Effects of Development Stage and Sodium Salts on the Antioxidant Properties of White Cabbage Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Microgreens
2.2. Soluble Dry Matter (SDM)
2.3. Extraction
2.4. Total Phenolic Content (TPC)
2.5. Total Anthocyanins Content (TAC)
2.6. Total Reducing Capacity (TRC)
2.7. DPPH Radical Scavenging Capacity (RSC)
2.8. Statistical Analysis
3. Results and Discussion
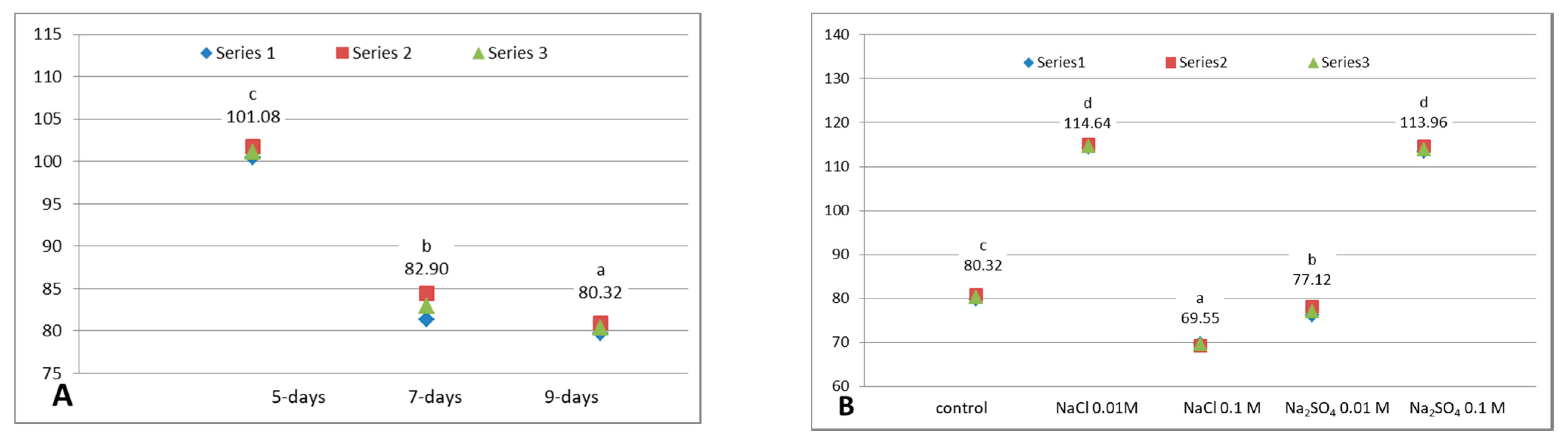
3.1. Soluble Dry Matter
3.2. Total Phenolic Content
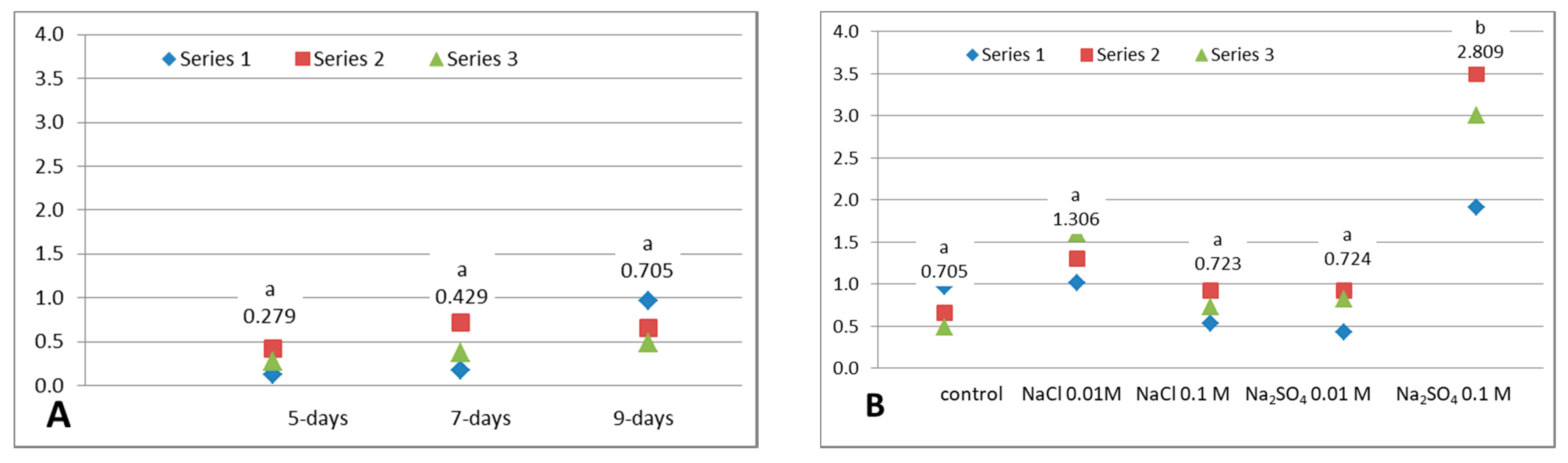
3.3. Total Anthocyanins Content
3.4. Total Reducing Capacity
3.5. DPPH Radical Scavenging Capacity
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šamec, D.; Pavlović, I.; Redovniković, I.R.; Salopek-Sondi, B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chem. 2018, 269, 96–102. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Hill, F. Microgreens: How to Grow Nature’s Own Superfood, 2nd ed.; Firefly Books: Richmond Hill, ON, Canada, 2016; pp. 3–11. [Google Scholar]
- Urban Cultivator. Microgreens and Sprouts Are Not the Same Thing. Available online: https://www.urbancultivator.net/microgreens-vs-sprouts/ (accessed on 8 January 2020).
