Monitoring of the PAL Enzymatic Activity and Polyphenolic Compounds in Leaves and Fruits of Two Myrtle Cultivars during Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents
2.3. Preparation of Extracts
2.4. Chemical Analysis
2.5. Extraction and Assays of Phenylalanine Ammonia-Lyase (PAL)
2.6. Data Analysis
3. Results
3.1. Chemical Analysis
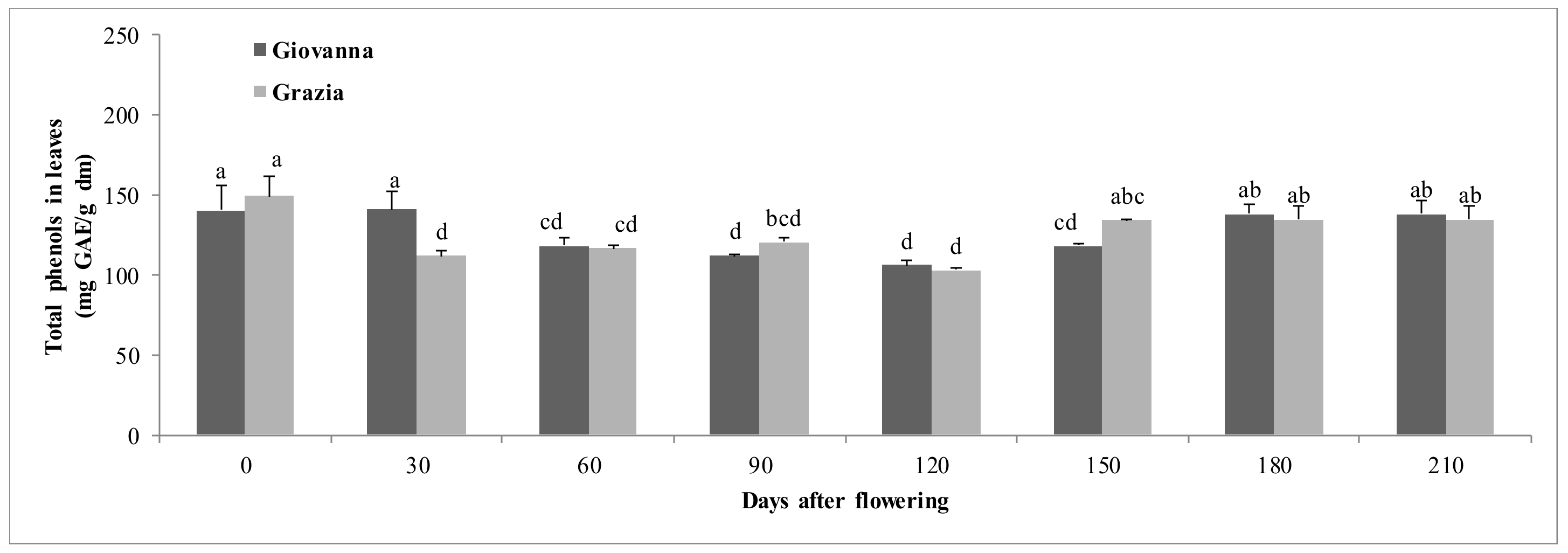
3.1.1. Total Polyphenols
3.1.2. Anthocyanins
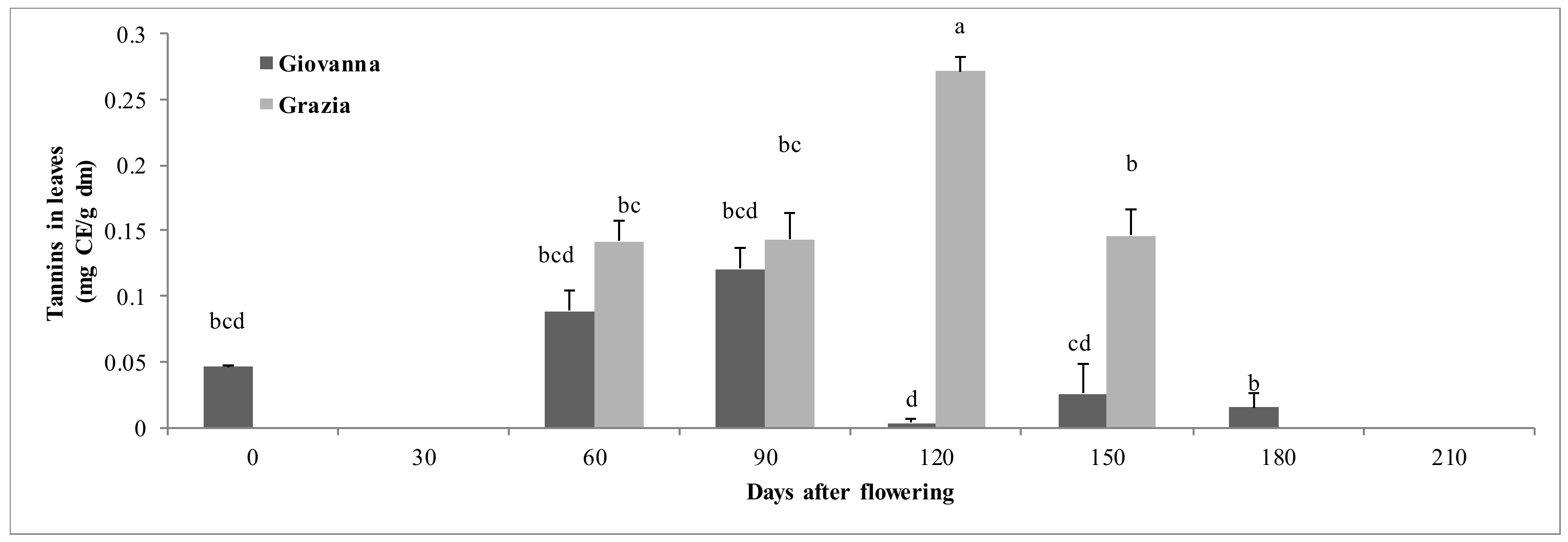
3.1.3. Tannins
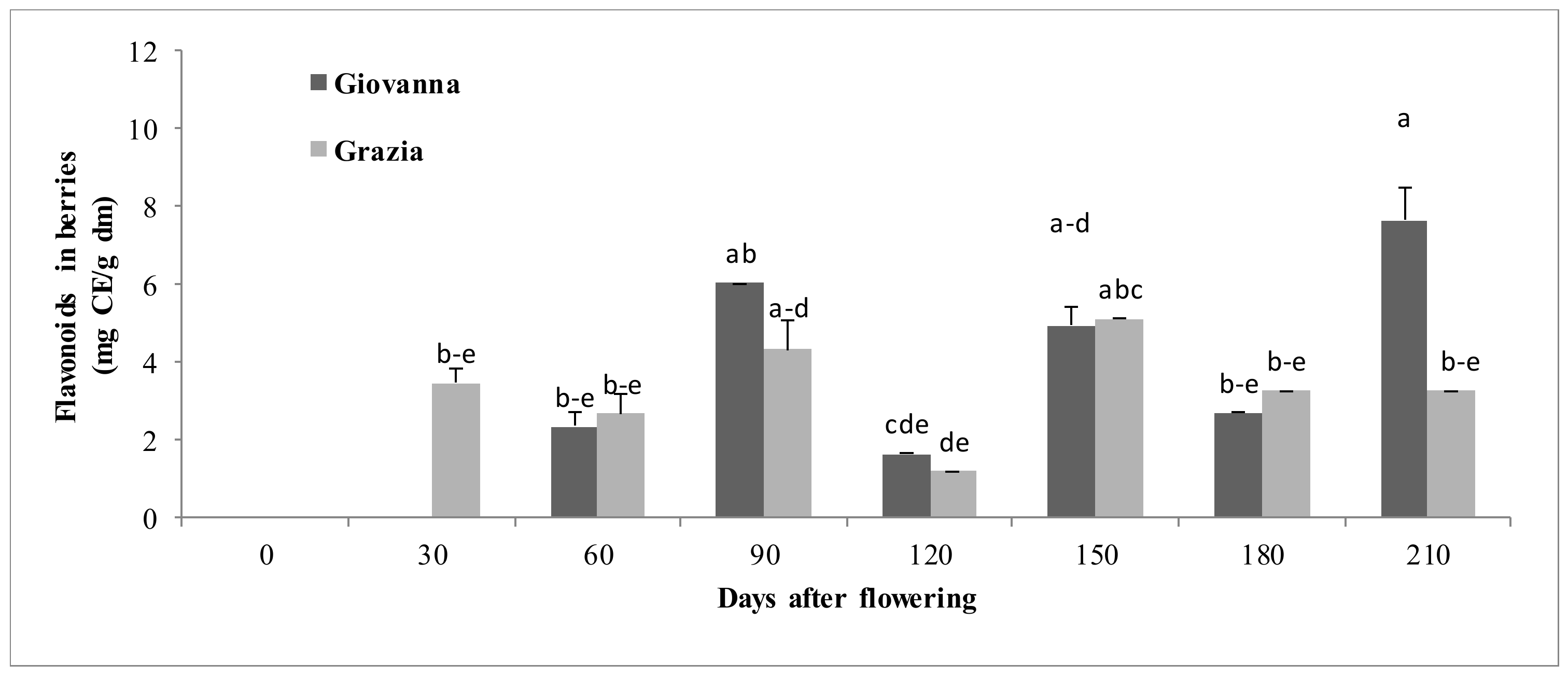
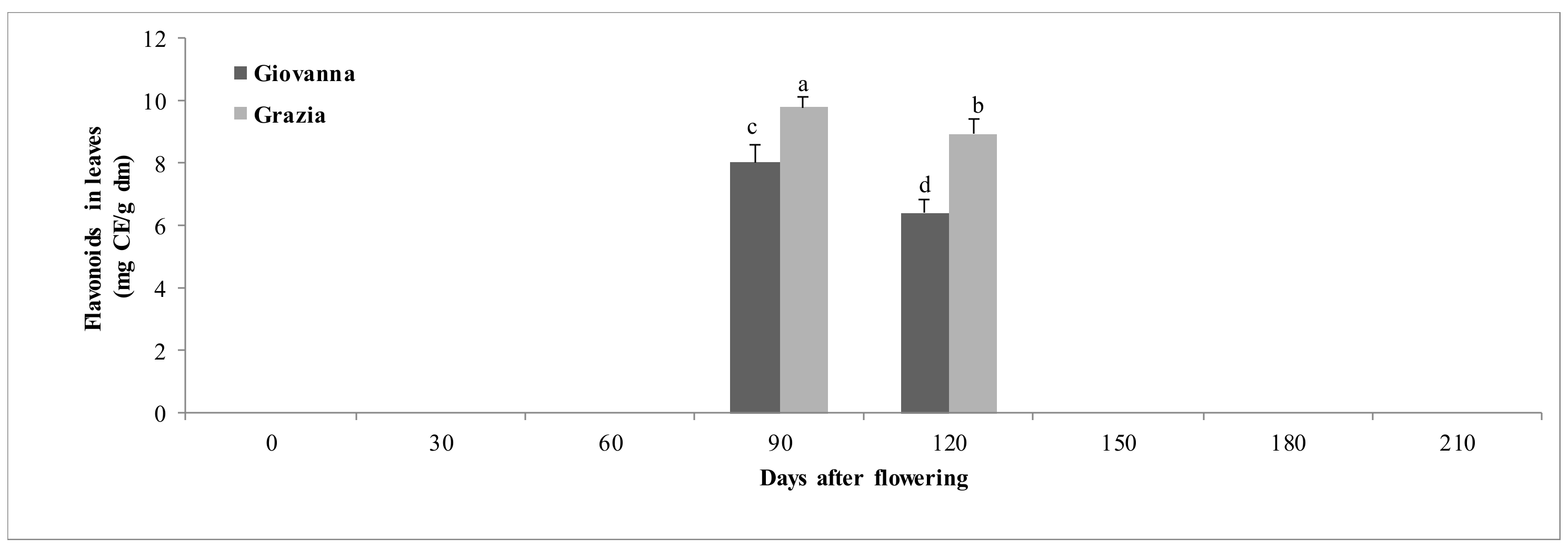
3.1.4. Flavonoids
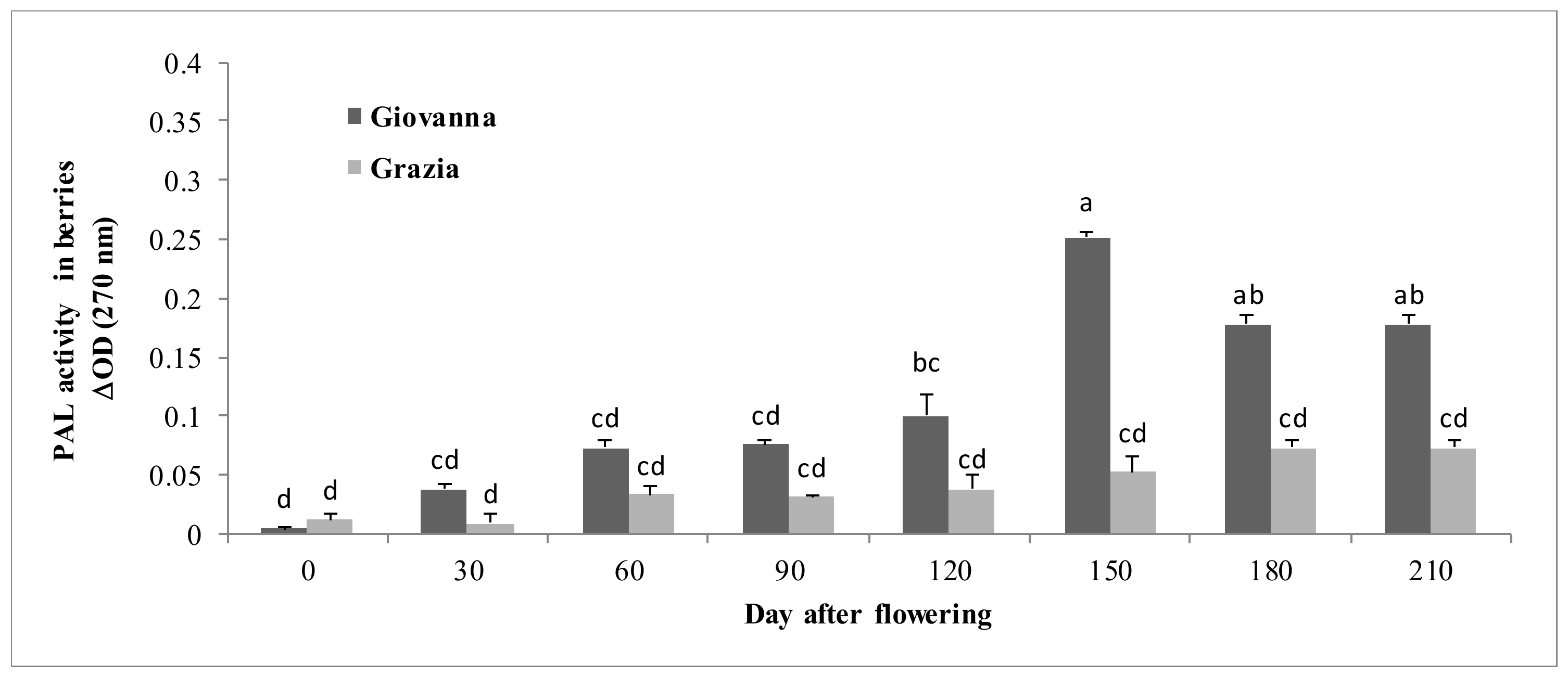
3.1.5. PAL Activity
3.2. Correlation between Phenolic Compounds and PAL
4. Discussion
4.1. Chemical Analysis
4.2. PAL Activity and Phenolic Compounds during Fruit Development and Ripening
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mulas, M. The myrtle (Myrtus communis L.) case: From a wild shrub to a new fruit crop. Acta Hortic. 2012, 948, 235–242. [Google Scholar] [CrossRef]
- Mulas, M.; Cani, M.R. Germplasm evaluation of spontaneous myrtle (Myrtus communis L.) for cultivar selection and crop development. J. Herbs Spices Med. Plants 1999, 6, 31–49. [Google Scholar] [CrossRef]
- Alipour, G.; Dashti, S.; Hosseinzadeh, H. Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytother. Res. 2014, 28, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Amira, S.; Dade, M.; Schinella, G.; Ríos, J.L. Anti-inflammatory, anti-oxidant, and apoptotic activities of four plant species used in folk medicine in the Mediterranean basin. Pak. J. Pharm. Sci. 2012, 25, 65–72. [Google Scholar] [PubMed]
- Cottiglia, F.; Casu, L.; Leonti, M.; Caboni, P.; Floris, C.; Busonera, B.; Farci, P.; Ouhtit, A.; Sanna, G. Cytotoxic Phloroglucinols from the Leaves of Myrtus communis. J. Nat. Prod. 2012, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Babaee, N.; Mansourian, A.; Momen-Heravi, F.; Moghadamnia, A.A.; Momen-Beitollahi, J. The efficacy of a paste containing Myrtus communis (Myrtle) in the management of recurrent aphthous stomatitis: A randomized controlled trial. Clin. Oral Investig. 2010, 14, 65–70. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.A.; Asif, M.; Saud, I.; Akhtar, M. Evaluation of Myrtus communis Linn. berries (common myrtle) in experimental ulcer models in rats. Hum. Exp. Toxicol. 2010, 29, 935–944. [Google Scholar] [CrossRef]
