Effective Management of Cucumber Powdery Mildew with Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Pathogen
2.2. Essential Oils
2.3. In Vitro Leaf Discs Bioassay
2.4. Greenhouse Experiment
2.4.1. Inoculum Preparation
2.4.2. Preparation of Essential Oil Emulsions
2.4.3. Experimental Procedure
2.4.4. Disease Assessment
2.4.5. Morphological Parameters
2.4.6. Physiological Measurements
Photosynthetic Pigments
2.4.7. Total Carbohydrate and Protein Content
2.4.8. Cell Membrane Stability
2.5. Data Analysis
3. Results
3.1. Identification of the Causal Pathogen
3.2. Fungitoxicity of Essential Oils on P. xanthii In Vitro
3.3. Effect of Essential Oils on Powdery Mildew Disease Severity and Incidence
3.4. Consequent Effect of Essential Oils on Cucumber Plant Morphology
3.5. Consequent Effect of Essential Oils on the Metabolic Activity of Plants
3.6. Consequent Effect of Essential Oils on Cell Membrane Stability
3.7. Potential Adverse Effect of Overdosing with Essential Oils
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, G.A. Using plant extracts to control powdery mildew disease that attack cucumber plants under protected houses. M. Sc. Fac. Agric. Moshtohor. Zagazig Univ. Benha Branch. 2005, 175. [Google Scholar]
- Essa, T.A.; El-Gamal, A.H.M.; Afifi, M.M.I. Control of cucumber downy mildew by some plant growth promoting Rhizobacteria under greenhouse conditions. Middle East J. Agri. Res. 2017, 6, 395–408. [Google Scholar]
- FOASTAT. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#search/Cucumbers%20and%20gherkins (accessed on 14 November 2021).
- Saudi-Arabia.Cropscience.Bayer Crop Science- Saudi Arabia. Available online: https://www.saudi-arabia.cropscience.bayer.com/ar/Crops/Cucumber.aspx (accessed on 1 September 2021).
- Pal, A.; Adhikary, R.; Shankar, T.; Sahu, A.K.; Maitra, S. Cultivation of Cucumber in Greenhouse. In Protected Cultivation and Smart Agriculture; Sagar Maitra, D.J.G.A.T.S., Ed.; New Delhi Publishers: New Delhi, India, 2020; pp. 139–145. [Google Scholar]
- Diab, Y.A.; Mousa, M.A.; Abbas, H.S. Greenhouse-grown Cucumber as an Alternative to Field Production and its Economic Feasibility in Aswan Governorate, Egypt. Assiut J. Agric. Sci. 2016, 47, 122–135. [Google Scholar]
- Al-Harbi, A.R.; Al-Omran, A.M.; Alharbi, K. Grafting improves cucumber water stress tolerance in Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture. Agriculture Statistical Yearbook; Ministry of Agriculture: Riyadh, Kingdom of Saudi Arabia, 2014; Volume 27. [Google Scholar]
- Elsharkawy, M.M.; Kamel, S.M.; El-Khateeb, N. M Biological control of powdery and downy mildews of cucumber under greenhouse conditions. Egypt. J. Biol. Pest Cont. 2014, 24, 301–308. [Google Scholar]
- Trecate, L.; Sedláková, B.; Mieslerová, B.; Manstretta, V.; Rossi, V.; Lebeda, A. Effect of temperature on infection and development of powdery mildew on cucumber. Plant Pathol. 2019, 68, 1165–1178. [Google Scholar] [CrossRef]
- Bettiol, W.; Silva, H.S.A.; Reis, R.C. Effectiveness of whey against zucchini squash and cucumber powdery mildew. Scientia Hort. 2008, 117, 82–84. [Google Scholar] [CrossRef] [Green Version]
- Lebeda, A.; Křístková, E.; Sedláková, B.; McCreight, J.D.; Kosman, E. Virulence variation of cucurbit powdery mildews in the Czech Republic–population approach. Eur. J. Plant Pathol. 2018, 152, 309–326. [Google Scholar] [CrossRef]
- Pérez-Garcia, A.; Olalla, L.; Rivera, E.; Del Pino, D.; Cánovas, I.; De Vicente, A.; Torés, J.A. Development of Sphaerotheca fusca on susceptible, resistant, and temperature-sensitive resistant melon cultivars. Mycol. Res. 2001, 105, 1216–1222. [Google Scholar] [CrossRef]
- Kuzuya, M.; Yashiro, K.; Tomita, K.; Ezura, H. Powdery mildew (Podosphaera xanthii) resistance in melon is categorized into two types based on inhibition of the infection processes. J. Exp. Bot. 2006, 57, 2093–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimati, H.; Amorim, L.; Bergamin Filho, A.; Camargo, L.E.A.; Rezende, J.A.M. Manual de Fitopatologia: Doenças das Plantas Cultivadas; Agronômica Ceres São Paulo: São Paulo, Brazil, 1997; Volume 2, p. 705. [Google Scholar]
- Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, Environmental Pollution, and Health. In Environmental Health Risk-Hazardous Factors to Living Species; IntechOpen: London, UK, 2016. [Google Scholar]
- Rur, M.; Rämert, B.; Hökeberg, M.; Vetukuri, R.R.; Grenville-Briggs, L.; Liljeroth, E. Screening of alternative products for integrated pest management of cucurbit powdery mildew in Sweden. Eur. J. Plant Pathol. 2018, 150, 127–138. [Google Scholar] [CrossRef]
- Sturchio, E.; Donnarumma, L.; Annesi, T.; Milano, F.; Casorri, L.; Masciarelli, E.; Zanellato, M.; Meconi, C.; Boccia, P. Essential oils: An alternative approach to management of powdery mildew diseases. Phytopath. Medit. 2014, 53, 385–395. [Google Scholar]
- IPM-Missouri Cucumber Downy Mildew. Available online: https://ipm.missouri.edu/MEG/2019/8/cucumberDownyMildew/ (accessed on 23 August 2021).
