Tomato Seed Coat Permeability: Optimal Seed Treatment Chemical Properties for Targeting the Embryo with Implications for Internal Seed-Borne Pathogen Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fluorescence Microscopy of Coumarin 1, 120, and 151 in Tomato Seeds
2.2. Chemicals and Synthesis of N-alkyl Piperonyl Amides
2.3. Sample Preparation for High-Performance Liquid Chromatography (HPLC) Analysis
2.3.1. Coating Tomato Seeds with Amides
2.3.2. Incubation and Harvest of Treated Seeds in Growth Chamber
2.3.3. Harvesting Tomato Seed Tissue for HPLC Analysis
2.3.4. HPLC Analysis of Tomato Seed Tissue
2.3.5. Tomato Seed Coat Permeability Data Calculation
3. Results
3.1. Fluorescence Microscopy of Coumarin 1, 120, and 151 in Tomato Seeds
3.2. Tomato Seed Coat Permeability
3.2.1. Maximal Uptake of Piperonyl Amides in Relation to log Kow
3.2.2. Uptake Efficiency (%) of Piperonyl Amides in Seed Tissue in Relation to Amount Applied
3.2.3. Percent of Piperonyl Amides in the Embryo Compared with the Entire Seed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maude, R.B. Seedborne Diseases and Their Control: Principles and Practice; CAB International: Wallingford, Oxon, UK, 1996. [Google Scholar]
- Elmer, W.H. Seeds as vehicles for pathogen importation. Biol. Invasions. 2001, 3, 263–271. [Google Scholar] [CrossRef]
- Gitaitis, R.; Walcott, R. The epidemiology and management of seedborne bacterial diseases. Ann. Rev. Phytopathol. 2007, 45, 371–397. [Google Scholar] [CrossRef]
