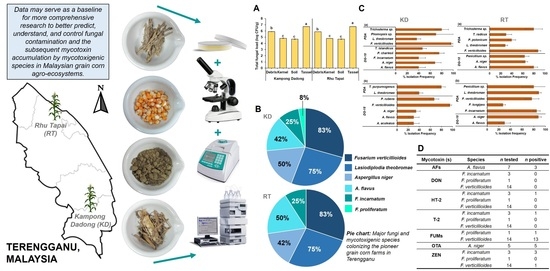
Diversity and Toxigenicity of Mycobiota in Grain Corn: A Case Study at Pioneer Grain Corn Plantations in Terengganu, Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Isolation and Enumeration of Indigenous Fungi
2.3. Morphological and Molecular Identification of Fungal Isolates
2.4. Preparation of Grain Corn Agar as Semi-Synthetic Growth Medium
2.5. Mycotoxigenic Potentials of Fungal Isolates
2.5.1. Extraction and Quantification of Aflatoxins
2.5.2. Extraction and Quantification of Ochratoxin A
2.5.3. Extraction, Derivatization, and Quantification of Fumonisins
2.5.4. Extraction and Quantification of Trichothecenes
2.5.5. Extraction and Quantification of Zearalenone
2.5.6. Linearity, Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.6. Statistical Analysis
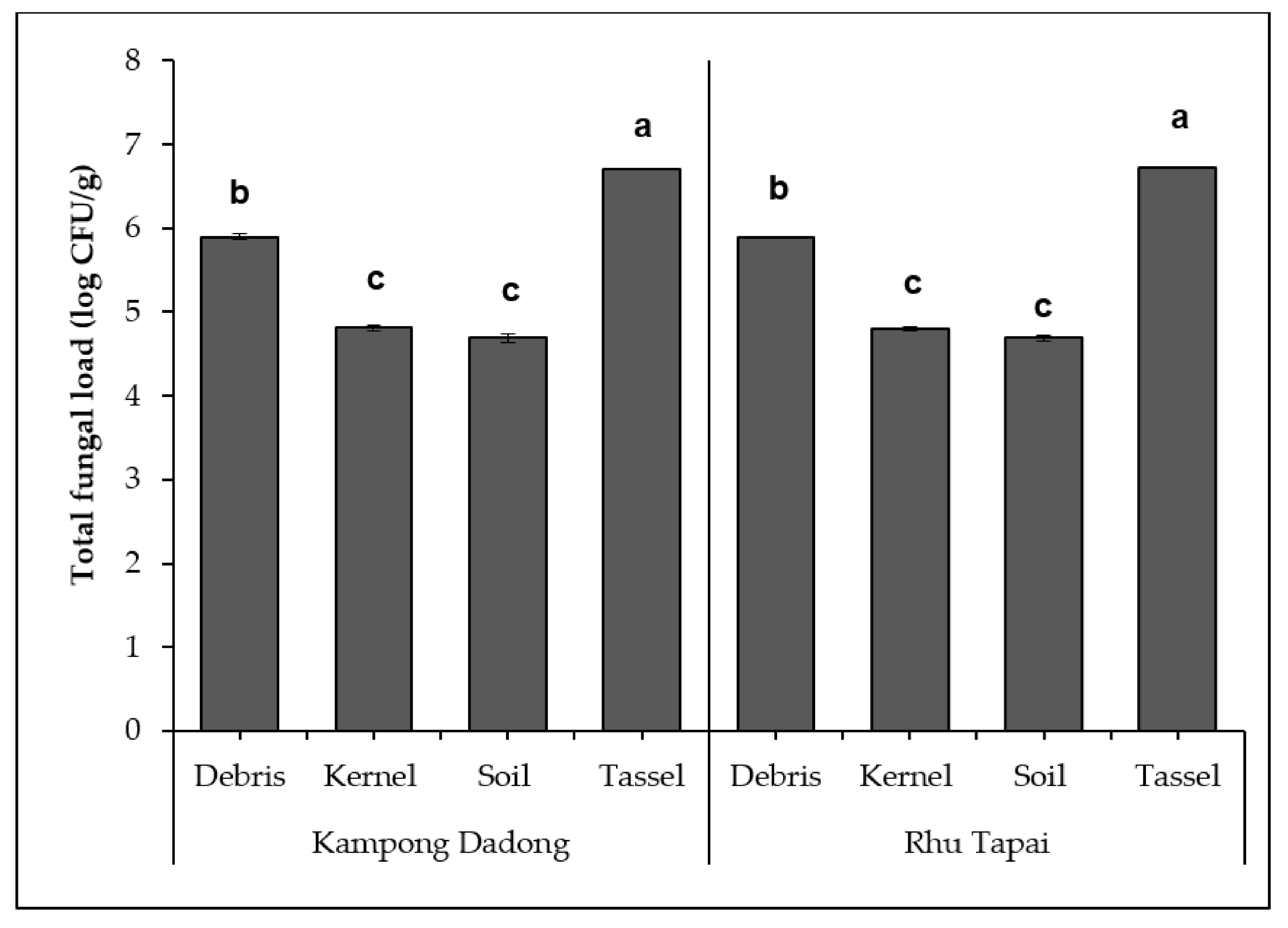
3. Results
3.1. Temperature, Relative Humidity, and Rainfall of Kampong Dadong and Rhu Tapai Grain Corn Farms during 2017 Cropping Season
3.2. Density and Diversity of Mycobiota Isolated from Kampong Dadong and Rhu Tapai Grain Corn Farms
3.3. Mycotoxigenic Potentials of Mycotoxigenic Species Isolated from Kampong Dadong and Rhu Tapai Grain Corn Farms
4. Discussion
4.1. Diversity of Mycobiota and the Occurrence of Economically Important Mycotoxigenic Species, Aspergillus flavus and Fusarium verticillioides, in Two Pioneer Grain Corn Farms of Terengganu, Malaysia
4.2. Mycotoxigenic Potential of Mycotoxigenic Isolates from Two Pioneer Grain Corn Farms in Terengganu, Malaysia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahari, M.W.; Wong, H.K. Research and development on animal feed in Malaysia. Wartazoa 2009, 19, 172–179. [Google Scholar] [CrossRef]
- Mohd Supaat, M.Z. Developmental Plan for Grain Corn Industry. In Proceedings of the National Seminar on Grain Corn Industrial Development, Kemaman, Terengganu, Malaysia, 5–7 July 2017. [Google Scholar]
- Bank Negara Malaysia. The 2018 Budget Speech. Available online: https://www.bnm.gov.my/files/2017/Budget2018.pdf (accessed on 15 January 2020).
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz, M.; Gil-Serna, J.; Vázquez, C.; Botia, M.N.; Patiño, B. A comprehensive study on the occurrence of mycotoxins and their producing fungi during the maize production cycle in Spain. Microorganisms 2020, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Mycotoxins in corn: Occurrence, impacts, and management. In Corn: Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Washington, DC, USA, 2019; pp. 235–287. ISBN 9780128119716. [Google Scholar]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Darnetty, T.; Salleh, B. Toxigenicity of Fusarium species in Gibberella fujikuroi species complex (GFSC) associated with stalk and ear rot disease of corn. Int. J. Phytopathol. 2013, 2, 147–154. [Google Scholar] [CrossRef]
