Above and Belowground Relative Yield Total of Clover–Ryegrass Mixtures Exceed One in Wet and Dry Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site, Plant Material, and Experimental Setting
2.2. Aboveground and Belowground Biomass and Root Length Density
2.3. FTIR Spectroscopy
2.4. Vertical Root Distribution
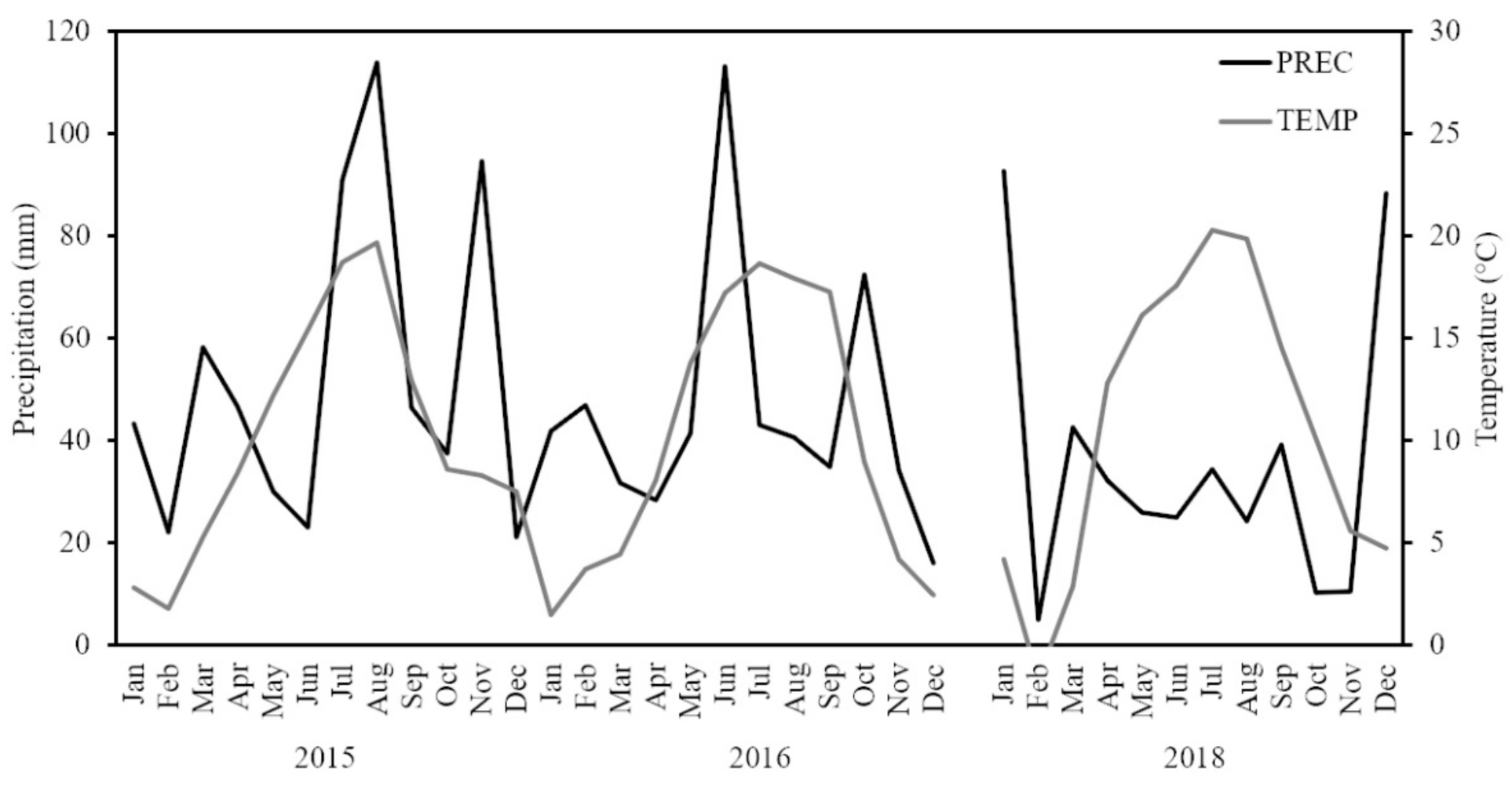
2.5. Weather
2.6. Data Analyses
3. Results
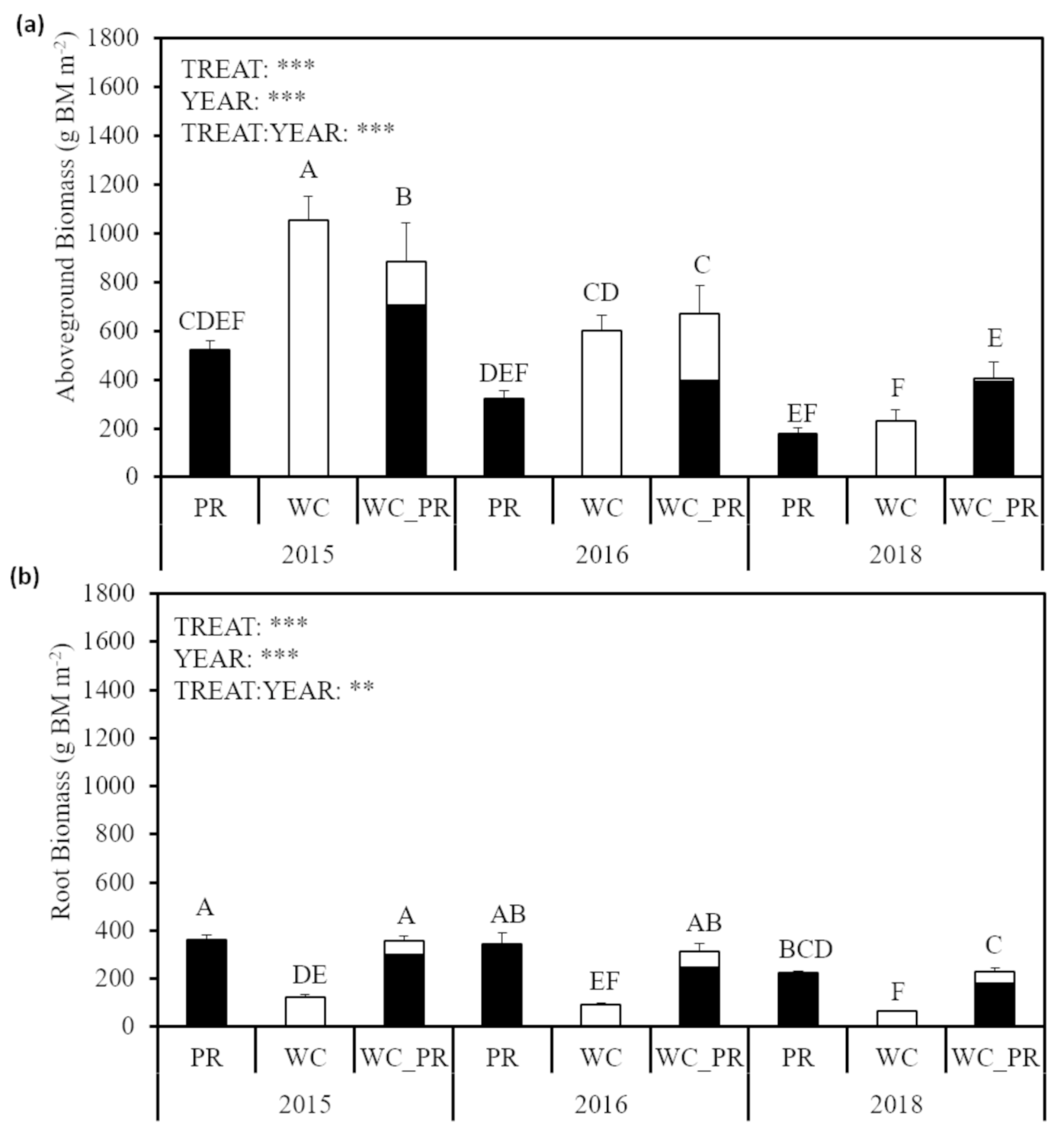
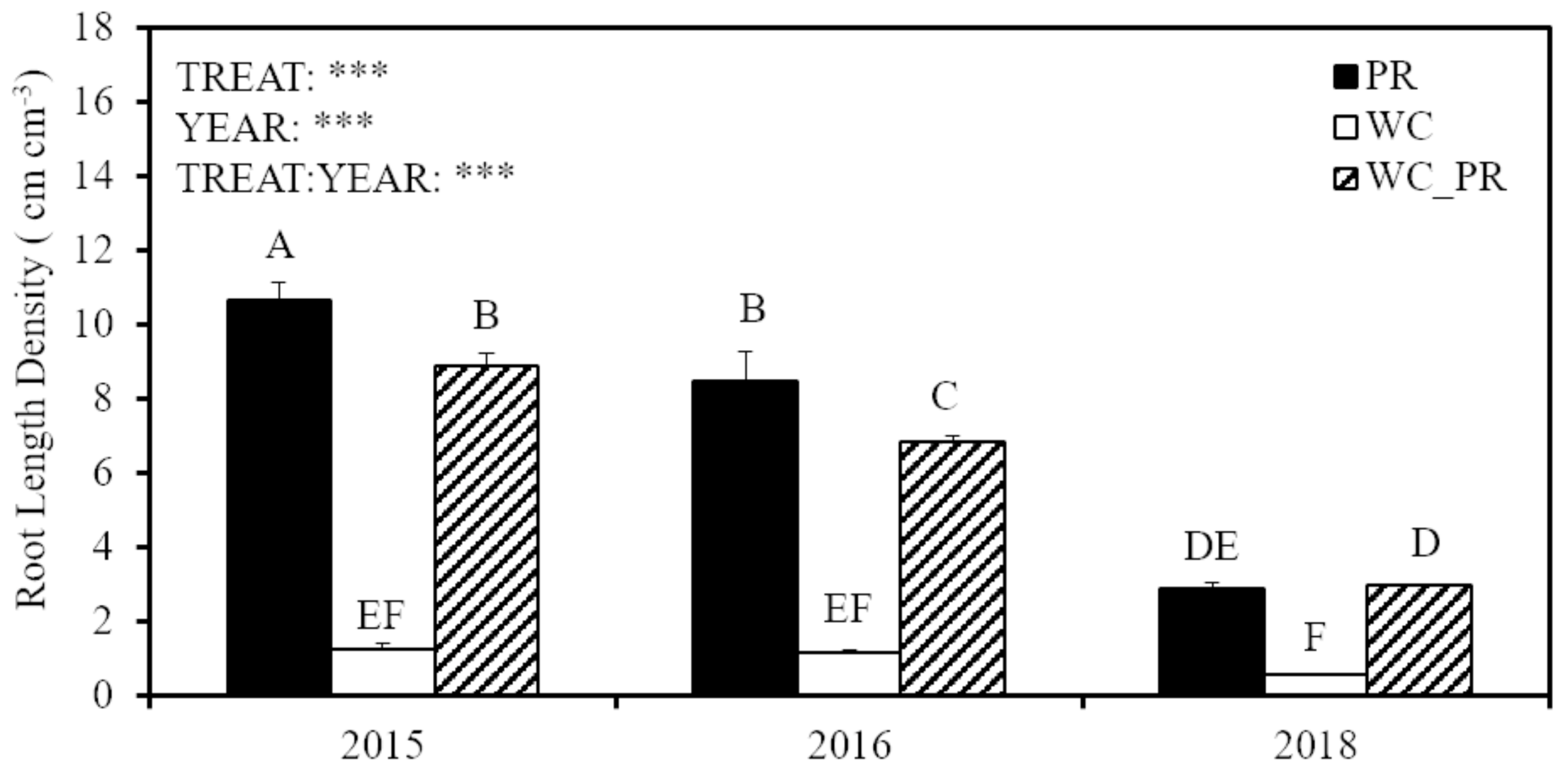
3.1. Aboveground and Belowground Biomass and Root Length Density
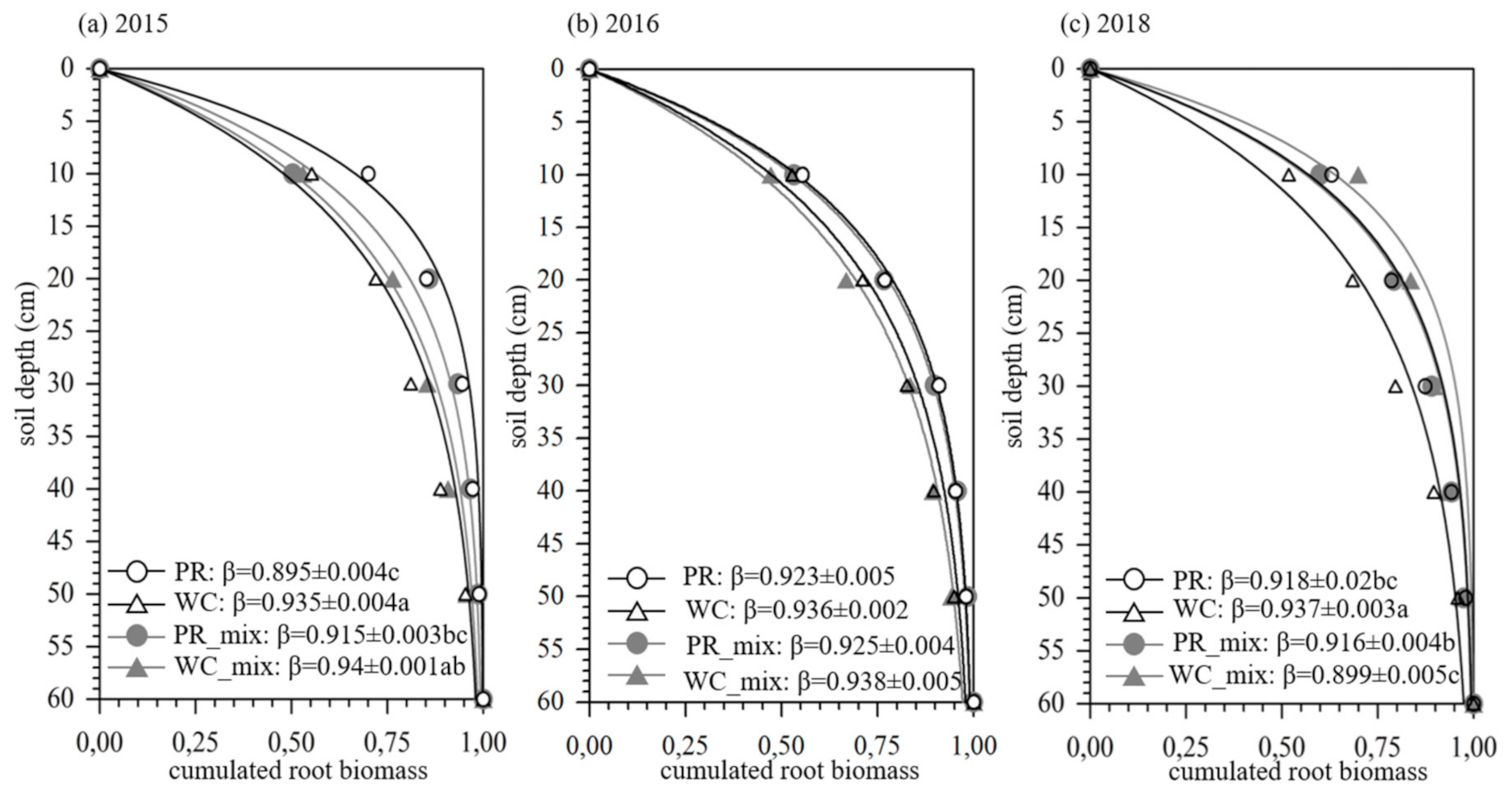
3.2. Vertical Root Distribution (β-Value) of White Clover and Perennial Ryegrass in Monocultures and Mixtures
3.3. Aboveground and Belowground Relative Yield Total
4. Discussion
4.1. Aboveground and Belowground Biomass
4.2. Vertical Root Distribution of White Clover and Perennial Ryegrass
4.2.1. Monocultures
4.2.2. Mixtures
4.3. Relative Yield Total
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| GT | Aboveground Biomass | Root Biomass | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Monocultures (g m−2) | WC–PR Mixtures (g m−2) | Monocultures (g m−2) | WC–PR Mixtures (g m−2) | ||||||
| AV | SE | AV | SE | AV | SE | AV | SE | ||
| 2015 | WC1 | 1084 | ±102 | 892 | ±118 | 160 | ±27 | 323 | ±11 |
| WC2 | 926 | ±15 | 797 | ±131 | 113 | ±29 | 389 | ±52 | |
| WC3 | 1078 | ±67 | 913 | ±143 | 84 | ±2 | 304 | ±18 | |
| WC4 | 811 | ±65 | 736 | ±45 | 108 | ±26 | 375 | ±11 | |
| WC5 | 1085 | ±119 | 782 | ±120 | 139 | ±42 | 316 | ±56 | |
| WC6 | 1279 | ±170 | 999 | ±289 | 69 | ±8 | 425 | ±31 | |