- Zhang, S.; Mersha, Z.; Vallad, G.E.; Huang, C.-H. Management of powdery mildew in squash by plant and alga extract biopesticides. Plant Pathol. J. 2016, 32, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koul, O. Microbial biopesticides: Opportunities and challenges. Biocont. News Inform. 2012, 33, 1R. [Google Scholar]
- Moyer, C.; Peres, N.A. Evaluation of biofungicides for control of powdery mildew of gerbera daisy. In Proceedings of the Florida State Horticultural Society, Fort Lauderdale, FL, USA, 1–4 June 2008; pp. 389–394. [Google Scholar]
- Cornell-Vegetables Biopesticides for Managing Diseases of Cucurbits Organically. Available online: https://www.vegetables.cornell.edu/ipm/diseases/biopesticides/biopesticides-for-managing-diseases-of-cucurbits-organically/ (accessed on 3 June 2021).
- Lamichhane, J.R.; Bischoff-Schaefer, M.; Bluemel, S.; Dachbrodt-Saaydeh, S.; Dreux, L.; Jansen, J.P.; Kiss, J.; Köhl, J.; Kudsk, P.; Malausa, T. Identifying obstacles and ranking common biological control research priorities for Europe to manage most economically important pests in arable, vegetable and perennial crops. Pest Manage. Sci. 2017, 73, 14–21. [Google Scholar] [CrossRef]
- Gilardi, G.; Baudino, M.; Garibaldi, A.; Gullino, M.L. Efficacy of biocontrol agents and natural compounds against powdery mildew of zucchini. Phytoparasitica 2012, 40, 147–155. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Hashem, M.; Moharam, A.M.; Zaied, A.A.; Saleh, F.E.M. Efficacy of essential oils in the control of cumin root rot disease caused by Fusarium spp. Crop Protect. 2010, 29, 1111–1117. [Google Scholar] [CrossRef]
- Znini, M.; Cristofari, G.; Majidi, L.; Mazouz, H.; Tomi, P.; Paolini, J.; Costa, J. Antifungal activity of essential oil from Asteriscus graveolens against postharvest phytopathogenic fungi in apples. Nat. Prod. Communic. 2011, 6, 1763. [Google Scholar] [CrossRef] [Green Version]
- Arshad, Z.; Hanif, M.A.; Qadri, R.W.K.; Khan, M.M.; Babarinde, A.; Omisore, G.O.; Babalola, J.O.; Syed, S.; Mahmood, Z.; Riaz, M. Role of essential oils in plant diseases protection: A review. Int. J. Chem. Biochem. Sci. 2014, 6, 11–17. [Google Scholar]
- Nguefack, J.; Nguikwie, S.; Fotio, D.; Dongmo, B.; Zollo, P.A.; Leth, V.; Nkengfack, A.; Poll, L. Fungicidal potential of essential oils and fractions from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris to control Alternaria padwickii and Bipolaris oryzae, two seed-borne fungi of rice (Oryza Sativa L.). J. Essent. Oil Res. 2007, 19, 581–587. [Google Scholar] [CrossRef]