- Shade, A.; Jacques, M.A.; Barret, M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Nelson, E.B.; Simoneau, P.; Barret, M.; Mitter, B.; Compant, S. Editorial special issue: The soil, the seed, the microbes and the plant. Plant. Soil. 2018, 422, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Barret, M.; Guimbaud, J.F.; Darrasse, A.; Jacques, M.A. Plant microbiota affects seed transmission of phytopathogenic microorganisms. Mol. Plant. Pathol. 2016, 17, 791. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, C.E.; Mitter, B.; Barret, M.; Sessitsch, A.; Compant, S. Commentary: Seed bacterial inhabitants and their routes of colonization. Plant. Soil. 2018, 422, 129–134. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Smith, E. Seed transmission of Tobamoviruses: Aspects of global disease distribution. Adv. Seed Biol. 2017, 233–260. [Google Scholar] [CrossRef] [Green Version]
- Nandi, M.; Macdonald, J.; Liu, P.; Weselowski, B.; Yuan, Z.C. Clavibacter michiganensis ssp. michiganensis: Bacterial canker of tomato, molecular interactions and disease management. Mol. Plant Patho. 2018, 19, 2036–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, T.; Ha Lee, B. Identification of fungus-infected tomato seeds based on full-field optical coherence tomography. Curr. Opt. Photonics 2019, 3, 571–576. [Google Scholar]
- Mink, G.I. Pollen and seed-transmitted viruses and viroids. Ann. Rev. Phytopathol. 1993, 31, 375–402. [Google Scholar] [CrossRef]
