Mycorrhizal Inoculation and Chemical Fertilizer Interactions in Pineapple under Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Mycorrhizal Inoculum
2.2. Experimental Site and Agroecological Characteristics
2.3. Treatments, Plot Size, and Experimental Design
2.4. Evaluation of Variables
2.5. Fruit Quality Evaluation
3. Results
3.1. Mycorrhizal Root Colonization Percentage
3.2. Dry Weight of D-Leaf (DWDL)
3.3. Fruit Weight, Size, and Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uriza-Ávila, D.E.; Torres-Ávila, A.; Aguilar-Ávila, J.; Santoyo-Cortés, V.H.; Zetina-Lezama, R.; Rebolledo-Martínez, A. La Piña Mexicana Frente al Reto de La Innovación. Avances y Retos en La Gestión de La Innovación; Universidad Autónoma Chapingo (UACh): Chapingo, México, 2018; 479p. [Google Scholar]
- Liu, Q.; Guo, Y.; Giesy, J.P. Spatio-temporal effects of fertilization in Anhui Province, China. Environ. Dev. Sustain. 2015, 17, 1197–1207. [Google Scholar] [CrossRef]
- Uriza, D.; Rebolledo, L. Integrated Control of Phytoparasitic Nematodes in Pineapple; System Technology for Product Sheet; Control Integrado de Nemátodos Fitoparásitos en Piña. Ficha Tecnológica por Sistema Producto SAGARPA-INIFAP; Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food-Forest Research Institute, Agricultural and Fishing, 2002.
- Benzonan, N.C.; Dalisay, L.C.S.; Reponte, K.C.C.; Mapanao, C.P.; Alvarez, L.V.; Rendon, A.O.; Zurbano, L.Y. Plant-parasitic nematodes associated with pineapple (Ananas comosus) in selected provinces in Luzon, Philippines. EJMCM 2021, 8, 945–957. [Google Scholar]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides—A review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Bora, M.; Lokhandwala, A. Mycorrhizal Association: A safeguard for plant pathogen. In Plant, Soil and Microbes; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 253–275. [Google Scholar]
- Pawlowski, M.L.; Hartman, G.L. Impact of arbuscular mycorrhizal species on Heterodera glycines. Plant Dis. 2020, 104, 2406–2410. [Google Scholar] [CrossRef]
- Kunze, A.; Lovato, P.E.; Costa, M.D.; Dal Vesco, L.L. Pineapple (Ananas comosus) cv. pérola ex vitro growth and mycorrhizal colonization affected by in vitro sucrose concentration. Rev. Bras. Frutic. 2014, 36, 766–770. [Google Scholar] [CrossRef] [Green Version]
- Moreira, B.C.; Junior, P.P.; Jordão, T.C.; Silva, M.d.C.S.d.; Ribeiro, A.P.F.; Stürmer, S.L.; Salomão, L.C.C.; Otoni, W.C.; Kasuya, M.C.M. Effect of inoculation of pineapple plantlets with arbuscular mycorrhizal fungi obtained from different inoculum sources multiplied by the on-farm method. Rev. Bras. Cienc. Solo 2019, 43. [Google Scholar] [CrossRef] [Green Version]
- Thamsurakul, S.; Nopamonbodi, O.; Charoensook, S.; Roenrungroeng, S. Increasing pineapple yield using va mycorrhizal fungi. Acta Hortic. 2000, 529, 199–202. [Google Scholar] [CrossRef]
- Jaizme-Vega, M.C.; Azcón, R. Responses of some tropical and subtropical cultures to endomycorrhizal fungi. Mycorrhiza 1995, 5, 213–217. [Google Scholar] [CrossRef]
- Moreira, B.C.; Mendes, F.C.; Mendes, I.R.; Paula, T.A.; Junior, P.P.; Salomão, L.C.C.; Stürmer, S.L.; Otoni, W.C.; Guarçoni, A.; Kasuya, M.C.M. The interaction between arbuscular mycorrhizal fungi and Piriformospora indica improves the growth and nutrient uptake in micropropagation-derived pineapple plantlets. Sci. Hortic. 2015, 197, 183–192. [Google Scholar] [CrossRef]
- Rodríguez-Romero, A.; Azcón, R.; Jaizme-Vega, M. Early mycorrhization of two tropical crops, papaya (Carica papaya L.) and pineapple [Ananas comosus (L.) Merr.], reduces the necessity of P fertilization during the nursery stage. Fruits 2011, 66, 3–10. [Google Scholar] [CrossRef]
- Trejo, D.; Bañuelos, J.; Gavito, M.E.; Sangabriel-Conde, W. High phosphorus fertilization reduces mycorrhizal colonization and plant biomass of three cultivars of pineapple. Terra Latinoam. 2020, 38, 853–858. [Google Scholar] [CrossRef]
- Maia, V.M.; Pegoraro, R.F.; Aspiazú, I.; Oliveira, F.S.; Nobre, D.A.C. Chapter 50- Diagnosis and management of nutrient constraints in pineapple. In Fruit Crops; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 739–760. [Google Scholar]
- Smith, S.E.; Anderson, I.C.; Smith, F.A. Mycorrhizal associations and phosphorus acquisition: From cells to ecosystems. In Annual Plant Reviews; Plaxton, W.C., Lambers, H., Eds.; Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 48, pp. 409–439. [Google Scholar]
- Sieverding, E. Vesicular-Arbuscular Mycorrhiza Management in Tropical Agrosystems; Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ) GmbH: Eschborn, Germany, 1991; 371p. [Google Scholar]
- FAO CODEX STAN 182-1993. Available online: http://www.fao.org/fao-who-codexalimentarius/about-codex/es/ (accessed on 13 June 2021).