- Kishore, G.K.; Pande, S.; Harish, S. Evaluation of essential oils and their components for broad-spectrum antifungal activity and control of late leaf spot and crown rot diseases in peanut. Plant Dis. 2007, 91, 375–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koul, O.; Walia, S.; Dhaliwal, G. Essential oils as green pesticides: Potential and constraints. Biopest. Int. 2008, 4, 63–84. [Google Scholar]
- Mohan, M.; Haider, S.Z.; Andola, H.C.; Purohit, V.K. Essential oils as green pesticides: For sustainable agriculture. Research J. Pharm. Biol. Chem. Sci. 2011, 2, 100–106. [Google Scholar]
- Braun, U.; Takamatsu, S. Phylogeny of Erysiphe, Microsphaera, Uncinula (Erysipheae) and Cystotheca, Podosphaera, Sphaerotheca (Cystotheceae) inferred from rDNA ITS sequences–Some taxonomic consequences. Schlechtendalia 2000, 4, 1–33. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.L. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Meth. Appl. 1990, 18, 315–322. [Google Scholar]
- Chen, R.-S.; Chu, C.; Cheng, C.-W.; Chen, W.-Y.; Tsay, J.-G. Differentiation of two powdery mildews of sunflower (Helianthus annuus) by a PCR-mediated method based on ITS sequences. Eur. J. Plant Pathol. 2008, 121, 1–8. [Google Scholar] [CrossRef]
- Nelson, H. Bioassay to detect small differences in resistance of tomato to late blight according to leaf age, leaf and leaflet position, and plant age. Aust. Plant Path. 2006, 35, 297–301. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Arendrup, M.C.; Arikan, S.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Denning, D.W.; Donnelly, J.P. EUCAST DEFINITIVE DOCUMENT E. DEF 9.1: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. Def. Sci. J. 2008, 9, 1–13. [Google Scholar]
- Ko, W.H.; Wang, S.Y.; Hsieh, T.F.; Ann, P.J. Effects of sunflower oil on tomato powdery mildew caused by Oidium neolycopersici. J. Phytopathol. 2003, 151, 144–148. [Google Scholar] [CrossRef]
- Morishita, M.; Sugiyama, K.; Saito, T.; Sakata, Y. Powdery mildew resistance in cucumber. Jpn. Agric. Res. Q. JARQ 2003, 37, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Waller, J.; Cannon, P. Fungi as Plant Pathogens. In Plant Pathologist’s Pocketbook, 3rd ed.; CABI Publishing: Boston, MA, USA, 2002; p. 85. [Google Scholar]
- Cho, Y.Y.; Oh, S.; Oh, M.M.; Son, J.E. Estimation of individual leaf area, fresh weight, and dry weight of hydroponically grown cucumbers (Cucumis sativus L.) using leaf length, width, and SPAD value. Scientia Hort. 2007, 111, 330–334. [Google Scholar] [CrossRef]