- Mulas, M.; Melis, R.A. Influence of growing area, year, season, and cultivar on the composition of myrtle leaves and infusions. HortScience 2008, 43, 549–553. [Google Scholar] [CrossRef]
- D’Urso, G.; Montoro, P.; Lai, C.; Piacente, S.; Sarais, G. LC-ESI/LTQOrbitrap/MS based metabolomics in analysis of Myrtus communis leaves from Sardinia (Italy). Ind. Crop. Prod. 2019, 128, 354–362. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Mulinacci, N.; Vincieri, F.F.; Tattini, M. Identification and quantitation of polyphenols in leaves of Myrtus communis L. Chromatografia 1999, 49, 17–20. [Google Scholar] [CrossRef]
- Barboni, T.; Cannac, M.; Massi, L.; Perez-Ramirez, Y.; Chiaramonti, N. Variability of polyphenol compounds in Myrtus Communis L. (Myrtaceae) berries from Corsica. Molecules 2010, 15, 7849–7860. [Google Scholar] [CrossRef] [PubMed]
- Sarais, G.; D’Urso, G.; Lai, C.; Pirisi, F.M.; Pizza, C.; Montoro, P. Targeted and untargeted mass spectrometric approaches in discrimination between Myrtus communis cultivars from Sardinia region. Int. J. Mass Spectrom. 2016, 51, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, S.; Lazzoi, M.R.; Mergola, L.; Di Bello, M.P.; Del Sole, R.; Vasapollo, G. Anthocyanins profile by Q-TOF LC/MS in Myrtus communis berries from Salento Area. Food Anal. Methods 2017, 10, 2404–2411. [Google Scholar] [CrossRef]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of free radical scavenging activity of plant extracts through DPPH assay: An EPR and UV–Vis study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Sanna, D.; Mulas, M.; Molinu, M.G.; Fadda, A. Oxidative stability of plant hydroalcoholic extracts assessed by EPR spin trapping under forced ageing conditions: A myrtle case study. Food Chem. 2019, 271, 753–761. [Google Scholar] [CrossRef]