- Maude, R.B.; Kyle, A.M. Seed treatments with benomyl and other fungicides for the control of Ascochyta pisi on peas. Ann. Appl. Biol. 1970, 66, 37–41. [Google Scholar] [CrossRef]
- Taylor, A.G. Seed treatments. In Encyclopedia of Applied Plant Sciences; Thomas, B., Murphy, D.J., Murray, B.G., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2003; pp. 1291–1298. ISBN 9780122270505. [Google Scholar]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern seed technology: Seed coating delivery systems for enhancing seed and Crop performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Su, W.H.; Fennimore, S.A.; Slaughter, D.C. Development of a systemic crop signaling system for automated real-time plant care in vegetable crops. Biosyst. Eng. 2020, 193, 62–74. [Google Scholar] [CrossRef]
- Wang, Z.; Amirkhani, M.; Avelar, S.A.; Yang, D.; Taylor, A.G. Systemic uptake of fluorescent tracers by soybean (Glycine max (L.) Merr.) seed and seedlings. Agriculture 2020, 10, 248. [Google Scholar] [CrossRef]
- Salanenka, Y.A.; Taylor, A.G. Seedcoat permeability: Uptake and post-germination transport of applied model tracer compounds. HortScience 2011, 46, 622–626. [Google Scholar] [CrossRef]
- Taylor, A.G.; Salanenka, Y.A. Seed treatments: Phytotoxicity amelioration and tracer uptake. Seed Sci. Res. 2012, 22, S86–S90. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.A.N.; Taylor, A.G.; Cicero, S.M. Uptake of systemic treatments by maize seeds evaluated with fluorescent tracers. Seed Sci. Technol. 2014, 42, 101–107. [Google Scholar]