- Wehner, T.C.; Guner, N. Growth stage, flowering pattern, yield, and harvest date prediction of four types of cucumber tested at 10 planting dates. In Proceedings of the XXVI International Horticultural Congress: Advances in Vegetable Breeding 637, Toronto, ON, Canada, 11–17 August 2002; pp. 223–230. [Google Scholar]
- Metzner, H.; Rau, H.; Senger, H. Untersuchungen zur synchronisierbarkeit einzelner pigmentmangel-mutanten von Chlorella. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Costache, M.A.; Campeanu, G.; Neata, G. Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Roman. Biotech. Lett. 2012, 17, 7702–7708. [Google Scholar]
- McLeroy-Etheridge, S.L.; McManus, G.B. Food type and concentration affect chlorophyll and carotenoid destruction during copepod feeding. Limnol. Oceanogr. 1999, 44, 2005–2011. [Google Scholar] [CrossRef] [Green Version]
- Fales, F.W. The assimilation and degradation of carbohydrates by yeast cells. J. Biol. Chem. 1951, 193, 113–124. [Google Scholar] [CrossRef]
- Schlegel, H.G. The recovery of organic acid by Chlorella in the light. Planta 1956, 47, 510–526. [Google Scholar] [CrossRef]
- Clegg, K. The application of the anthrone reagent to the estimation of starch in cereals. J. Sci. Food Agric. 1956, 7, 40–44. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Snedecor, G.; Cochran, W. Statistical Methods; Iowa State University: Ames, IA, USA, 1980. [Google Scholar]
- Tripathi, A.K.; Prajapati, V.; Kumar, S. Bioactivities of l-carvone, d-carvone, and dihydrocarvone toward three stored product beetles. J. Econ. Entomol. 2003, 96, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B.; Machial, C.M. Pesticides based on plant essential oils: From traditional practice to commercialization. Adv. Phytomed. 2006, 3, 29–44. [Google Scholar]
- Raj, K.; Shukia, D.S. Evaluation of some innovative vis a vis powdery mildew of opium poppy incited by Erysiphe polygoni. J. Living World 1996, 3, 12–17. [Google Scholar]
- Nada, M.G.A. Studies on Antifungal Activities of Some Egyptian Medical and Aromatic plants. Ph. D. Thesis, Faculty of Agriculture, Zagazig University, Zagazig, Egypt,, 2002; p. 163. [Google Scholar]
- Abd El-Sayed, M.H.F. Studies on Powdery Mildew Disease of Cucurbits Under Protected Cultivation. Master’s Thesis, Plant Pathology Department, Faculty of Agriculture, Cairo University,, Cairo, Egypt, 2000. [Google Scholar]
- Utkhede, R.S.; Koch, C.A. Reduction of powdery mildew caused by Podosphaera xanthii on greenhouse cucumber plants by foliar sprays of various biological and chemical agents. J. Horticult. Sci. Biotechnol. 2006, 81, 23–26. [Google Scholar]