- Sideris, C.P.; Krauss, B.H. The classification and nomenclature of groups of pineapple leaves, sections of leaves and section of stems based on morphological and anatomical differences. Pineapple Q. 1936, 6, 56–66. [Google Scholar]
- Phillips, J.M.; Hayman, D.A. Improved procedures for clearing roots and standing parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.T.; Fitter, A.H. Ecological specificity of vesicular-arbuscular mycorrhizal association. Mycol. Res. 1990, 94, 120–122. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mäder, P.; Boller, T.; Wiemken, A. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 2003, 69, 2816–2824. [Google Scholar] [CrossRef] [Green Version]
- Sharmah, D.; Jha, D.K. Diversity of arbuscular mycorrhizal fungi in undisturbed forest, slash-and-burn field, and monoculture forest of Indo-Burma megadiverse region. Rev. Bras. Bot. 2014, 37, 339–351. [Google Scholar] [CrossRef]
- Trejo, D.; Lara-Capistrán, L.; Maldonado-Mendoza, I.; Zulueta-Rodríguez, R.; Sangabriel-Conde, W.; Mancera-López, M.; Negrete-Yankelevich, S.; Barois, I. Loss of arbuscular mycorrhizal fungal diversity in trap cultures during long-term subculturing. IMA Fungus 2013, 4, 161–167. [Google Scholar] [CrossRef]
- Sasvári, Z.; Hornok, L.; Posta, K. The community structure of arbuscular mycorrhizal fungi in roots of maize grown in a 50-year monoculture. Biol. Fertil. Soils 2011, 47, 167–176. [Google Scholar] [CrossRef]
- Johnson, N.C.; Copeland, P.J.; Crookston, R.K.; Pfleger, F.L. Mycorrhizae: Possible explanation for yield decline with continuous corn and soybean. Agron. J. 1992, 84, 387–390. [Google Scholar] [CrossRef]
- Barrer, B.S.E. El uso de hongos micorrizicos arbusculares como una alternativa para la agricultura. Fac. Cienc. Agropecu. 2009, 7, 123–132. [Google Scholar]
- Higo, M.; Azuma, M.; Kamiyoshihara, Y.; Kanda, A.; Tatewaki, Y.; Isobe, K. 2020. Impact of phosphorus fertilization on tomato growth and arbuscular mycorrhizal fungal communities. Microorganisms 2020, 8, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansa, J.; Wiemken, A.; Frossard, E. The effects of agricultural practices on arbuscular mycorrhizal fungi. Geol. Soc. Spec. Publ. 2006, 266, 89–115. [Google Scholar] [CrossRef]
- Williams, A.; Manoharan, L.; Rosenstock, N.P.; Olsson, P.A.; Hedlund, K. Long-term agricultural fertilization alters arbuscular mycorrhizal fungal community composition and barley (Hordeum vulgare) mycorrhizal carbon and phosphorus exchange. New Phytol. 2017, 213, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Kjöller, R.; Rosendahl, S. Effects of fungicides on arbuscular mycorrhizal fungi: Differential reponses in alkaline phosphatase activity of external and internal hyphae. Biol. Fertil. Soils 2000, 31, 361–365. [Google Scholar] [CrossRef]
- Sukarno, N.; Smith, F.A.; Smith, S.E.; Scott, E.S. The effect of fungicides on vesicular-arbuscular mycorrhizal symbiosis. II. The effects on area of interface and efficiency of p uptake and transfer to plant. New Phytol. 1996, 132, 583–592. [Google Scholar] [CrossRef]
- Sreenivasa, M.N.; Bagyaraj, D.J. Use of pesticides for mass production of vesicular-arbuscular mycorrhizal inoculum. Plant Soil 1989, 119, 127–132. [Google Scholar] [CrossRef]
- Rabab, A.M.; Reda, E.A. Impact of Ridomil, Bavistin and Agrothoate on arbuscular mycorrhizal fungal colonization, biochemical changes and potassium content of cucumber plants. Ecotoxicology 2019, 28, 487–498. [Google Scholar] [CrossRef]
- Mishra, U.; Dhar, D.W. Biodiversity and biological degradation of soil. Resonance 2004, 9, 26–33. [Google Scholar] [CrossRef]
- Osman, A.A. The role of soil solarization in the scope of Meloidogyne spp. integrated control under sandy soil conditions. In FAO Plant Protection and Production Paper No. 109; FAO: Rome, Italy, 1990. [Google Scholar]
- Veeraswamy, J.; Padmavathi, T.; Venkateswarlu, K. Effect of selected insecticides on plant growth and mycorrhizal development in sorghum. Agric. Ecosyst. Environ. 1993, 43, 337–343. [Google Scholar] [CrossRef]
- FIRA. Sistema de Costos Agrícolas. Cultivo de Piña Ciclo: PN, 2020. Available online: https://www.fira.gob.mx/Nd/Agrocostos.jsp (accessed on 3 December 2020).