| WC7 | 1023 | ±123 | 918 | ±78 | 186 | ±31 | 359 | ±20 | |
| WC8 | 1130 | ±142 | 1038 | ±109 | 143 | ±35 | 349 | ±30 | |
| PR | 521 | ±102 | 361 | ±20 | |||||
| 2016 | WC1 | 634 | ±57 | 617 | ±82 | 97 | ±13 | 317 | ±25 |
| WC2 | 612 | ±40 | 833 | ±103 | 100 | ±22 | 317 | ±71 | |
| WC3 | 644 | ±95 | 583 | ±42 | 72 | ±9 | 323 | ±18 | |
| WC4 | 405 | ±30 | 679 | ±76 | 83 | ±7 | 305 | ±33 | |
| WC5 | 608 | ±96 | 631 | ±43 | 102 | ±14 | 352 | ±26 | |
| WC6 | 658 | ±40 | 625 | ±71 | 77 | ±10 | 318 | ±29 | |
| WC7 | 564 | ±81 | 584 | ±56 | 113 | ±4 | 340 | ±43 | |
| WC8 | 675 | ±88 | 806 | ±39 | 103 | ±8 | 295 | ±13 | |
| PR | 322 | ±32 | 345 | ±45 | |||||
| 2018 | WC1 | 175 | ±18 | 428 | ±47 | 65 | ±17 | 230 | ±43 |
| WC2 | 335 | ±83 | 436 | ±108 | 80 | ±7 | 134 | ±46 | |
| WC3 | 203 | ±35 | 333 | ±29 | 83 | ±17 | 239 | ±38 | |
| WC4 | 270 | ±43 | 412 | ±24 | 82 | ±11 | 241 | ±26 | |
| WC5 | 312 | ±59 | 428 | ±138 | 68 | ±15 | 195 | ±33 | |
| WC6 | 160 | ±44 | 354 | ±06 | 44 | ±13 | 24 | ±30 | |
| WC7 | 253 | ±54 | 545 | ±94 | 61 | ±10 | 312 | ±80 | |
| WC8 | 138 | ±30 | 314 | ±41 | 55 | ±5 | 160 | ±23 | |
| PR | 177 | ±26 | 224 | ±7 | |||||
References
- Heuermann, D.; Gentsch, N.; Boy, J.; Schweneker, D.; Feuerstein, U.; Groß, J.; Bauer, B.; Guggenberger, G.; Wirén, N. von. Interspecific competition among catch crops modifies vertical root biomass distribution and nitrate scavenging in soils. Sci. Rep. 2019, 9, 11531. [Google Scholar] [CrossRef] [Green Version]
- Giehl, R.F.H.; von Wirén, N. Root nutrient foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Dimitrakopoulos, P.G.; Schmid, B. Biodiversity effects increase linearly with biotope space. Ecol. Lett. 2004, 7, 574–583. [Google Scholar] [CrossRef]