- Jee, H.-J.; Shim, C.-K.; Ryu, K.-Y.; Park, J.-H.; Lee, B.-M.; Choi, D.-H.; Ryu, G.-H. Control of powdery and downy mildews of cucumber by using cooking oils and yolk mixture. Plant Pathol. J. 2009, 25, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Villaverde, J.J.; Sandín-España, P.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides from natural products: Current development, legislative framework, and future trends. BioResources 2016, 11, 5618–5640. [Google Scholar] [CrossRef] [Green Version]
- Kordali, S.; Cakir, A.; Mavi, A.; Kilic, H.; Yildirim, A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J. Agric. Food Chem. 2005, 53, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Cheah, L.H.; Page, B.B.C.; Cox, J.K. Epidemiology of powdery mildew (Sphaerotheca fuliginea) of squash. N. Z. Plant Protec. Soc. 1996, 49, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Herger, G.; Klingauf, F.; Mangold, D.; Pommer, E.H.; Scherer, M. Efficacy of extracts of Reynoutria sachalinensis (F. Schmidt) Nakai (Polygonaceae), against fungal diseases, especially powdery mildews. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 1988, 40, 56–60. [Google Scholar]
- Hashem, M.; Hamada, A.M. Evaluation of two biologically active compounds for control of wheat root rot and its causal pathogens. Mycobiology 2002, 30, 233–239. [Google Scholar] [CrossRef] [Green Version]
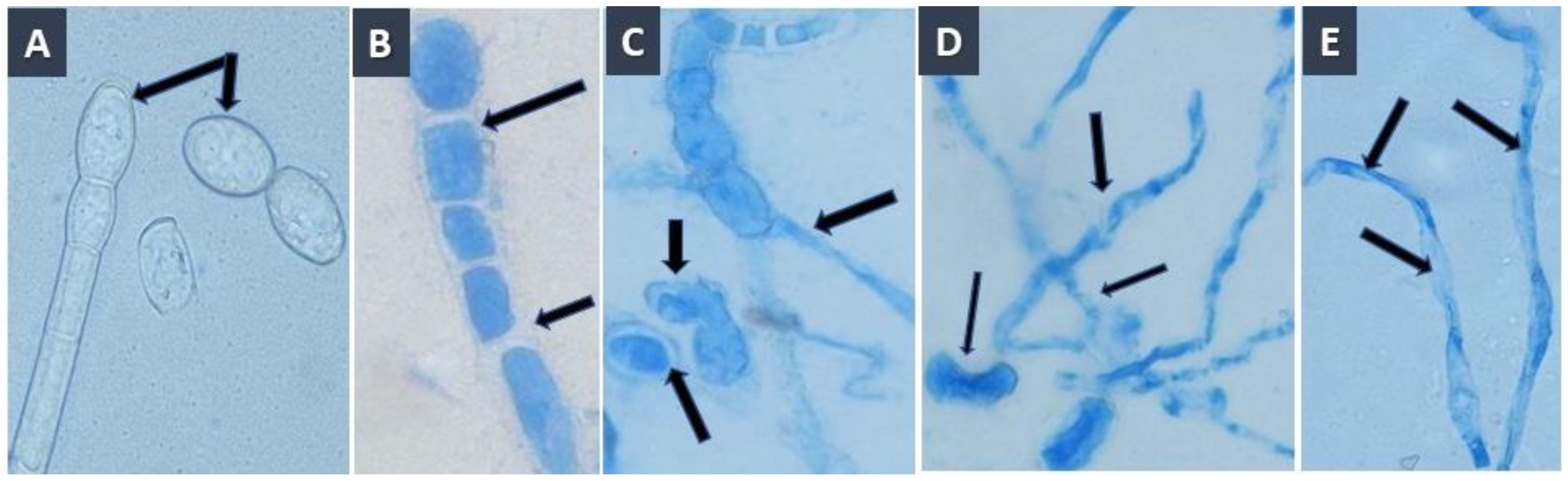

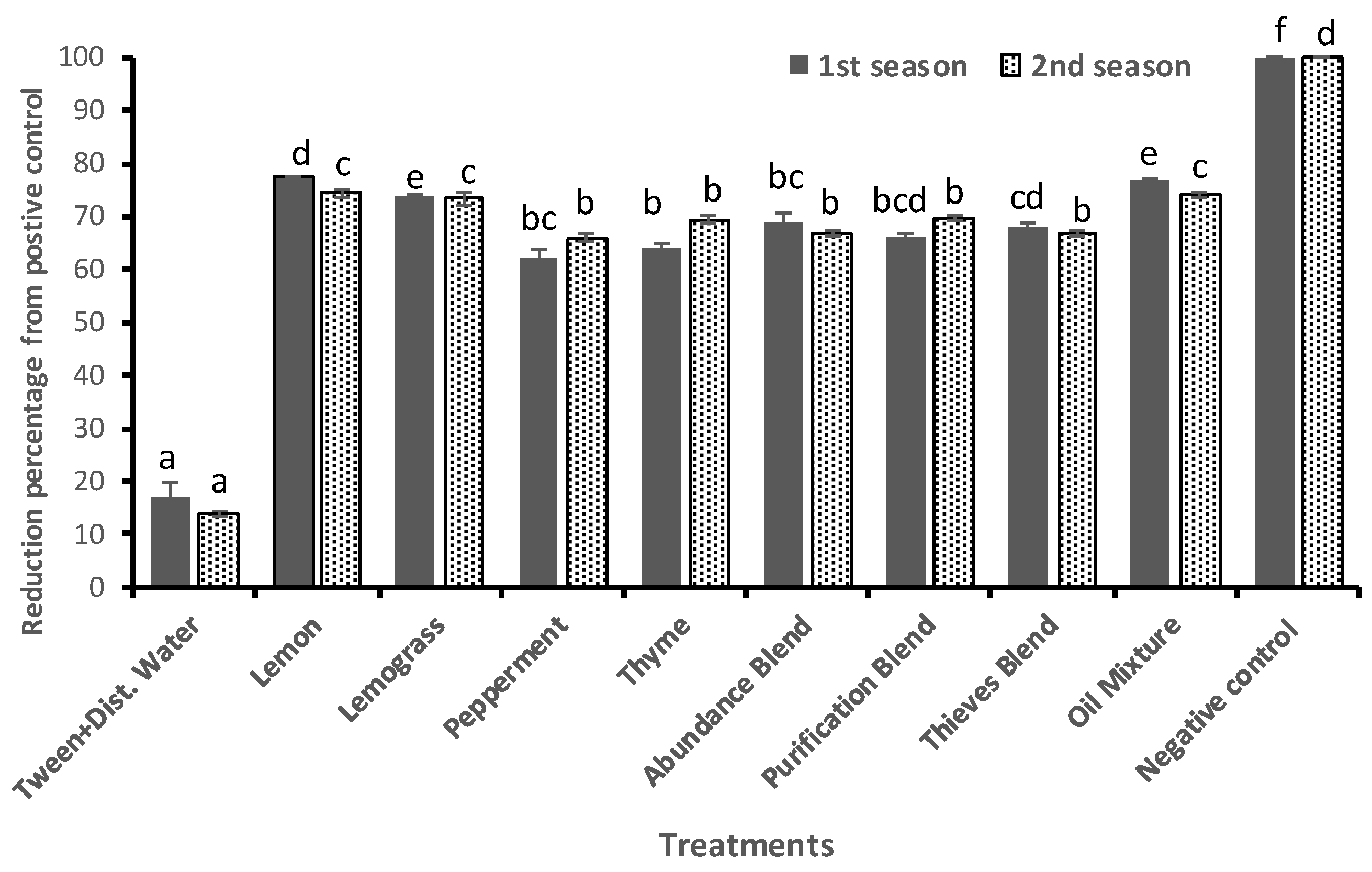
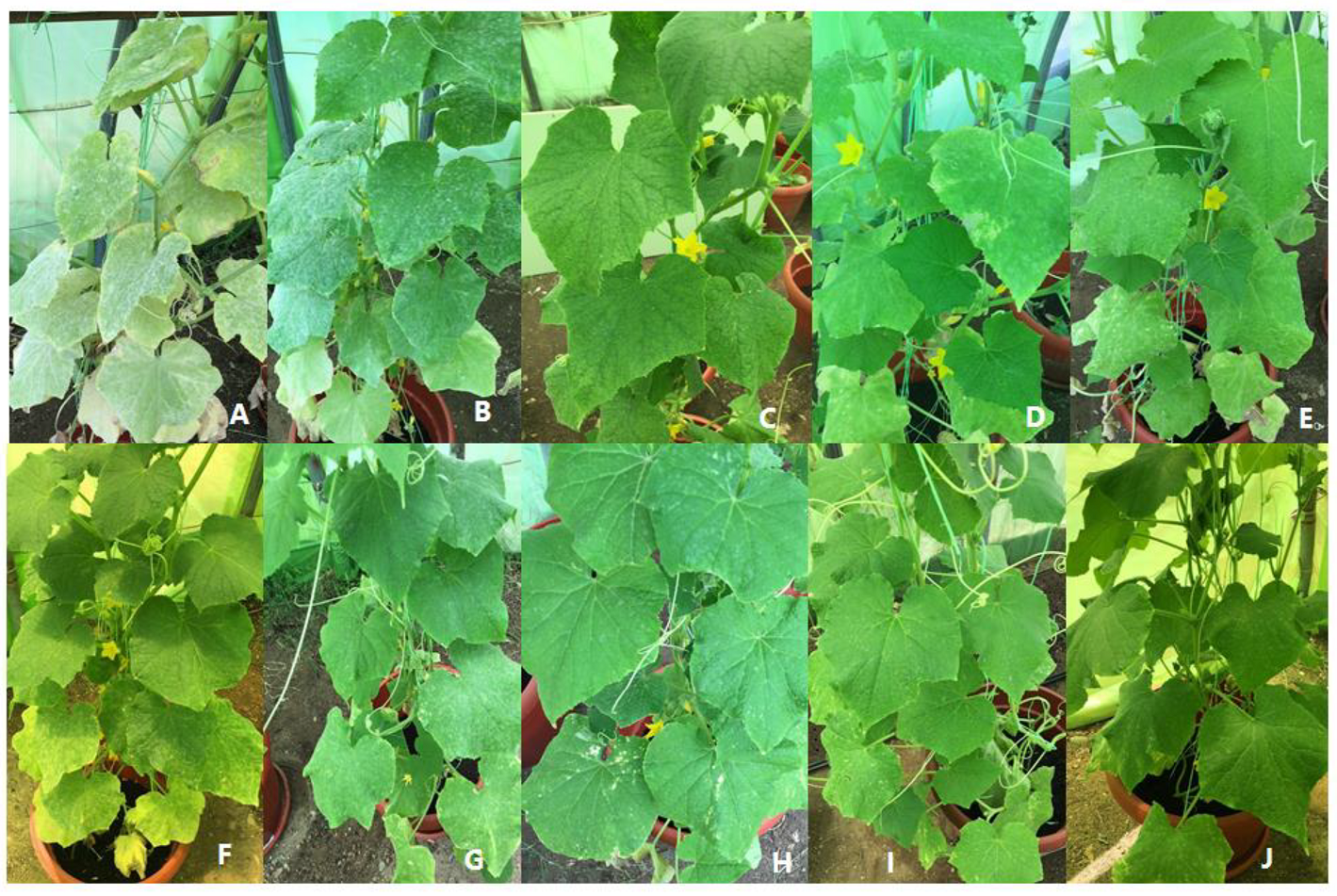
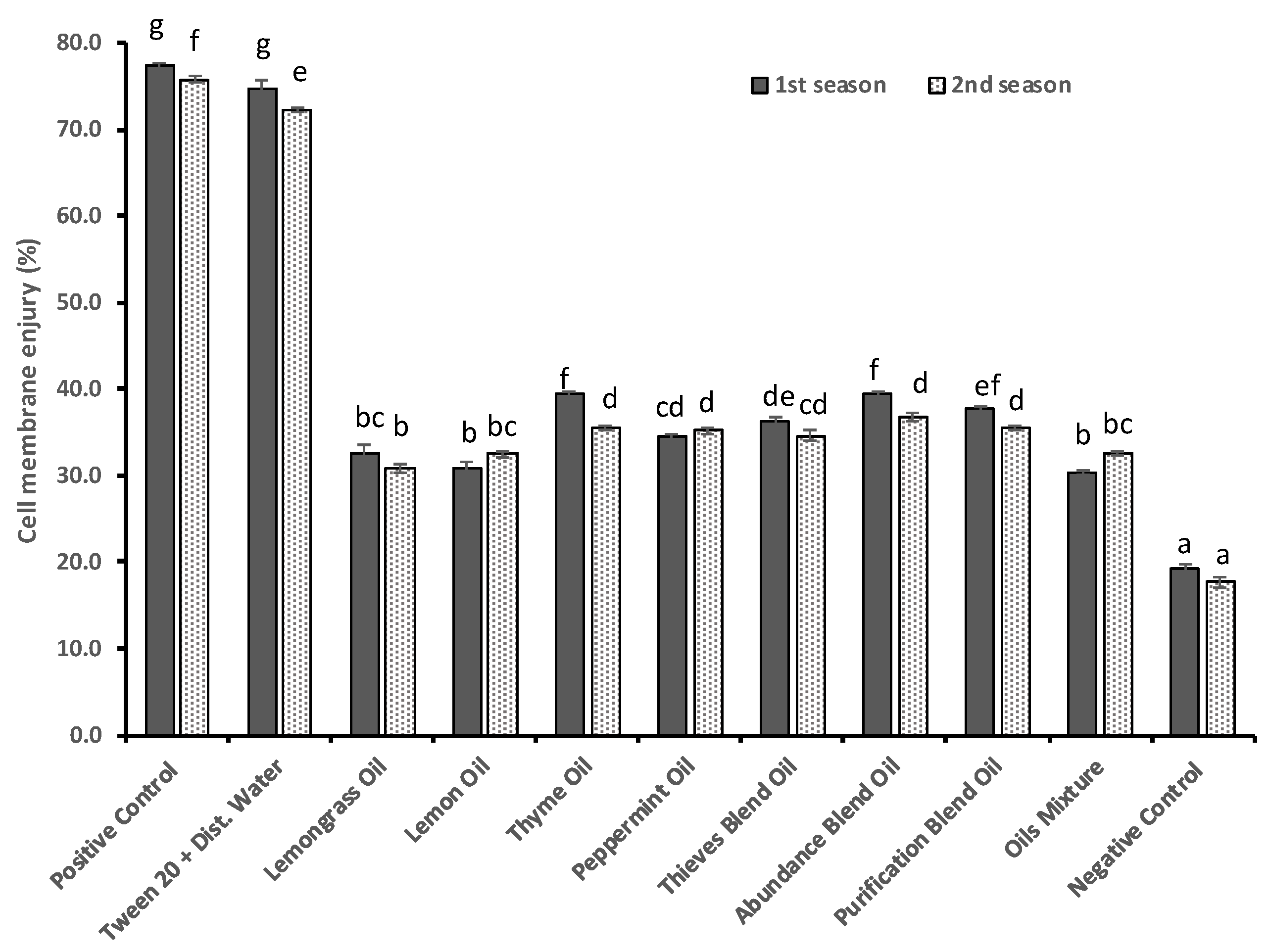

| No. of Treatment | Description |
|---|---|
| 1 | Positive control (PMC) * |
| 2 | PMC + Distilled water + Tween 20 |
| 3 | PMC + Thyme essential oil |
| 4 | PMC + Lemongrass essential oil |
| 5 | PMC + lemon essential oil |
| 3 | PMC + Peppermint essential oil |
| 7 | PMC + Abundance blend essential oil |
| 8 | PMC + Purification blend essential oil |
| 9 | PMC + Thieves blend essential oil |
| 10 | PMC + oil mixture |
| 11 | Negative control |
| Treatment | Concentration in mL/L | |||
|---|---|---|---|---|
| 1.0 | 1.5 | 2.0 | 2.5 | |
| Control | − | − | − | − |
| Tween 20 + Dist. Water | − | − | − | − |
| Lemongrass | + | ++ | ++ | +++ |
| Lemon | + | ++ | ++ | +++ |
| Thyme | + | + | ++ | ++ |
| Peppermint | + | + | ++ | +++ |
| Thieves Blend | + | ++ | ++ | +++ |
| Abundance Blend | + | ++ | ++ | +++ |
| Purification Blend | + | ++ | ++ | +++ |