- Yang, D.; Avelar, S.A.; Taylor, A.G. Systemic seed treatment uptake during imbibition by corn and soybean. Crop. Sci. 2018, 58, 2063–2070. [Google Scholar] [CrossRef]
- Briggs, G.G.; Bromilow, R.H.; Evans, A.A. Relationships between lipophilicity and root uptake and translocation of non-ionised chemicals by barley. Pesticide Sci. 1982, 13, 495–504. [Google Scholar] [CrossRef]
- Clarke, E.D.; Delaney, J.S. Physical and molecular properties of agrochemicals: An analysis of screen inputs, hits, leads, and products. CHIMIA Int. J. Chem. 2003, 57, 731–734. [Google Scholar] [CrossRef]
- Yang, D.; Donovan, S.; Black, B.C.; Cheng, L.; Taylor, A.G. Relationships between compound lipophilicity on seed coat permeability and embryo uptake by soybean and corn. Seed Sci. Res. 2018, 28, 229–235. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 23, 3–26. [Google Scholar] [CrossRef]
- Aazam, E.S. Synthesis and characterization of mononuclear and binuclear metal complexes of a new fluorescent dye derived from 2-hydroxy-1-naphthaldehyde and 7-amino-4-methylcoumarin. J. King Abdulaziz Univ. Sci. 2010, 148, 1–32. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of absorption and fluorescence spectra of >300 common compounds for use in photochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, S.F.; Pescatore, M.C. Method for measuring the logarithm of the octanol–water partition coefficient by using short octadecyl–poly (vinyl alcohol) high-performance liquid chromatography columns. J. Chromatogr. A 2002, 952, 47–61. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC. Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barceló, A.; Amaya, I.; Medina, M.I.; Alonso, F.J.; de Forchetti, S.M.; Tigier, H.; Valpuesta, V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant. Physiol. 2000, 122, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Beresniewicz, M.B.; Taylor, A.G.; Goffinet, M.C.; Koeller, W.D. Chemical nature of a semipermeable layer in seed coats of leek, onion (Liliaceae), tomato and pepper (Solanaceae). Seed Sci. Technol. 1995, 23, 135–145. [Google Scholar]
- Beresniewicz, M.B.; Taylor, A.G.; Goffinet, M.C.; Terhune, B.T. Characterization and location of a semipermeable layer in seed coats of leek, onion, tomato and pepper. Seed Sci. Technol. 1995, 23, 123–134. [Google Scholar]
- Kiesselbach, T.A.; Walker, E.R. Structure of certain specialized tissues in the kernel of corn. Am. J. Bot. 1952, 39, 561–569. [Google Scholar] [CrossRef]
- Wróbel-Marek, J.; Kurczyńska, E.; Płachno, B.J.; Kozieradzka-Kiszkurno, M. Identification of symplasmic domains in the embryo and seed of Sedum acre L. (Crassulaceae). Planta 2017, 245, 491–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouden, S.; Kappers, I.F.; Klinkhamer, P.G.L.; Leiss, K.A. Cultivar variation in tomato seed coat permeability is an important determinant of jasmonic acid elicited defenses against western flower thrips. Front. Plant. Sci. 2020, 11, 576505. [Google Scholar] [CrossRef] [PubMed]
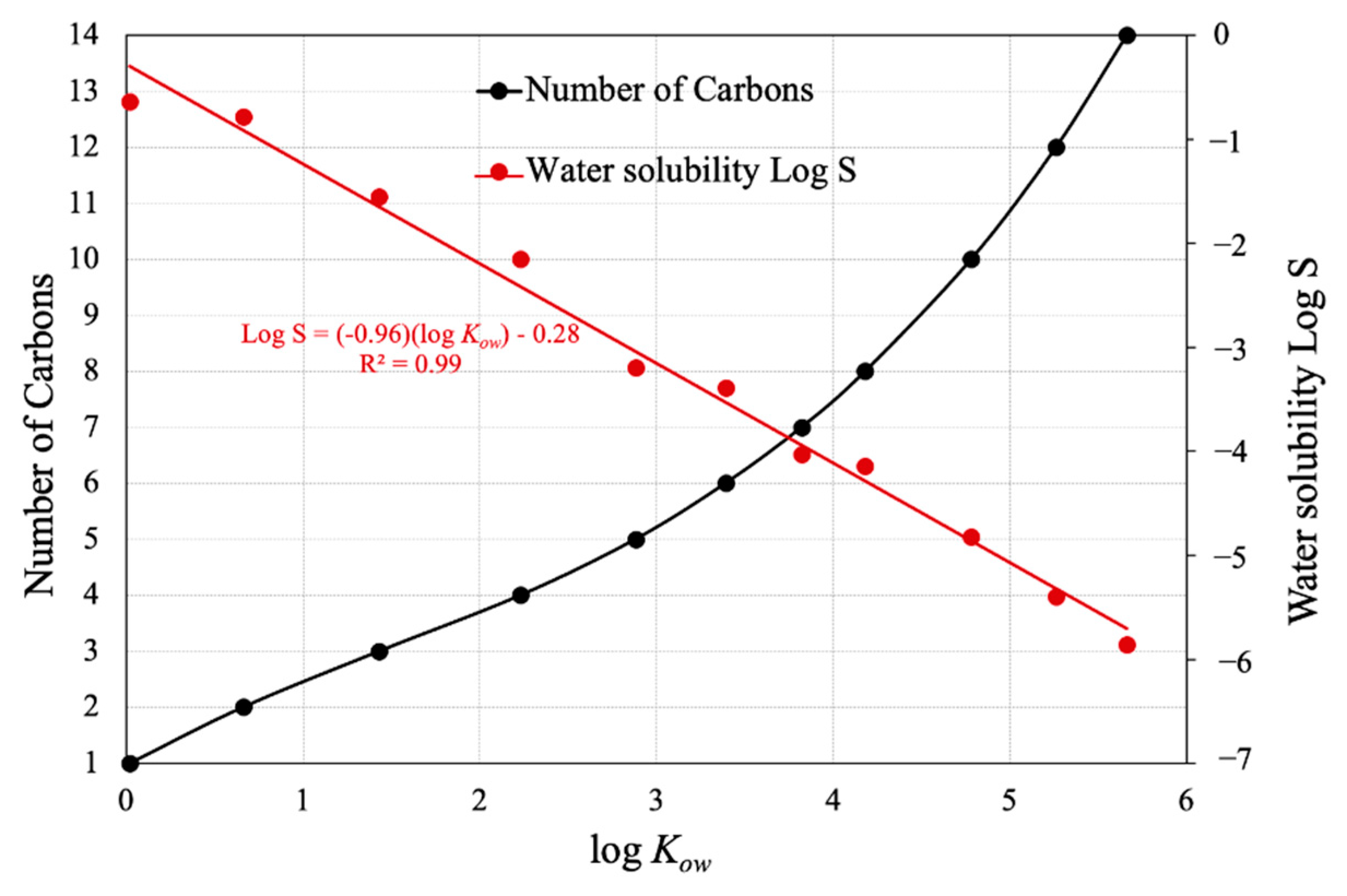