- Hsuan, H.M.; Salleh, B.; Zakaria, L. Molecular identification of Fusarium species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 6722–6732. [Google Scholar] [CrossRef] [PubMed]
- Nur Ain Izzati, M.Z.; Azmi, A.R.; Siti Nordahliawate, M.S.; Norazlina, J. Contribution to the knowledge of diversity of Fusarium associated with maize in Malaysia. Plant Prot. Sc. 2011, 47, 20–24. [Google Scholar] [CrossRef]
- Zainudin, N.A.I.M.; Sidique, S.N.M.; Johari, N.A.; Darnetty; Razak, A.A.; Salleh, B. Isolation and identification of Fusarium species associated with Fusarium ear rot disease of corn. Pertanika J. Trop. Agric. Sci. 2011, 34, 325–330. [Google Scholar]
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Nesci, A.; Barros, G.; Castillo, C.; Etcheverry, M. Soil fungal population in preharvest maize ecosystem in different tillage practices in Argentina. Soil Tillage Res. 2006, 91, 143–149. [Google Scholar] [CrossRef]
- Warris, M.N.; Azami, A.; Abdul Wahab, N.M.A.; Abd Rahman, N.; Abdul Wahab, N.H.; Mohd Zin, M.F. Production Economy and Management Model of Grain Corn. Presented at the National Seminar on Grain Corn Industrial Development, Kemaman, Terengganu, Malaysia, 5–7 July 2017. [Google Scholar]
- Carranza, C.S.; Barberis, C.L.; Chiacchiera, S.M.; Dalcero, A.M.; Magnoli, C.E. Isolation of culturable mycobiota from agricultural soils and determination of tolerance to glyphosate of non-toxigenic Aspergillus section Flavi strains. J. Environ. Sci. Health B 2016, 51, 35–43. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Microbiological Specifications for Foods. Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions (ISO 6887—1:2017). Available online: https://www.iso.org/standard/63335.html (accessed on 1 December 2020).
- International Commission on Microbiological Specifications for Foods. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95 (ISO 21527—1:2008). Available online: https://www.iso.org/standard/38275.html (accessed on 1 December 2020).
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: Heidelberg, Germany, 2009; ISBN 9780387922065. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010; ISBN 9789070351823. [Google Scholar]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470276464. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439804209. [Google Scholar]
- Yazid, S.N.E.; Thanggavelu, H.; Mahror, N.; Selamat, J.; Samsudin, N.I.P. Formulation of maize-and peanut-based semi-synthetic growth media for the ecophysiological studies of aflatoxigenic Aspergillus flavus in maize and peanut agro-ecosystems. Int. J. Food Microbiol. 2018, 282, 57–65. [Google Scholar] [CrossRef]
- Bernáldez, V.; Córdoba, J.J.; Magan, N.; Peromingo, B.; Rodríguez, A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. LWT 2017, 83, 283–291. [Google Scholar] [CrossRef]