| Oils Mixture | + | ++ | ++ | +++ |
| Treatment | Plant Length (cm) | Leaf Area (cm2) | Fresh Weight (g) | Dry Weight (g) | Number of Flower/Plant | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Experiment | 2nd Experiment | 1st Experiment | 2nd Experiment | 1st Experiment | 2nd Experiment | 1st Experiment | 2nd Experiment | 1st Experiment | 2nd Experiment | |
| Positive Control | 150.7 * ± 0.67 a ** | 153.0 ± 0.58 a | 24.3 ± 0.33 a | 24.2 ± 0.44 a | 66.0 ± 0.58 a | 66.7 ± 0.88 a | 13.7 ± 0.17 a | 14.7 ± 0.13 a | 7.3 ± 0.33 a | 8.3 ± 0.33 a |
| Tween 20 + Dist. Water | 152.7 ± 0.67 a | 155.3 ± 0.33 a | 24.6 ± 0.40 ab | 25.8 ± 0.44 abc | 70.3 ± 0.33 b | 71.2 ± 0.60 b | 15.7 ± 0.33 b | 16.2 ± 0.17 b | 8.0 ± 0.58 a | 8.3 ± 0.33 a |
| Lemongrass | 187.0 ± 0.15 f | 185.3 ± 0.33 d | 26.8 ± 0.17 bc | 26.6 ± 0.58 bc | 116.3 ± 0.88 d | 117.0 ± 0.58 d | 21.5 ± 0.29 cd | 22.2 ± 0.44 d | 12.7 ± 0.33 bc | 12.3 ± 0.33 bc |
| Lemon | 183.2 ± 0.60 e | 183.2 ± 0.17 d | 27.8 ± 0.44 c | 26.2 ± 0.44 abc | 121.7 ± 0.88 ef | 123.3 ± 0.88 e | 21.5 ± 0.47 cd | 20.5 ± 0.29 c | 12.7 ± 0.33 bc | 13.7 ± 0.33 c |
| Thyme | 173.2 ± 1.59 d | 175.7 ± 0.33 c | 26.7 ± 0.67 abc | 25.3 ± 0.67 abc | 119.2 ± 0.73 e | 116.8 ± 0.44 d | 20.7 ± 0.33 c | 20.7 ± 0.33 c | 11.0 ± 0.58 b | 12.3 ± 0.67 bc |
| Peppermint | 162.5 ± 0.50 b | 165.2 ± 2.46 b | 27.5 ± 0.29 c | 26.7 ± 0.44 bc | 108.7 ± 0.33 c | 112.3 ± 0.67 c | 20.3 ± 0.33 c | 21.2 ± 0.44 cd | 10.7 ± 0.88 b | 11.3 ± 0.33 b |
| Thieves Blend | 175.7 ± 0.33 d | 174.7 ± 1.33 c | 27.0 ± 0.58 bc | 25.0 ± 0.03 ab | 115.3 ± 0.33 d | 116.3 ± 0.33 d | 20.7 ± 0.33 c | 21.7 ± 0.33 cd | 16.0 ± 0.58 d | 16.3 ± 0.33 d |
| Abundance Blend | 175.7 ± 0.72 d | 178.0 ± 0.58 c | 27.0 ± 1.00 bc | 26.5 ± 0.74 bc | 123.0 ± 0.58 f | 126.0 ± 1.15 f | 20.0 ± 0.58 c | 22.5 ± 0.29 d | 13.0 ± 0.58 bc | 13.0 ± 0.58 bc |
| Purification Blend | 166.5 ± 0.29 c | 167.7 ± 1.20 b | 26.0 ± 0.58 abc | 26.8 ± 0.44 bc | 116.0 ± 0.58 d | 118.3 ± 0.33 d | 22.7 ± 0.33 d | 21.3 ± 0.33 cd | 12.7 ± 0.33 bc | 13.0 ± 0.00 bc |
| Oil Mixture | 188.8 ± 0.17 fg | 185.0 ± 1.53 d | 27.6 ± 0.49 c | 27.3 ± 0.17 c | 123.0 ± 1.00 f | 126.3 ± 0.33 f | 22.7 ± 0.33 d | 22.2 ± 0.17 d | 13.7 ± 0.33 c | 13.7 ± 0.33 c |
| Negative Control | 190.3 ± 0.40 g | 187.4 ± 1.58 d | 27.93 ± 0.32 c | 27.57 ± 0.21 c | 123.2 ± 0.42 f | 126.3 ± 0.16 f | 23.07 ± 0.07 d | 22.27 ± 0.14 d | 14.3 ± 0.27 cd | 14.0 ± 0.46 c |
| Treatment | Chlorophyll a | Chlorophyll b | Carotenoids | |||
|---|---|---|---|---|---|---|