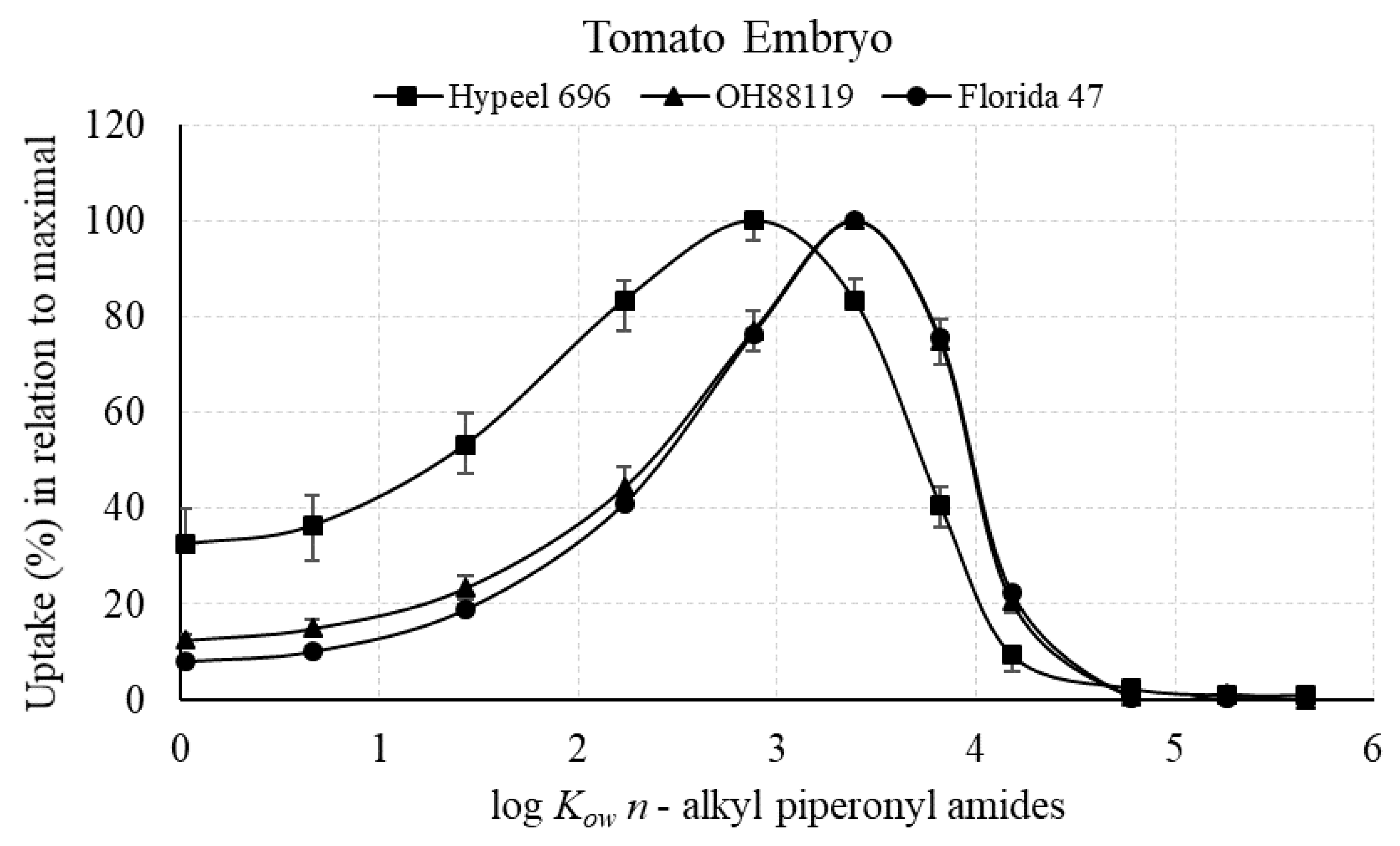
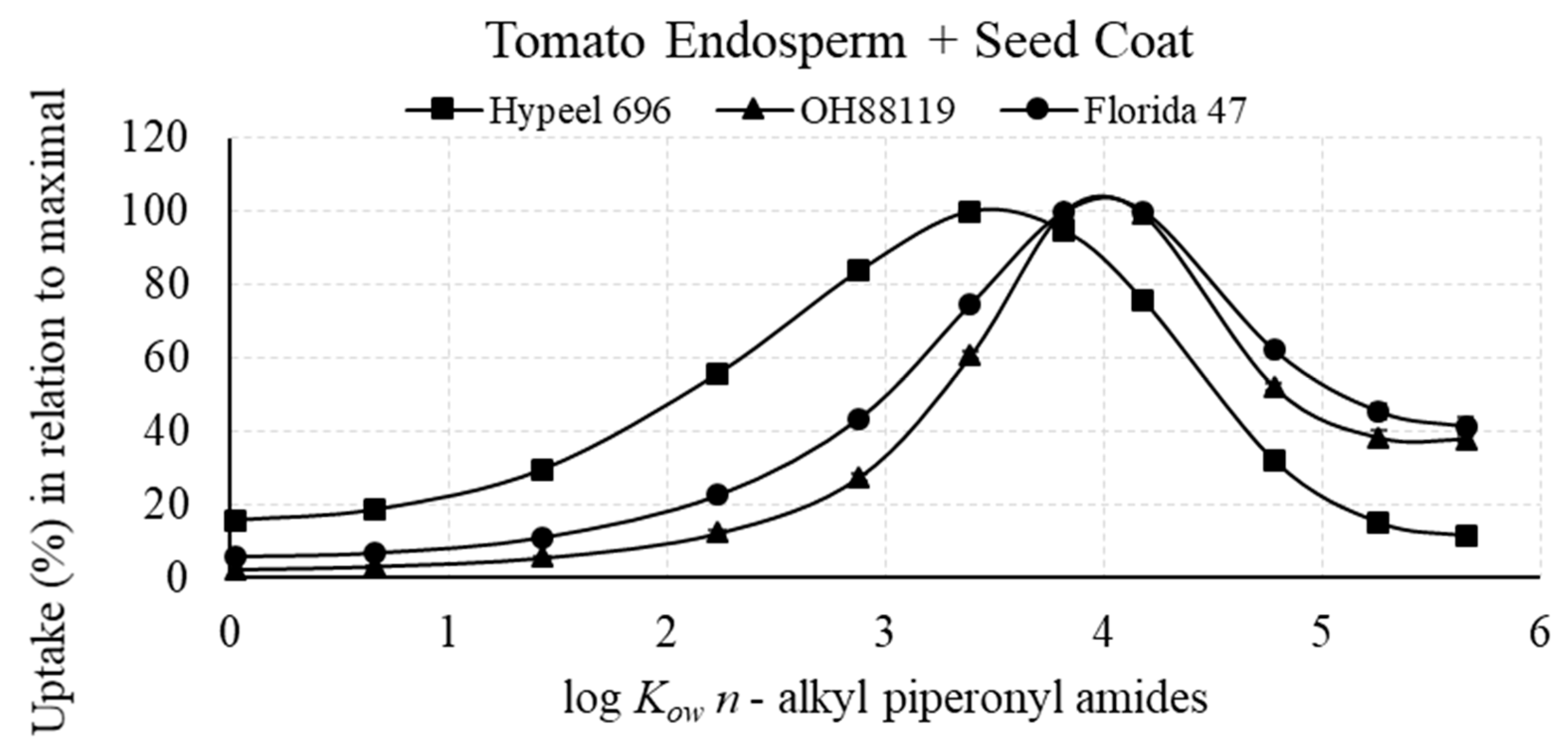

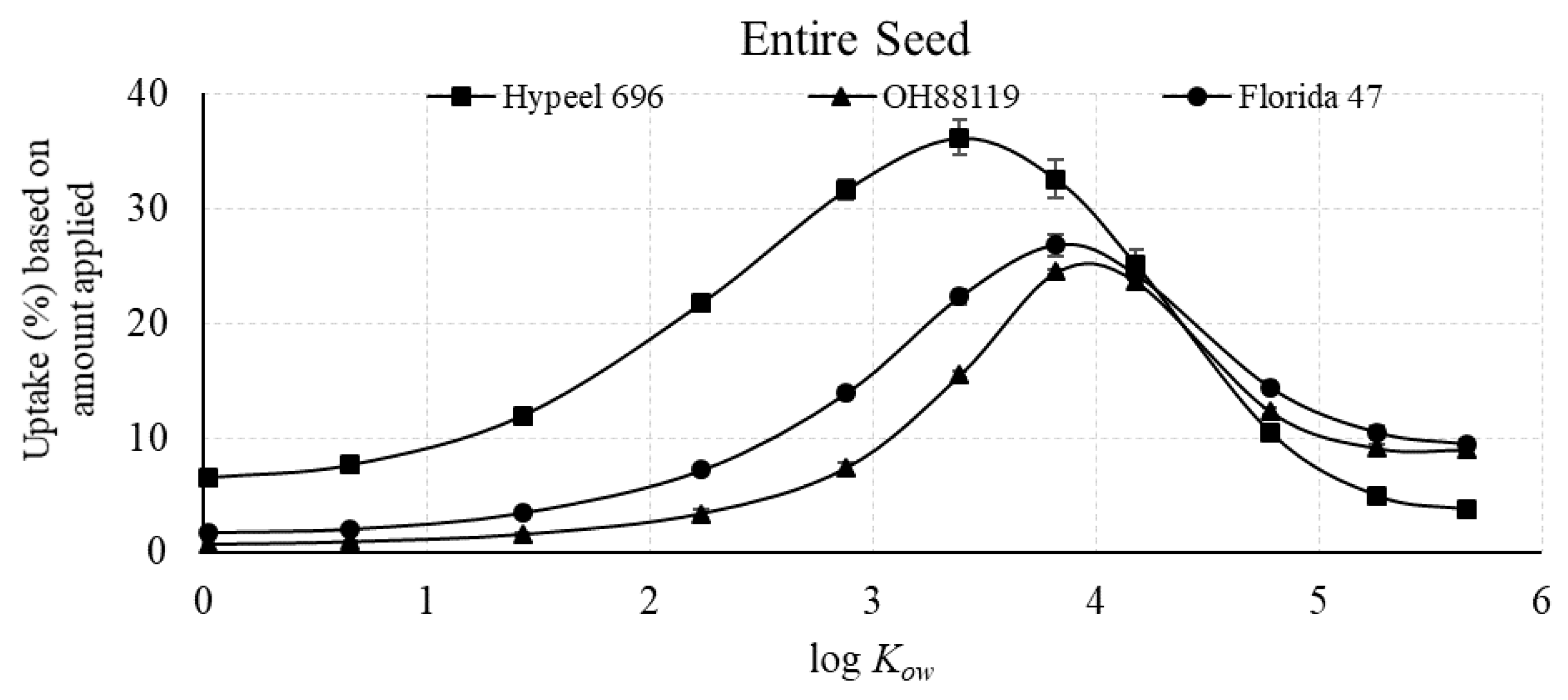

| Coumarin Compound | CAS Number | MW, g/mol | * Log Kow | * Water Solubility, Log S | Excitation/Emission Max, nm | Molar Abs Coefficient, cm−1 | Quantum Yield |
|---|---|---|---|---|---|---|---|
| 120 | 26093-31-2 | 175.2 | 1.25 | 1.25 | 342/409 | 3.50 × 108 | 0.63 |
| 151 | 53518-15-3 | 229.2 | 1.62 | −3.56 | 364/460 | 4.58 × 108 | 0.53 |
| 1 | 91-44-1 | 231.3 | 2.90 | −3.69 | 369/431 | 4.63 × 108 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayton, H.; Amirkhani, M.; Yang, D.; Donovan, S.; Taylor, A.G. Tomato Seed Coat Permeability: Optimal Seed Treatment Chemical Properties for Targeting the Embryo with Implications for Internal Seed-Borne Pathogen Control. Agriculture 2021, 11, 199. https://doi.org/10.3390/agriculture11030199
Mayton H, Amirkhani M, Yang D, Donovan S, Taylor AG. Tomato Seed Coat Permeability: Optimal Seed Treatment Chemical Properties for Targeting the Embryo with Implications for Internal Seed-Borne Pathogen Control. Agriculture. 2021; 11(3):199. https://doi.org/10.3390/agriculture11030199
Chicago/Turabian StyleMayton, Hilary, Masoume Amirkhani, Daibin Yang, Stephen Donovan, and Alan G. Taylor. 2021. "Tomato Seed Coat Permeability: Optimal Seed Treatment Chemical Properties for Targeting the Embryo with Implications for Internal Seed-Borne Pathogen Control" Agriculture 11, no. 3: 199. https://doi.org/10.3390/agriculture11030199