- von Felten, S.; Schmid, B. Complementarity among species in horizontal versus vertical rooting space. J. Plant Ecol. 2008, 1, 33–41. [Google Scholar] [CrossRef]
- Kemper, R.; Bublitz, T.A.; Müller, P.; Kautz, T.; Döring, T.F.; Athmann, M. Vertical Root Distribution of Different Cover Crops Determined with the Profile Wall Method. Agriculture 2020, 10, 503. [Google Scholar] [CrossRef]
- Parrish, J.; Bazzaz, F.A. Underground Niche Separation in Successional Plants. Ecology 1976, 57, 1281–1288. [Google Scholar] [CrossRef]
- Berendse, F. Interspecific Competition and Niche Differentiation Between Plantago Lanceolata and Anthoxanthum Odoratum in a Natural. J. Ecol. 1983, 71, 379–390. [Google Scholar] [CrossRef]
- Berendse, F. Competition between Plant Populations with Different Rooting Depths II. Pot Experiments. Oecologia 1981, 48, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; HilleRisLambers, J. The importance of niches for the maintenance of species diversity. Nature 2009, 461, 254–257. [Google Scholar] [CrossRef]
- Gersani, M.; Arbramsky, Z.; Falik, O. Density-dependent habitat selection in plants. Evolut. Ecol. 1998, 12, 223–234. [Google Scholar] [CrossRef]
- Semchenko, M.; John, E.A.; Hutchings, M.J. Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytol. 2007, 176, 644–654. [Google Scholar] [CrossRef]
- Mommer, L.; van Ruijven, J.; de Caluwe, H.; Smit-Tiekstra, A.E.; Wagemaker, C.A.; Joop Ouborg, N.; Bögemann, G.M.; van der Weerden, G.M.; Berendse, F.; de Kroon, H. Unveiling below-ground species abundance in a biodiversity experiment: A test of vertical niche differentiation among grassland species. J. Ecol. 2010, 98, 1117–1127. [Google Scholar] [CrossRef]