- Hassini, I.; Baenas, N.; Moreno, D.A.; Carvajal, M.; Boughanmi, N.; Martinez Ballesta, M.D.C. Effects of seed priming, salinity and methyl jasmonate treatment on bioactive composition of Brassica oleracea var. capitata (white and red varieties) sprouts. J. Sci. Food Agric. 2017, 97, 2291–2299. [Google Scholar] [CrossRef]
- López-Cervantes, J.; Tirado-Noriega, L.G.; Sánchez-Machado, D.I.; Campas-Baypoli, O.N.; Cantú-Soto, E.U.; Núñez-Gastélum, J.A. Biochemical composition of broccoli seeds and sprouts at different stages of seedling development. Int. J. Food Sci. Technol. 2013, 48, 2267–2275. [Google Scholar] [CrossRef]
- Sousa, C.; Valentão, P.; Pereira, D.M.; Taveira, M.; Ferreres, F.; Pereira, J.A.; Bento, A.; Seabra, R.M.; Andrade, P.B. Phytochemical and antioxidant characterization of Brassica oleraceae var. costata extracts. In Recent Progress on Medicinal Plants—Standardization of Herbal/Ayurvedic Formulations; Govil, J.N., Singh, V.K., Eds.; Studium Press LLC: Houston, TX, USA, 2009; Volume 24, pp. 311–339. [Google Scholar]
- Vale, A.P.; Santos, J.; Brito, N.V.; Marinho, C.; Amorim, V.; Rosa, E.; Oliveira, M.B.P. Effect of refrigerated storage on the bioactive compounds and microbial quality of Brassica oleraceae sprouts. Postharvest Biol. Technol. 2015, 109, 120–129. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [Green Version]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yao, S.; You, Y.; Xiao, G.; You, Q. Antioxidant activity of isothiocyanate extracts from broccoli. Chin. J. Chem. Eng. 2010, 18, 312–321. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Velasco, P.; Lema, M.; Francisco, M.; Soengas, P.; Cartea, M.E. In vivo and in vitro effects of secondary metabolites against Xanthomonas campestris pv. campestris. Molecules 2013, 18, 11131–11143. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, D.J.; Basu, A. Phenolic compounds: Potential health benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste, 1st ed.; Vuong, Q., Ed.; CRC Press Taylor and Francis Group: London, UK, 2017; pp. 27–59. [Google Scholar]
- Traka, M.H. Health benefits of glucosinolates. In Advances in Botanical Research, 1st ed.; Popriva, S., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 80, pp. 247–279. [Google Scholar]
- Domínguez-Perles, R.; Mena, P.; Garcia-Viguera, C.; Moreno, D.A. Brassica foods as a dietary source of vitamin C: A review. Crit. Rev. Food Sci. 2014, 54, 1076–1091. [Google Scholar] [CrossRef]
- Butnariu, M.; Caunii, A. Design management of functional foods for quality of life improvement. Ann. Agric. Environ. Med. 2013, 20, 736–741. [Google Scholar]
- Bantis, F.; Fotelli, M.; Ilić, Z.S.; Koukounaras, A. Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution. Agriculture 2020, 10, 574. [Google Scholar] [CrossRef]
- Butnariu, M. Detection of the polyphenolic components in Ribes nigrum L. Ann. Agric. Environ. Med. 2014, 21, 11–14. [Google Scholar] [PubMed]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Rehab, F.M. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris Medik.) sprouts. Acta Scient. Pol. Technol. Alim. 2015, 14, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassini, I.; Martinez-Ballesta, M.C.; Boughanmi, N.; Moreno, D.A.; Carvajal, M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci. Hort. 2017, 226, 141–151. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Patras, A.; Stoleru, V.; Filimon, R.V.; Padureanu, S.; Chelariu, E.L.; Biliaderis, C.G. Influence of sodium and maturity stage on the antioxidant properties of cauliflower and broccoli sprouts. Not. Bot. Horti. Agrobo. 2017, 45, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic compounds. In Handbook of Enology, the Chemistry of Wine Stabilization and Treatments, 1st ed.; Ribéreau-Gayon, P., Ed.; John Wiley Sons Ltd: Chichester, UK, 2006; Volume 2, pp. 141–203. [Google Scholar]