- Babou, L.; Hadidi, L.; Grosso, C.; Zaidi, F.; Valentão, P.; Andrade, P.B.; Grosso, A.C. Study of phenolic composition and antioxidant activity of myrtle leaves and fruits as a function of maturation. Eur. Food Res. Technol. 2016, 242, 1447–1457. [Google Scholar] [CrossRef]
- Škrovánková, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Saxena, M.; Saxena, J.; Pradhan, A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res. 2012, 16, 130–134. [Google Scholar]
- Dai, J.; Mumper, R. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Patel, J.M. Review of potential health benefits of flavonoids. LURJ 2008, 3, 1–5. [Google Scholar]
- Cruciani, S.; Santaniello, S.; Fadda, A.; Sale, L.; Sarais, G.; Sanna, D.; Mulas, M.; Ginesu, G.C.; Cossu, M.L.; Serra, P.A.; et al. Extracts from myrtle liqueur processing waste modulate stem cells pluripotency under stressing conditions. BioMed Res. Int. 2019, 2019, 5641034. [Google Scholar] [CrossRef] [PubMed]
- Babenko, L.M.; Smirnov, O.E.; Romanenko, K.O.; Trunova, O.K.; Kosakivska, I.V. Phenolic compounds in plants: Biogenesis and functions. Ukr. Biochem. J. 2019, 91, 5–18. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- González-De-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Palma, M.; Amores-Arrocha, A.; Palma, M.; Barbero, G.F.; Jiménez-Cantizano, A. Discrimination of myrtle ecotypes from different geographic areas according to their morphological characteristics and anthocyanins composition. Plants 2019, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Bertrams, J.; Kunz, N.; Müller, M.; Kammerer, D.; Stintzing, F.C. Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. J. Appl. Bot. Food. Qual. 2013, 86, 143–153. [Google Scholar] [CrossRef]
- Milivojević, J.; Rakonjac, V.; Akšić, M.M.F.; Pristov, J.B.; Maksimović, V. Classification and fingerprinting of different berries based on biochemical profiling and antioxidant capacity. Pesqui. Agropecu. Bras. 2013, 48, 1285–1294. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Xie, R.; Zheng, L.; He, S.; Zheng, Y.; Yi, S.; Deng, L. Anthocyanin biosynthesis in fruit tree crops: Genes and their regulation. Afr. J. Biotechnol. 2011, 10, 19890–19897. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- Macdonald, M.J.; D’Cunha, G.B. A modern view of phenylalanine ammonia lyase. Biochem. Cell Biol. 2007, 85, 273–282. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, S.; Rani, A.; Gulati, A.; Ahuja, P.S. Phenylalanine ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H) and catechins (flavan-3-ols) accumulation in tea. Funct. Integr. Genom. 2009, 9, 125–134. [Google Scholar] [CrossRef]
- Kataoka, I.; Kubo, Y.; Sugiura, A.; Tomana, T. Changes in L-phenylalanine ammonia-lyase activity and anthocyanin synthesis during berry ripening of three grape cultivars. J. Jpn. Soc. Hortic. Sci. 1983, 52, 273–279. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, C.; Yao, Y.; Mao, Z.; Sun, G.; Dai, Z. Red anthocyanins contents and the relationships with phenylalanine ammonia lyase (PAL) activity, soluble sugar and chlorophyll contents in carmine radish (Raphanus sativus L.). Hortic. Sci. 2019, 46, 17–25. [Google Scholar] [CrossRef]
- Cheng, G.W.; Breen, P.J. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hortic. Sci. 1991, 116, 865–869. [Google Scholar] [CrossRef]
- Sirin, S.; Aslım, B. Determination of antioxidant capacity, phenolic acid composition and antiproliferative effect associated with phenylalanine ammonia lyase (PAL) activity in some plants naturally growing under salt stress. Med. Chem. Res. 2019, 28, 229–238. [Google Scholar] [CrossRef]
- Ghasemi, S.; Kumleh, H.H.; Kordrostami, M. Changes in the expression of some genes involved in the biosynthesis of secondary metabolites in Cuminum cyminum L. under UV stress. Protoplasma 2019, 256, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lei, Y. Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol. Plant 2006, 50, 775–778. [Google Scholar] [CrossRef]
- Nadernejad, N.; Ahmadimoghadam, A.; Hosseinifard, J.; Pourseyedi, S. Phenylalanin ammonia lyase activity, total phenolic and flavonoid content in flowers, leaves, hulls, and kernels of three pistachio (Pistacia vera L.) cultivars. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 807–814. [Google Scholar]
