz, M.; Ribaudo, C.M.; Pardo, M.A.; Cantore, M.L.; Ciampi, L.; Curá, J.A. Plant growth promoting properties of a strain of Enterobacter ludwigii isolated from Lolium perenne rhizosphere. Soil Biol. Biochem. 2009, 41, 1768–1774. [Google Scholar] [CrossRef]
- Hwangbo, H.; Park, R.D.; Kim, Y.W.; Rim, Y.S.; Park, K.H.; Kim, T.H.; Kim, K.Y. 2-Ketogluconic acid production and phosphate solubilization by Enterobacter intermedium. Curr. Microbiol. 2003, 47, 0087–0092. [Google Scholar]
- Vassilev, N.; Toro, M.; Vassileva, M.; Azcon, R.; Barea, J.M. Rock phosphate solubilization by immobilized cells of Enterobacter sp. in fermentation and soil conditions. Bioresour. Technol 1997, 61, 29–32. [Google Scholar] [CrossRef]
- Camacho-Chab, J.C.; Castañeda-Chávez, M.D.R.; Chan-Bacab, M.J.; Aguila-Ramírez, R.N.; Galaviz-Villa, I.; Bartolo-Pérez, P.; Ortega-Morales, B.O. Biosorption of cadmium by non-toxic extracellular polymeric substances (EPS) synthesized by bacteria from marine intertidal biofilms. Int. J. Environ. Res. Public Health 2018, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Mishra, R.R.; Sethi, B.K.; Dutta, S.K.; Thatoi, H.N. Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J. Genet. Eng. Biotechnol. 2017, 15, 169–178. [Google Scholar] [CrossRef]
- Cordero-Elvia, J.; Ortega-Rodés, P.; Ortega, E. La inoculación de plantas con Pantoea sp., bacteria solubilizadora de fosfatos, incrementa la concentración de P en los tejidos foliares. Rev. Colomb. Biotecnol. 2008, 10, 111–121. [Google Scholar]
- Nowack, B. Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533–2546. [Google Scholar] [CrossRef]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action. Biological Processes in Soil Phosphorus Cycling, 1st ed.; Büneman, E.K., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243. [Google Scholar]
- Crowley, D. Microbial siderophores in the plan rhizosphere. In Iron Nutrition in Plants and Rhizospheric Microorganisms, 1st ed.; Barton, L., Abadia, J., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 169–198. [Google Scholar]
- Sasirekha, B.; Srividya, S. Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chilli. Agric. Nat. Resour. 2016, 50, 250–256. [Google Scholar] [CrossRef]
- Kumar, P.; Thakur, S.; Dhingra, G.K.; Singh, A.; Kumar-Pal, M.; Harshvardhan, K.; Dubey, R.C.; Maheshwari, D. Inoculation of siderophore producing rhizobacteria and their consortium for growth enhancement of wheat plant. Biocatal. Agric. Biotechnol. 2018, 15, 264–269. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, C.; Son, H. Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plan growth-promoting activities. Lett. Appl. Microbiol. 2009, 49, 222–228. [Google Scholar] [CrossRef]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, H.; Ali, Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016, 7, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shikano, I.; Hoover, K.; Liu, T.X.; Felton, G.W. Enterobacter ludwigii, isolated from the gut microbiota of Helicoverpa zea, promotes tomato plant growth and yield without compromising anti-herbivore defenses. Arthropod Plant Interact. 2019, 13, 271–278. [Google Scholar] [CrossRef]
- Borham, A.; Belal, E.; Metwaly, M. Phosphate Solubilization by Enterobacter cloacae and Its Impact on Growth and Yield of Wheat Plants. J. Sustain. Agric. Sci. 2017, 43, 89–103. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).