- Schwinning, S.; Parsons, A.J. Analysis of the Coexistence Mechanisms for Grasses and Legumes in Grazing Systems. J. Ecol. 1966, 84, 799–813. [Google Scholar] [CrossRef]
- Del Río, M.; Pretzsch, H.; Ruíz-Peinado, R.; Ampoorter, E.; Annighöfer, P.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; Drössler, L.; et al. Species interactions increase the temporal stability of community productivity in Pinus sylvestris-Fagus sylvatica mixtures across Europe. J. Ecol. 2017, 105, 1032–1043. [Google Scholar] [CrossRef] [Green Version]
- Dennis, W.D.; Woledge, J. The effect of nitrogen in spring on shoot number and leaf area of white clover in mixtures. Grass Forage Sci. 1987, 42, 265–269. [Google Scholar] [CrossRef]
- Davidson, I.A.; Robson, M.J. Short-term effects of nitrogen on the growth and nitrogen nutrition of small swards of white clover and perennial ryegrass in spring. Grass Forage Sci. 1990, 45, 413–421. [Google Scholar] [CrossRef]
- Streit, J.; Meinen, C.; Rauber, R. Intercropping effects on root distribution of eight novel winter faba bean genotypes mixed with winter wheat. Field Crop. Res. 2019, 235, 1–10. [Google Scholar] [CrossRef]
- Streit, J.; Meinen, C.; Nelson, W.C.D.; Siebrecht-Schöll, D.J.; Rauber, R. Above- and belowground biomass in a mixed cropping system with eight novel winter faba bean genotypes and winter wheat using FTIR spectroscopy for root species discrimination. Plant Soil 2019, 436, 141–158. [Google Scholar] [CrossRef]