- Lafuente, M.T.; Zacarías, L.; Martínez-Téllez, M.A.; Sanchez-Ballesta, M.T.; Dupille, E. Phenylalanine ammonia-lyase as related to ethylene in the development of chilling symptoms during cold storage of citrus fruits. J. Agric. Food Chem. 2001, 49, 6020–6025. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Asghari, M.; Farmani, B.; Mohayeji, M.; Moradbeygi, H. Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci. Hortic. 2012, 144, 116–120. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Fadda, A.; Mulas, M. Chemical profile changes during myrtle (Myrtus communis L.) fruit development and ripening. Sci. Hortic. 2010, 125, 477–485. [Google Scholar] [CrossRef]
- Ballester, A.R.; Lafuente, M.T.; González-Candelas, L. Spatial study of antioxidant enzymes, peroxidase and phenylalanine ammonia-lyase in the citrus fruit–Penicillium digitatum interaction. Postharvest Biol. Technol. 2006, 39, 115–124. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Aradhya, S. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005, 93, 319–324. [Google Scholar] [CrossRef]
- Ibrahim, K.E.; Abu-Goukh, A.A.; Yusuf, K.S. Use of ethylene, acetylene and ethrel on banana fruit ripening. Univ. Khartoum J. Agric. Sci. 1994, 2, 73–92. [Google Scholar]
- Belwal, T.; Pandey, A.; Bhatt, I.D.; Rawal, R.S.; Luo, Z. Trends of polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Sci. Rep. 2019, 9, 5894. [Google Scholar] [CrossRef] [PubMed]
- Bashir, H.A.; Abu-Goukh, A.B.A. Compositional changes during guava fruit ripening. Food Chem. 2003, 80, 557–563. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Morelló, J.R.; Romero, M.P.; Ramo, T.; Motilva, M.J.; Romero, M.P. Evaluation of l-phenylalanine ammonia-lyase activity and phenolic profile in olive drupe (Olea europaea L.) from fruit setting period to harvesting time. Plant Sci. 2005, 168, 65–72. [Google Scholar] [CrossRef]
- Lakshminarayana, S.; Mathew, A.G.; Parpia, H.A.B. Changes in polyphenols of sapota fruit (Achras zapota L.) during maturation. J. Sci. Food Agric. 1969, 20, 651–653. [Google Scholar] [CrossRef]