- Jaffri, S.B.; Ahmad, K.S.; Jabeen, A. Biofertilizers’ functionality in organic agriculture entrenching sustainability and ecological protection. In Biofertilizers; Woodhead Publishing: Sawston, UK, 2021; pp. 211–219. [Google Scholar]
- Juntahum, S.; Jongrungklang, N.; Kaewpradit, W.; Ekprasert, J.; Boonlue, S. Improved physiological performances of sugarcane during maturation and ripening phase by inoculation of arbuscular mycorrhizal fungi. Sugar Tech. 2021, 23, 336–342. [Google Scholar] [CrossRef]
- Mathimaran, N.; Jegan, S.; Thimmegowda, M.N.; Prabavathy, V.R.; Yuvaraj, P.; Kathiravan, R.; Sivakumar, M.N.; Manjunatha, B.N.; Bhavitha, N.C.; Sathish, A.; et al. Intercropping transplanted pigeon pea with finger millet: Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria boost yield while reducing fertilizer input. Front. Sustain. Food Syst. 2020, 4, 88. [Google Scholar] [CrossRef]
- Saborío, A.D.; Camacho, B.O. Descripción del manejo poschosecha y factores de rechazo de piña (Ananas comosus L.) var Cayena Lisa y cion Champaka para exportación de la zona norte de Costa Rica. Agronoía Costarric. 1996, 20, 67–73. [Google Scholar]

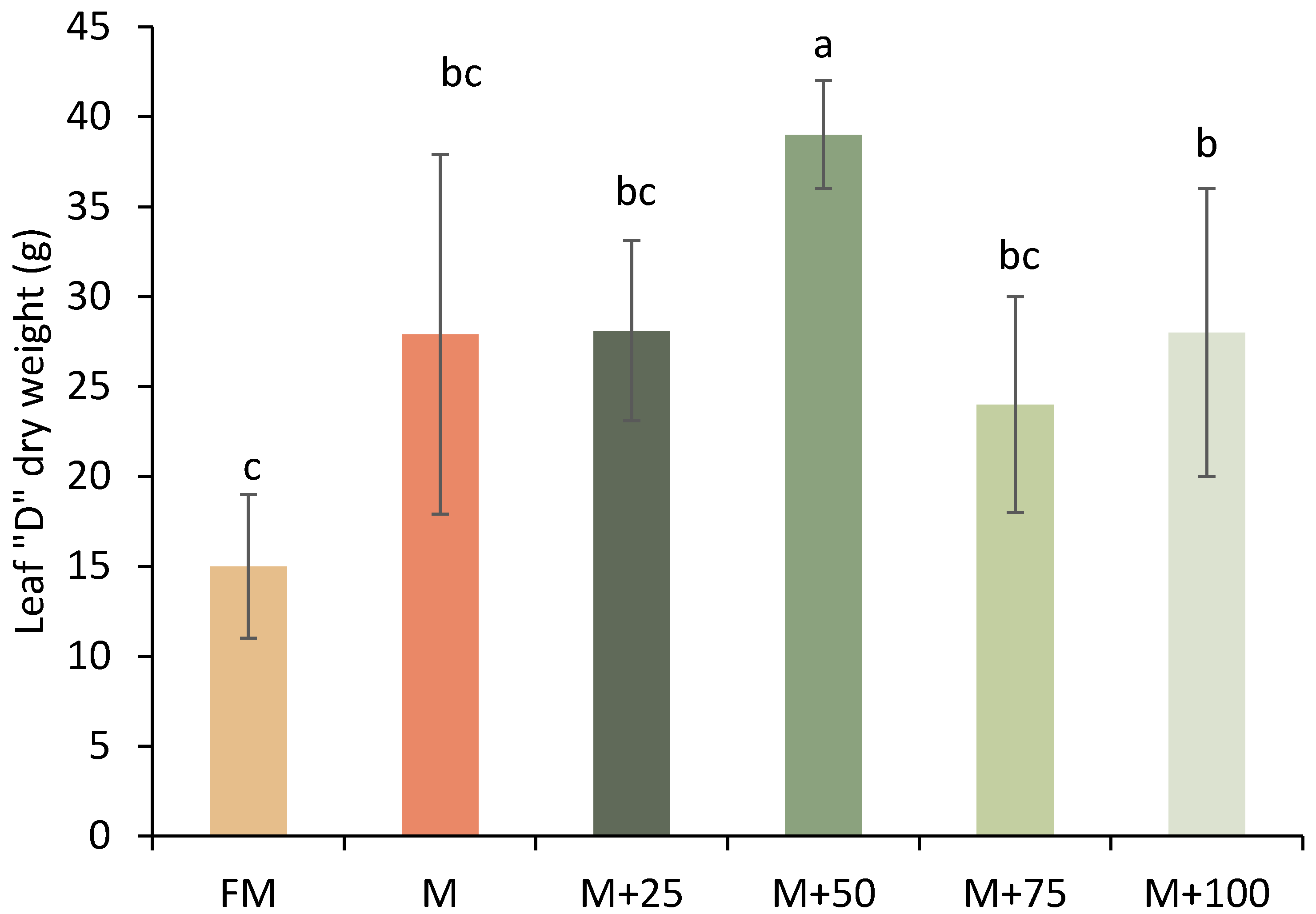
| Treatments | Fruit Weight (kg) | Crownless Fruit Weight (kg) | Fruit Lenght (cm) | Fruit Diameter (cm) | Peduncle Diameter (cm) |
|---|---|---|---|---|---|
| FM + 100% | 2.18 (0.28) b | 1.98 (0.27) b | 19.06 (1.94) b | 11.9.79 (12.89) ab | 0.34 (3.48) a |
| M | 1.65 (0.20) c | 1.43 (0.22) c | 16.08 (1.92) c | 11.3.92 (15.58) c | 0.35 (1.71) a |
| M + 25% | 2.20 (0.20) b | 2.02 (0.21) b | 18.96 (1.18) b | 11.8.23 (5.43) abc | 0.34 (1.85) a |