- Ragusa, L.; Picchi, V.; Tribulato, A.; Cavallaro, C.; Lo Scalzo, R.; Branca, F. The effect of the germination temperature on the phytochemical content of broccoli and rocket sprouts. Int. J. Food Sci. Nutr. 2016, 68, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Giuffrida, F.; Agnello, M.; Mauro, R.P.; Ferrante, A.; Leonardi, C. Cultivation under salt stress conditions influences postharvest quality and glucosinolates content of fresh-cut cauliflower. Sci. Hort. 2018, 236, 166–174. [Google Scholar] [CrossRef]
- Di Gioia, F.; Rosskopf, E.N.; Leonardi, C.; Giuffrida, F. Effects of application timing of saline irrigation water on broccoli production and quality. Agric. Water Manag. 2018, 203, 97–104. [Google Scholar] [CrossRef]
- Butu, M.; Rodino, S.; Butu, A.; Butnariu, M. Screening of bioflavonoid and antioxidant activity of Lens culinaris medikus. Dig. J. Nanomater. Biostruct. 2014, 9, 519–529. [Google Scholar]
- Aleixandre-Tudo, J.L.; Du Toit, W. The role of UV-visible spectroscopy for phenolic compounds quantification in winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages, 1st ed.; Solís-Oviedo, R.L., De La Cruz Pech-Canul, A., Eds.; IntechOpen: London, UK, 2019; pp. 1–22. [Google Scholar]
- Korus, A. Level of vitamin C, polyphenols, and antioxidant and enzymatic activity in three varieties of kale (Brassica oleracea L. var Acephala) at different stages of maturity. Int. J. Food Prop. 2011, 14, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Kusznierewicz, B.; Bartoszek, A.; Wolska, L.; Drzewiecki, J.; Gorinstein, S.; Namieśnik, J. Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT-Food Sci. Technol. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; LI, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant. Sci. 2016, 7, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Hughes, N.M.; Carpenter, K.L.; Cannon, J.G. Estimating contribution of anthocyanin pigments to osmotic adjustment during winter leaf reddening. J. Plant. Physiol. 2013, 170, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Maldini, M.; Nardini, M.; Azzini, E.; Foddai, M.S.; Giusti, A.M.; Baima, S.; Morelli, G.; Scaccini, C. Improvement of the nutraceutical quality of broccoli sprouts by elicitation. Food Chem. 2016, 201, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Influence of light on health-promoting phytochemicals of broccoli sprouts. J. Sci. Food Agric. 2008, 88, 904–910. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C.; Frias, J. Time dependence of bioactive compounds and antioxidant capacity during germination of different cultivars of broccoli and radish seeds. Food Chem. 2010, 120, 710–716. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Wang, Z.; Guo, Q.; Gu, Z. Effect of NaCl stress on health-promoting compounds and antioxidant activity in the sprouts of three broccoli cultivars. Int. J. Food Sci. Nutr. 2014, 65, 476–481. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Ghasemnezhad, A.; Barani, M.; Telmadarrehei, T. Effect of salinity on phenolic composition and antioxidant activity of artichoke (Cynara scolymus L.) leaves. Res. J. Med. Plant. 2012, 6, 245–252. [Google Scholar] [CrossRef] [Green Version]


| Variant of White Cabbage Microgreens | SDM (°Bx) | |
|---|---|---|
| untreated | 5-days | 3.41 ± 0.01 d |
| 7-days | 3.11 ± 0.14 c | |
| 9-days | 2.97 ± 0.15 b | |
| 9-days treated | NaCl, 0.01 M | 2.83 ± 0.15 a |
| NaCl, 0.1 M | 2.91 ± 0.01 a,b | |
| Na2SO4, 0.01 M | 3.03 ± 0.06 b,c | |
| Na2SO4, 0.1 M | 5.01 ± 0.01 e | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patras, A. Effects of Development Stage and Sodium Salts on the Antioxidant Properties of White Cabbage Microgreens. Agriculture 2021, 11, 200. https://doi.org/10.3390/agriculture11030200
Patras A. Effects of Development Stage and Sodium Salts on the Antioxidant Properties of White Cabbage Microgreens. Agriculture. 2021; 11(3):200. https://doi.org/10.3390/agriculture11030200
Chicago/Turabian StylePatras, Antoanela. 2021. "Effects of Development Stage and Sodium Salts on the Antioxidant Properties of White Cabbage Microgreens" Agriculture 11, no. 3: 200. https://doi.org/10.3390/agriculture11030200