- Vlaic, R.A.; Mureşan, V.; Muresan, A.E.; Muresan, C.C.; Păucean, A.; Mitre, V.; Chis, S.M.; Muste, S. The changes of polyphenols, flavonoids, anthocyanins and chlorophyll content in plum peels during growth Phases: From fructification to ripening. Not. Bot. Horti Agrobot. 2018, 46, 148–155. [Google Scholar] [CrossRef]
- Kanoun, K.; Belyagoubi-Benhammou, N.; Ghembza, N.; Atik Bekkara, F. Comparative studies on antioxidant activities of extracts from the leaf, stem and berry of Myrtus communis L. Int. Res. Food. J. 2014, 21, 1957–1962. [Google Scholar]
- Amensour, M.; Sendra, E.; Abrini, J.; Bouhdid, S.; Pérez-Alvarez, J.A.; Fernández-López, J. Total phenolic content and antioxidant activity of myrtle (Myrtus communis) extracts. Nat. Prod. Commun. 2009, 4, 819–824. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Sriti, J.; Ben Jemia, M.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef]
- Gardeli, C.; Vassiliki, P.; Athanasios, M.; Kibouris, T.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Snoussi, A.; Essaidi, I.; Koubaier, H.B.H.; Chaabouni, M.M.; Bouzouita, N. Chemical composition and antioxidant activity of essential oils and ethanol extracts of Myrtus communis L. organs (Berries, Leaves and Floral buds). J. Soc. Chim. Tunis. 2012, 14, 69–76. [Google Scholar]
- Andersen, O.M.; Markham, K.R. (Eds.) The anthocyanins. In Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006; pp. 471–473. [Google Scholar]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Wannes, W.A.; Marzouk, B. Differences between myrtle fruit parts (Myrtus communis var. italica) in phenolics and antioxidant contents. J. Food Biochem. 2013, 37, 1745–4514. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Onodera, H.; Kawai, Y.; Kubo, T.; Itoh, H.; Wada, R. Enzyme activity changes during anthocyanin synthesis in ′Olympia′ grape berries. Sci. Hortic. 2001, 90, 255–264. [Google Scholar] [CrossRef]
- Lister, C.E.; Lancaster, J.E.; Walker, J.R. Phenylalanine ammonia-lyase (PAL) activity and its relationship to anthocyanin and flavonoid levels in New Zealand-grown apple cultivars. J. Am. Soc. Hortic. Sci. 1996, 121, 281–285. [Google Scholar] [CrossRef]