- Lynch, J.P. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Hodge, A.; Griffiths, B.S.; Fitter Alastair, H. Plant Root Proliferation in Nitrogen-Rich Patches Confers Competitive Advantage. Proc. Biol. Sci. 1999, 226, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Junker, A.; Seiler, C.; Altmann, T. Phenotyping roots in darkness: Disturbance-free root imaging with near infrared illumination. Funct. Plant Biol. 2018, 45, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Mueller, K.E.; Tilman, D.; Fornara, D.A.; Hobbie, S.E. Root depth distribution and the diversity-productivity relationship in a long-term grassland experiment. Ecology. 2013, 94, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Frame, J.; Newbould, P. Agronomy of white clover. Adv. Agron. 1986, 40, 1–88. [Google Scholar] [CrossRef]
- Lane, L.A.; Ayres, J.F.; Lovett, J.V. The pastoral significance, adaptive characteristics, and grazing value of white clover (Trifolium repens L.) in dryland environments in Australia: A review. Aust. J. Exp. Agric. 2000, 40, 1033. [Google Scholar] [CrossRef]
- Marshall, A.H.; Collins, R.P.; Humphreys, M.W.; Scullion, J. A new emphasis on root traits for perennial grass and legume varieties with environmental and ecological benefits. Food Energy Secur. 2016, 5, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Sun, J.; Zhang, F.; Guo, T.; Bao, X.; Smith, F.A.; Smith, S.E. Root distribution and interactions between intercropped species. Oecologia 2006, 147, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Vinther, F.P. Effects of cutting frequency on plant production, N-uptake and N2 fixation in above- and below-ground plant biomass of perennial ryegrass–white clover swards. Grass Forage Sci. 2006, 61, 154–163. [Google Scholar] [CrossRef]