- Bragulat, M.R.; Abarca, M.L.; Cabañes, F.J. An easy screening method for fungi producing ochratoxin A in pure culture. Int. J. Food Microbiol. 2001, 71, 139–144. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Arzandeh, S.; Mirhosseini, H. Optimization of HPLC conditions for quantitative analysis of aflatoxins in contaminated peanut. Food Control 2011, 22, 381–388. [Google Scholar] [CrossRef]
- Mohale, S.; Medina, A.; Rodríguez, A.; Sulyok, M.; Magan, N. Mycotoxigenic fungi and mycotoxins associated with stored maize from different regions of Lesotho. Mycotoxin Res. 2013, 29, 209–219. [Google Scholar] [CrossRef]
- Visconti, A.; Solfrizzo, M.; Girolamo, A.D. Determination of fumonisins B1 and B2 in corn and corn flakes by liquid chromatography with immunoaffinity column cleanup: Collaborative study. J. AOAC Int. 2001, 84, 1828–1837. [Google Scholar] [CrossRef]
- Moazami, E.F.; Jinap, S. Natural occurrence of deoxynivalenol (DON) in wheat based noodles consumed in Malaysia. Microchem. J. 2009, 93, 25–28. [Google Scholar] [CrossRef]
- Medina, A.; Magan, N. Temperature and water activity effects on production of T-2 and HT-2 by Fusarium langsethiae strains from north European countries. Food Microbiol. 2011, 28, 392–398. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Jinap, S.; Rashid, U. Detection of aflatoxins and zearalenone contamination in wheat derived products. Food Control 2014, 35, 223–226. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Beuchat, L.R. Media for detecting and enumerating yeasts and moulds. Int. J. Food Microbiol. 1992, 17, 145–158. [Google Scholar] [CrossRef]
- Thompson, M.E.; Raizada, M.N. Fungal pathogens of maize gaining free passage along the silk road. Pathogens 2018, 7, 81. [Google Scholar] [CrossRef]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Ma, H.X.; Zhang, H.J.; Shi, J.; Dang, J.J.; Chang, J.Y.; Chen, D.; Hu, Q.Y.; Guo, N.; Han, H.L. First report of Lasiodiplodia theobromae causing maize ear rot in Hainan Province in Southern China. Plant Dis. 2016, 100, 2160–2161. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Alves, A.; Andolfi, A. Secondary metabolites of Lasiodiplodia theobromae: Distribution, chemical diversity, bioactivity, and implications of their occurrence. Toxins 2020, 12, 457. [Google Scholar] [CrossRef]
- Yazid, S.N.E.; Selamat, J.; Ismail, S.I.; Magan, N.; Samsudin, N.I.P. Phytopathogenic organisms and mycotoxigenic fungi: Why do we control one and neglect the other? A biological control perspective in Malaysia. Compr. Rev. Food Sci. Food Saf. 2020, 19, 643–669. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Desjardins, A.E. Fumonisins in maize: Can we reduce their occurrence? Plant Dis. 1997, 81, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Degraeve, S.; Madege, R.R.; Audenaert, K.; Kamala, A.; Ortiz, J.; Kimanya, M.; Tiisekwa, B.; Meulenaer, B.D.; Haesaert, G. Impact of local pre-harvest management practices in maize on the occurrence of Fusarium species and associated mycotoxins in two agro-ecosystems in Tanzania. Food Control 2016, 59, 225–233. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Salvi, S. Infection by mycotoxigenic fungal species and mycotoxin contamination of maize grain in Umbria, central Italy. Food Chem. Toxicol. 2011, 49, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Ghiasian, S.A.; Rezayat, S.M.; Kord-Bacheh, P.; Maghsood, A.H.; Yazdanpanah, H.; Shephard, G.S.; Westhuizen, L.V.D.; Vismer, H.F.; Marasas, W.F. Fumonisin production by Fusarium species isolated from freshly harvested corn in Iran. Mycopathologia 2005, 159, 31–40. [Google Scholar] [CrossRef]
- Reyes Gaige, A.; Todd, T.; Stack, J.P. Interspecific competition for colonization of maize plants between Fusarium proliferatum and Fusarium verticillioides. Plant Dis. 2020, 104, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Marín, P.; Magan, N.; Vázquez, C.; González-Jaén, M.T. Differential effect of environmental conditions on the growth and regulation of the fumonisin biosynthetic gene FUM1 in the maize pathogens and fumonisin producers Fusarium verticillioides and Fusarium proliferatum. FEMS Microbiol. Ecol. 2010, 73, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Climate change in Malaysia: Trends, contributors, impacts, mitigation and adaptations. Sci. Total Environ. 2019, 650, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium verticillioides: Managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Cotton, T.K.; Munkvold, G.P. Survival of Fusarium moniliforme, F. proliferatum, and F. subglutinans in maize stalk residue. Phytopathology 1998, 88, 550–555. [Google Scholar] [CrossRef]
- Marín, S.; Sanchis, V.; Arnau, F.; Ramos, A.J.; Magan, N. Colonisation and competitiveness of Aspergillus and Penicillium species on maize grain in the presence of Fusarium moniliforme and Fusarium proliferatum. Int. J. Food Microbiol. 1998, 45, 107–117. [Google Scholar] [CrossRef]
- Giorni, P.; Bertuzzi, T.; Battilani, P. Impact of fungi co-occurrence on mycotoxin contamination in maize during the growing season. Front. Microbiol. 2019, 10, 1265–1274. [Google Scholar] [CrossRef]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef]
- Clear, R.M.; Patrick, S.K.; Gaba, D.; Roscoe, M.; Demeke, T.; Pouleur, S.; Couture, L.; Ward, T.J.; O’Donnell, K.; Turkington, T.K. Trichothecene and zearalenone production, in culture, by isolates of Fusarium pseudograminearum from western Canada. Can. J. Plant Pathol. 2006, 28, 131–136. [Google Scholar] [CrossRef]
- Khan, R.; Mohamad Ghazali, F.; Mahyudin, N.A.; Samsudin, N.I.P. Morphological characterization and determination of aflatoxigenic and non-aflatoxigenic Aspergillus flavus isolated from sweet corn kernels and soil in Malaysia. Agriculture 2020, 10, 450. [Google Scholar] [CrossRef]