- Ding, C.K.; Chachin, K.; Ueda, Y.; Imahori, Y.; Wang, C.Y. Metabolism of phenolic compounds during loquat fruit development. J. Agric. Food Chem. 2001, 49, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Parthiban, R. Changes in enzyme activities (polyphenol oxidase and phenylalanine ammonia lyase) with type of tea leaf and during black tea manufacture and the effect of enzyme supplementation of dhool on black tea quality. Food Chem. 1998, 62, 277–281. [Google Scholar] [CrossRef]
- Yu, X.Z.; Fan, W.J.; Lin, Y.J.; Zhang, F.F.; Gupta, D.K. Differential expression of the PAL gene family in rice seedlings exposed to chromium by microarray analysis. Ecotoxicology 2018, 27, 325–335. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the arabidopsis pal gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Olsen, K.M.; Lea, U.S.; Slimestad, R.; Verheul, M.; Lillo, C. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental-triggered flavonoid synthesis. J. Plant Physiol. 2008, 165, 1491–1499. [Google Scholar] [CrossRef]
- Ortega García, F.; Blanco, S.; Peinado, M.Á.; Peragón, J. Phenylalanine ammonia-lyase and phenolic compounds in leaves and fruits of Olea europaea L. cv. Picual during ripening. J. Sci. Food Agric. 2009, 89, 398–406. [Google Scholar] [CrossRef]
- Ju, Z.G.; Yuan, Y.B.; Liou, C.L.; Xin, S.H. Relationships among phenylalanine ammonia-Iyase activity, simple phenol concentrations and anthocyanin accumulation in apple. Sci. Hortic. 1995, 61, 215–226. [Google Scholar] [CrossRef]
- Margna, U. Control at the level of substrate supply—An alternative in the regulation of phenylpropanoid accumulation in plant cells. Phytochemistry 1977, 16, 419–426. [Google Scholar] [CrossRef]
- Samanta, T.; Kotamreddy, J.N.R.; Ghosh, B.C.; Mitra, A. Changes in targeted metabolites, enzyme activities and transcripts at different developmental stages of tea leaves: A study for understanding the biochemical basis of tea shoot plucking. Acta Physiol. Plant. 2017, 39, 11. [Google Scholar] [CrossRef]
- Wei, H.; Chen, X.; Zong, X.; Shu, H.; Gao, D.; Liu, Q. Comparative transcriptome analysis of genes involved in anthocyanin biosynthesis in the red and yellow fruits of sweet cherry (Prunus avium L.). PLoS ONE 2015, 10, e0121164. [Google Scholar] [CrossRef] [PubMed]