- Naumann, A.; Heine, G.; Rauber, R. Efficient discrimination of oat and pea roots by cluster analysis of Fourier transform infrared (FTIR) spectra. Field Crop. Res. 2010, 119, 78–84. [Google Scholar] [CrossRef]
- Meinen, C.; Rauber, R. Root discrimination of closely related crop and weed species using FT MIR-ATR spectroscopy. Front. Plant Sci. 2015, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legner, N.; Meinen, C.; Rauber, R. Root Differentiation of Agricultural Plant Cultivars and Proveniences Using FTIR Spectroscopy. Front. Plant Sci. 2018, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heshmati, S.; Tonn, B.; Isselstein, J. White clover population effects on the productivity and yield stability of mixtures with perennial ryegrass and chicory. Field Crop. Res. 2020, 252, 107802. [Google Scholar] [CrossRef]
- De Wit, C.T. On competition. Versl. Landbouwk. Onderzoek. 1960, 66, 1–82. [Google Scholar]
- De Wit, C.T.; van den Bergh, J.P. Competition between herbage plants. Neth. J. Agric. Sci. 1965, 13, 212–221. [Google Scholar] [CrossRef]
- Gale, M.R.; Grigal, D.F. Vertical root distributions of northern tree species in relation to successional status. Can. J. For. Res. 1987, 829–834. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- DWD—Climate Data Center. Climate Data Center (CDC)—German Meteorological Service (DWD). 2020. Available online: https://cdc.dwd.de/portal/ (accessed on 12 February 2020).