| 1st Experiment | 2nd Experiment | 1st Experiment | 2nd Experiment | 1st Experiment | 2nd Experiment | |
| Positive Control | 2.63 * ± 0.03 a ** | 2.43 ± 0.03 a | 5.18 ± 0.02 f | 5.12 ± 0.01 g | 5.30 ± 0.03 f | 5.03 ± 0.01 g |
| Tween 20 + Dist. Water | 2.85 ± 0.03 a | 2.77 ± 0.05 b | 3.43 ± 0.01 b | 3.33 ± 0.01 b | 5.52 ± 0.00 g | 1.43 ± 0.00 a |
| Lemongrass | 5.22 ± 0.23 e | 5.70 ± 0.06 g | 3.14 ± 0.01 a | 3.17 ± 0.01 a | 3.31 ± 0.01 bc | 3.62 ± 0.02 d |
| Lemon | 4.77 ± 0.03 cd | 4.59 ± 0.00 d | 4.09 ± 0.03 d | 4.49 ± 0.03 e | 4.58 ± 0.01 d | 4.14 ± 0.01 e |
| Thyme | 4.47 ± 0.03 c | 4.69 ± 0.01 de | 3.09 ± 0.01 a | 3.93 ± 0.03 d | 3.26 ± 0.00 b | 3.32 ± 0.02 c |
| Peppermint | 4.02 ± 0.01 b | 4.16 ± 0.02 c | 3.74 ± 0.03 c | 3.64 ± 0.03 c | 3.43 ± 0.00 c | 3.33 ± 0.00 c |
| Thieves Blend | 5.13 ± 0.01 e | 5.69 ± 0.09 g | 3.67 ± 0.03 c | 3.63 ± 0.01 c | 2.04 ± 0.01 a | 2.12 ± 0.01 b |
| Abundance Blend | 4.75 ± 0.01 cd | 4.88 ± 0.03 f | 4.94 ± 0.04 e | 4.98 ± 0.01 f | 4.72 ± 0.02 d | 4.42 ± 0.00 f |
| Purification Blend | 4.82 ± 0.04 d | 4.77 ± 0.01 ef | 5.64 ± 0.02 g | 5.44 ± 0.02 h | 5.09 ± 0.02 e | 5.12 ± 0.01 h |
| Oil Mixture | 5.78 ± 0.01 f | 5.79 ± 0.03 g | 3.40 ± 0.00 b | 3.32 ± 0.02 b | 4.70 ± 0.00 d | 4.46 ± 0.02 f |
| Negative Control | 5.83 ± 0.01 f | 5.84 ± 0.03 g | 3.37 ± 0.02 b | 3.27 ± 0.02 b | 4.62 ± 0.80 d | 4.48 ± 0.03 f |
| Treatment | Water Soluble Carbohydrates | Total Insoluble Carbohydrates | Starch | |||
|---|---|---|---|---|---|---|
| 1st Experiment | 2nd Experiment | 1st Experiment | 2nd Experiment | 1st Experiment | 2nd Experiment | |
| Positive Control | 4.75 * ± 0.01 b** | 5.16 ± 0.02 a | 24.11 ± 0.01 b | 27.15 ± 0.01 b | 17.43 ± 0.04 b | 19.81 ± 0.02 b |
| Tween 20 + Dist. Water | 4.48 ± 0.01 a | 5.54 ± 0.01 b | 23.42 ± 0.02 a | 26.34 ± 0.01 a | 17.04 ± 0.02 a | 18.72 ± 0.01 a |
| Lemongrass | 5.48 ± 0.01 e | 6.48 ± 0.00 de | 29.07 ± 0.01 e | 28.25 ± 0.01 d | 21.49 ± 0.02 e | 22.86 ± 0.02 i |
| Lemon | 5.19 ± 0.01 c | 6.31 ± 0.00 d | 29.33 ± 0.01 e | 28.31 ± 0.01 e | 21.73 ± 0.01 e | 20.79 ± 0.02 e |
| Thyme | 5.32 ± 0.00 d | 5.32 ± 0.01 ab | 27.46 ± 0.01 c | 28.15 ± 0.01 c | 20.84 ± 0.02 d | 20.45 ± 0.02 c |
| Peppermint | 5.59 ± 0.00 f | 5.44 ± 0.01 b | 27.13 ± 0.01 c | 29.23 ± 0.02 f | 19.39 ± 0.01 c | 20.53 ± 0.02 d |
| Thieves Blend | 5.59 ± 0.01 f | 5.12 ± 0.01 a | 28.35 ± 0.01 d | 30.15 ± 0.02 g | 20.49 ± 0.01 d | 21.64 ± 0.01 f |
| Abundance Blend | 5.33 ± 0.00 d | 5.88 ± 0.01 c | 31.74 ± 0.01 f | 30.25 ± 0.02 h | 23.77 ± 0.01 g | 21.93 ± 0.01 g |