- He, J.J.; Liu, Y.X.; Pan, Q.H.; Cui, X.Y.; Duan, C.Q. Different anthocyanin profiles of the skin and the pulp of yan73 (Muscat Hamburg × Alicante Bouschet) grape berries. Molecules 2010, 15, 1141–1153. [Google Scholar] [CrossRef] [PubMed]




| Total Phenols of Leaves | Total Phenols of Berries | Tannins of Leaves | Tannins of Berries | Anthocyanins of Berries | Flavonoids of Leaves | Flavonoids of Berries | PAL of Leaves | |
|---|---|---|---|---|---|---|---|---|
| Total phenols of berries | 0.340 * | |||||||
| Tannins of leaves | −0.439 *** | −0.054 | ||||||
| Tannins of berries | −0.439 *** | −0.115 | 0.633 **** | |||||
| Anthocyanins of berries | 0.219 | −0.292 * | −0.267 | −0.380 ** | ||||
| Flavonoids of leaves | −0.509 **** | −0.238 | 0.532 ** | 0.369 ** | −0.251 | |||
| Flavonoids of berries | −0.130 | −0.471 **** | 0.031 | 0.070 | 0.468 **** | 0.086 | ||
| PAL of leaves | 0.102 | −0.094 | 0.082 | −0.058 | −0.080 | 0.078 | −0.233 | |
| PAL of berries | −0.001 | −0.445 *** | −0.235 | −0.259 | 0.695 **** | −0.142 | 0.444 ** | −0.139 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medda, S.; Dessena, L.; Mulas, M. Monitoring of the PAL Enzymatic Activity and Polyphenolic Compounds in Leaves and Fruits of Two Myrtle Cultivars during Maturation. Agriculture 2020, 10, 389. https://doi.org/10.3390/agriculture10090389
Medda S, Dessena L, Mulas M. Monitoring of the PAL Enzymatic Activity and Polyphenolic Compounds in Leaves and Fruits of Two Myrtle Cultivars during Maturation. Agriculture. 2020; 10(9):389. https://doi.org/10.3390/agriculture10090389
Chicago/Turabian StyleMedda, Silvia, Leonarda Dessena, and Maurizio Mulas. 2020. "Monitoring of the PAL Enzymatic Activity and Polyphenolic Compounds in Leaves and Fruits of Two Myrtle Cultivars during Maturation" Agriculture 10, no. 9: 389. https://doi.org/10.3390/agriculture10090389
APA StyleMedda, S., Dessena, L., & Mulas, M. (2020). Monitoring of the PAL Enzymatic Activity and Polyphenolic Compounds in Leaves and Fruits of Two Myrtle Cultivars during Maturation. Agriculture, 10(9), 389. https://doi.org/10.3390/agriculture10090389