- Schils, R.; Vellinga, T.V.; Kraak, T. Dry-matter yield and herbage quality of a perennial ryegrass/white clover sward in a rotational grazing and cutting system. Grass Forage Sci. 1999, 54, 19–29. [Google Scholar] [CrossRef]
- Elgersma, A.; Nassiri, M.; Schlepers, H. Competition in perennial ryegrass–white clover mixtures under cutting. 1. Dry-matter yield, species composition and nitrogen fixation. Grass Forage Sci. 1998, 53, 353–366. [Google Scholar] [CrossRef]
- Elgersma, A.; Schlepers, H. Performance of white clover/perennial ryegrass mixtures under cutting. Grass Forage Sci. 1997, 52, 134–146. [Google Scholar] [CrossRef]
- Goh, K.M.; Bruce, G.E. Comparison of biomass production and biological nitrogen fixation of multi-species pastures (mixed herb leys) with perennial ryegrass-white clover pasture with and without irrigation in Canterbury, New Zealand. Agric. Ecosyst. Environ. 2005, 110, 230–240. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Ma, X. Different response on drought tolerance and post-drought recovery between the small-leafed and the large-leafed white clover (Trifolium repens L.) associated with antioxidative enzyme protection and lignin metabolism. Acta Physiol. Plant. 2013, 35, 213–222. [Google Scholar] [CrossRef]
- Lucero, D.W. Water deficit and plant competition effects on growth and water-use efficiency of white clover (Trifolium repens, L.) and ryegrass (Lolium perenne, L.). Plant Soil 2000, 227, 1–15. [Google Scholar] [CrossRef]
- Ren, H.; Gao, T.; Hu, J.; Yang, G. The effects of arbuscular mycorrhizal fungi and root interaction on the competition between Trifolium repens and Lolium perenne. PeerJ 2017, 5, e4183. [Google Scholar] [CrossRef] [Green Version]
- Siebenkäs, A.; Roscher, C. Functional composition rather than species richness determines root characteristics of experimental grasslands grown at different light and nutrient availability. Plant Soil 2016, 404, 399–412. [Google Scholar] [CrossRef]
- Crush, J.R.; Easton, H.S.; Waller, J.E.; Hume, D.E.; Faville, M.J. Genotypic variation in patterns of root distribution, nitrate interception and response to moisture stress of a perennial ryegrass (Lolium perenne L.) mapping population. Grass Forage Sci. 2007, 62, 265–273. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. Are differences in root growth of nitrogen catch crops important for their ability to reduce soil nitrate-N content, and how can this be measured? Plant Soil 2001, 230, 185–195. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. Effect of deep and shallow root systems on the dynamics of soil inorganic N during 3-year crop rotations. Plant Soil 2006, 288, 233–248. [Google Scholar] [CrossRef]
- Crush, J.R.; Waller, J.E.; Care, D.A. Root distribution and nitrate interception in eleven temperate forage grasses. Grass Forage Sci. 2005, 60, 385–392. [Google Scholar] [CrossRef]
- Wardle, D.A.; Peltzer, D.A. Interspecific interactions and biomass allocation among grassland plant species. Oikos 2003, 100, 497–506. [Google Scholar] [CrossRef]
- Shmida, A.; Ellner, S. Coexistence of Plant Species with Similar Niches. Vegetatio 1984, 58, 29–55. [Google Scholar]
- Chesson, P. A need for niches? Trends Ecol. Evol. 1991, 6, 26–28. [Google Scholar] [CrossRef]
- Rasmussen, J.; Gylfadóttir, T.; Loges, R.; Eriksen, J.; Helgadóttir, Á. Spatial and temporal variation in N transfer in grass–white clover mixtures at three Northern European field sites. Soil Biol. Biochem. 2013, 57, 654–662. [Google Scholar] [CrossRef]
- Dhamala, N.R.; Rasmussen, J.; Carlsson, G.; Søegaard, K.; Eriksen, J. N transfer in three-species grass-clover mixtures with chicory, ribwort plantain or caraway. Plant Soil 2017, 413, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.S. Grain yield, symbiotic N2 fixation and interspecific competition for inorganic N in pea-barley intercrops. Plant Soil 1996, 182, 25–38. [Google Scholar] [CrossRef]