| Purification Blend | 5.23 ± 0.01 c | 5.97 ± 0.01 c | 28.99 ± 0.01 e | 30.43 ± 0.01 i | 21.39 ± 0.02 e | 22.01 ± 0.01 h |
| Oil Mixture | 5.46 ± 0.04 e | 6.57 ± 0.02 e | 32.62 ± 0.01 g | 32.41 ± 0.01 j | 22.85 ± 0.00 f | 23.64 ± 0.01 j |
| Negative Control | 5.56 ± 0.03 f | 6.65 ± 0.14 e | 32.97 ± 0.29 g | 33.13 ± 0.03 k | 23.5 ± 0.24 g | 24.17 ± 0.03 k |
| Treatment | Soluble Protein | Total Protein | ||
|---|---|---|---|---|
| 1st Experiment | 2nd Experiment | 1st Experiment | 2nd Experiment | |
| Positive Control | 85.67 * ± 0.08 a ** | 88.31 ± 0.03 a | 204.32 ± 0.04 a | 203.32 ± 0.53 a |
| Tween 20 + Dist. Water | 94.86 ± 0.07 b | 99.58 ± 0.12 c | 207.86 ± 0.03 b | 202.52 ± 0.31 a |
| Lemongrass | 109.17 ± 0.02 g | 108.30 ± 0.06 g | 233.30 ± 0.12 d | 243.20 ± 0.46 d |
| Lemon | 107.53 ± 0.07 f | 108.80 ± 0.06 h | 235.33 ± 0.08 e | 238.53 ± 0.69 c |
| Thyme | 106.45 ± 0.03 ef | 104.60 ± 0.06 f | 229.43 ± 0.09 c | 235.33 ± 0.14 b |
| Peppermint | 97.97 ± 0.07 c | 98.60 ± 0.06 b | 263.43 ± 0.12 j | 258.35 ± 0.03 e |
| Thieves Blend | 105.77 ± 0.01 e | 103.37 ± 0.09 e | 254.17 ± 0.09 h | 263.24 ± 0.72 f |
| Abundance Blend | 100.29 ± 0.01 d | 108.30 ± 0.06 g | 238.73 ± 0.69 f | 243.54 ± 0.41 d |
| Purification Blend | 99.07 ± 0.01 cd | 102.96 ± 0.01 d | 242.82 ± 0.04 g | 239.54 ± 0.32 c |
| Oil Mixture | 106.85 ± 0.22 ef | 108.40 ± 0.06 g | 253.20 ± 0.06 h | 266.34 ± 0.22 g |
| Negative Control | 107.20 ± 0.82 f | 108.93 ± 0.18 h | 255.60 ± 0.29 i | 266.80 ± 0.72 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, Y.S.; Hashem, M.; Alshehri, A.M.; Alamri, S.; Eid, E.M.; Ziedan, E.-S.H.E.; Alrumman, S.A. Effective Management of Cucumber Powdery Mildew with Essential Oils. Agriculture 2021, 11, 1177. https://doi.org/10.3390/agriculture11111177
Mostafa YS, Hashem M, Alshehri AM, Alamri S, Eid EM, Ziedan E-SHE, Alrumman SA. Effective Management of Cucumber Powdery Mildew with Essential Oils. Agriculture. 2021; 11(11):1177. https://doi.org/10.3390/agriculture11111177
Chicago/Turabian StyleMostafa, Yasser S., Mohamed Hashem, Ali M. Alshehri, Saad Alamri, Ebrahem M. Eid, El-Sayed H.E. Ziedan, and Sulaiman A. Alrumman. 2021. "Effective Management of Cucumber Powdery Mildew with Essential Oils" Agriculture 11, no. 11: 1177. https://doi.org/10.3390/agriculture11111177
APA StyleMostafa, Y. S., Hashem, M., Alshehri, A. M., Alamri, S., Eid, E. M., Ziedan, E.-S. H. E., & Alrumman, S. A. (2021). Effective Management of Cucumber Powdery Mildew with Essential Oils. Agriculture, 11(11), 1177. https://doi.org/10.3390/agriculture11111177