| M + 50% | 2.70 (0.32) a | 2.49 (0.32) a | 21.46 (1.89) a | 12.9.20 (4.80) a | 0.29 (3.02) b |
| M + 75% | 2.09 (0.36) b | 1.89 (0.35) b | 18.03 (2.06) bc | 11.6.95 (6.21) bc | 0.28 (2.63) b |
| M + 100% | 1.99 (0.30) b | 1.81 (0.27) b | 17.63 (1.94) bc | 11.6.57 (6.28) c | 0.25 (1.26) c |
| Treatments | °Brix | pH | Titratable Acidity (% Citric Acid) | Ascorbic Acid (mg in 100 mL) |
|---|---|---|---|---|
| FM + 100% | 7.86 (2.67) c | 4.06 (0.25) a | 0.44 (0.05) a | 4.84 (3.08) c |
| M | 10.99 (0.84) b | 3.84 (0.03) a | 0.41 (0.03) a | 11.77 (4.92) c |
| M + 25% | 12.61 (0.75) b | 3.97 (0.03) a | 0.37 (0.10) a | 7.01 (2.14) bc |
| M + 50% | 15.97 (0.78) a | 3.28 (0.34) b | 0.33 (0.066) b | 18.87 (1.22) a |
| M + 75% | 11.76 (1.23) b | 3.90 (0.10) a | 0.39 (0.15) a | 7.99 (1.32) b |
| M + 100% | 11.57 (0.86) b | 3.99 (0.22) a | 0.34 (0.11) a | 5.09 (3.41) bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trejo, D.; Sangabriel-Conde, W.; Gavito-Pardo, M.E.; Banuelos, J. Mycorrhizal Inoculation and Chemical Fertilizer Interactions in Pineapple under Field Conditions. Agriculture 2021, 11, 934. https://doi.org/10.3390/agriculture11100934
Trejo D, Sangabriel-Conde W, Gavito-Pardo ME, Banuelos J. Mycorrhizal Inoculation and Chemical Fertilizer Interactions in Pineapple under Field Conditions. Agriculture. 2021; 11(10):934. https://doi.org/10.3390/agriculture11100934
Chicago/Turabian StyleTrejo, Dora, Wendy Sangabriel-Conde, Mayra E. Gavito-Pardo, and Jacob Banuelos. 2021. "Mycorrhizal Inoculation and Chemical Fertilizer Interactions in Pineapple under Field Conditions" Agriculture 11, no. 10: 934. https://doi.org/10.3390/agriculture11100934