- Suter, M.; Connolly, J.; Finn, J.A.; Loges, R.; Kirwan, L.; Sebastià, M.-T.; Lüscher, A. Nitrogen yield advantage from grass-legume mixtures is robust over a wide range of legume proportions and environmental conditions. Glob. Chang. Biol. 2015, 21, 2424–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høgh-Jensen, H.; Schjoerring, J.K. Below-ground nitrogen transfer between different grassland species: Direct quantification by 15N leaf feeding compared with indirect dilution of soil 15N. Plant Soil 2000, 227, 171–183. [Google Scholar] [CrossRef]
- Jørgensen, F.V.; Jensen, E.S.; Schjoerring, J.K. Dinitrogen fixation in white clover grown in pure stand and mixture with ryegrass estimated by the immobilized 15N isotope dilution method. Plant Soil 1999, 208, 293–305. [Google Scholar] [CrossRef]
- Xiao-Qing, T.U.O.; Li, H.E.; Ying-Ning, Z.O.U. Alleviation of Drought Stress in White Clover after Inoculation with Arbuscular Mycorrhizal Fungi. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Husse, S.; Huguenin-Elie, O.; Buchmann, N.; Lüscher, A. Larger yields of mixtures than monocultures of cultivated grassland species match with asynchrony in shoot growth among species but not with increased light interception. Field Crop. Res. 2016, 194, 1–11. [Google Scholar] [CrossRef]
- Collins, R.P.; Rhodes, I. Influence of root competition on compatibility between white clover and perennial ryegrass populations during seedling establishment. Grass Forage Sci. 1994, 49, 506–509. [Google Scholar] [CrossRef]
- Davidson, I.A.; Robson, M.J. Effect of Nitrogen Supply on the Grass and Clover Components of Simulated Mixed Swards Grown under Favourable Environmental Conditions II. Nitrogen Fixation and Nitrate Uptake. Ann. Bot. 1985, 55, 697–703. [Google Scholar] [CrossRef]
| Year | Aboveground Biomass | Belowground Biomass | Root Length Density | ||||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| WC1–WC8 | 2015 | 1.58 | 0.19 | 2.00 | 0.10 | 0.93 | 0.50 |
| 2016 | 1.49 | 0.22 | 1.36 | 0.27 | 0.58 | 0.76 | |
| 2018 | 2.14 | 0.08 | 1.26 | 0.31 | 0.98 | 0.47 | |
| WC1_PR–WC8_PR | 2015 | 0.53 | 0.80 | 1.55 | 0.20 | 1.54 | 0.20 |
| 2016 | 2.10 | 0.08 | 0.25 | 0.97 | 1.02 | 0.45 | |
| 2018 | 0.96 | 0.48 | 1.58 | 0.19 | 0.91 | 0.52 | |
| Aboveground Biomass | Belowground Biomass | Root Length Density | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| TREAT | 15.7 | ≤0.0001 | 260.2 | ≤0.0001 | 613.6 | ≤0.0001 |
| YEAR | 64.3 | ≤0.0001 | 27.6 | ≤0.0001 | 165.4 | ≤0.0001 |
| TREAT:YEAR | 8.7 | ≤0.0001 | 4.6 | ≤0.001 | 53.5 | ≤0.0001 |
| 2015 | 2016 | 2018 | Average | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RY WC | RY PR | RYT | RY WC | RY PR | RYT | RY WC | RY PR | RYT | RY WC | RY PR | RYT | |
| Shoot Yield | 0.17 * ± 0.04 | 1.36 * ± 0.09 | 1.53 * ± 0.12 | 0.46 ± 0.08 | 1.28 * ± 0.17 | 1.74 * ± 0.18 | 0.07 * ± 0.01 | 2.26 * ± 0.20 | 2.33 * ± 0.19 | 0.23 * ± 0.02 | 1.63 * ± 0.14 | 1.87 * ± 0.24 |
| Root Yield | 0.55 ± 0.06 | 1.00 ± 0.15 | 1.55 * ± 0.15 | 0.90 * ± 0.09 | 0.65 ± 0.09 | 1.56 * ± 0.14 | 0.65 ± 0.11 | 0.86 * ± 0.02 | 1.50 * ± 0.12 | 0.70 ± 0.04 | 0.84 * ± 0.06 | 1.54 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dirks, I.; Streit, J.; Meinen, C. Above and Belowground Relative Yield Total of Clover–Ryegrass Mixtures Exceed One in Wet and Dry Years. Agriculture 2021, 11, 206. https://doi.org/10.3390/agriculture11030206
Dirks I, Streit J, Meinen C. Above and Belowground Relative Yield Total of Clover–Ryegrass Mixtures Exceed One in Wet and Dry Years. Agriculture. 2021; 11(3):206. https://doi.org/10.3390/agriculture11030206
Chicago/Turabian StyleDirks, Inga, Juliane Streit, and Catharina Meinen. 2021. "Above and Belowground Relative Yield Total of Clover–Ryegrass Mixtures Exceed One in Wet and Dry Years" Agriculture 11, no. 3: 206. https://doi.org/10.3390/agriculture11030206