- James, M.G.; Robertson, D.S.; Myers, A.M. Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 1995, 7, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Cao, A.; Malvar, R.A.; Reid, L.M.; Butrón, A. Assessment of corn resistance to fumonisin accumulation in a broad collection of inbred lines. Field Crops Res. 2013, 149, 193–202. [Google Scholar] [CrossRef]
- Burge, H.A. An update on pollen and fungal spore aerobiology. J. Allergy Clin. Immunol. 2002, 110, 544–552. [Google Scholar] [CrossRef]
- McCartney, H.A. Dispersal of spores and pollen from crops. Grana 1994, 33, 76–80. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gilbert, G.S. Meteorological factors associated with abundance of airborne fungal spores over natural vegetation. Atmos. Environ. 2017, 162, 87–99. [Google Scholar] [CrossRef]
- Bock, C.H.; Mackey, B.; Cotty, P.J. Population dynamics of Aspergillus flavus in the air of an intensively cultivated region of south-west Arizona. Plant Pathol. 2004, 53, 422–433. [Google Scholar] [CrossRef]
- Proctor, R.H.; Plattner, R.D.; Desjardins, A.E.; Busman, M.; Butchko, R.A. Fumonisin production in the maize pathogen Fusarium verticillioides: Genetic basis of naturally occurring chemical variation. J. Agric. Food Chem. 2006, 54, 2424–2430. [Google Scholar] [CrossRef]
- Nelson, P.E.; Plattner, R.D.; Shackelford, D.D.; Desjardins, A.E. Production of fumonisins by Fusarium moniliforme strains from various substrates and geographic areas. Appl. Environ. Microbiol. 1991, 57, 2410–2412. [Google Scholar] [CrossRef]
- Ismail, N.A.; Mohd, M.H.; Nor, N.M.I.M.; Zakaria, L. Fumonisin B1-producing Fusarium species from agricultural crops in Malaysia. Crop Prot. 2017, 98, 70–75. [Google Scholar] [CrossRef]
- Duan, C.; Qin, Z.; Yang, Z.; Li, W.; Sun, S.; Zhu, Z.; Wang, X. Identification of pathogenic Fusarium spp. causing maize ear rot and potential mycotoxin production in China. Toxins 2016, 8, 186. [Google Scholar] [CrossRef]
- Covarelli, L.; Stifano, S.; Beccari, G.; Raggi, L.; Lattanzio, V.M.T.; Albertini, E. Characterization of Fusarium verticillioides strains isolated from maize in Italy: Fumonisin production, pathogenicity and genetic variability. Food Microbiol. 2012, 31, 17–24. [Google Scholar] [CrossRef]
- Agbetiameh, D.; Ortega-Beltran, A.; Awuah, R.T.; Atehnkeng, J.; Cotty, P.J.; Bandyopadhyay, R. Prevalence of aflatoxin contamination in maize and groundnut in Ghana: Population structure, distribution, and toxigenicity of the causal agents. Plant Dis. 2018, 102, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Allameh, A.; Kazeroon-Shiri, A.; Ranjbar-Bahadori, S.; Mirzahoseini, H.; Rezaee, M.B. A survey on distribution of Aspergillus section Flavi in corn field soils in Iran: Population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia 2006, 161, 183–192. [Google Scholar] [CrossRef]
- Cotty, P.J. Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycol. Res. 1997, 101, 698–704. [Google Scholar] [CrossRef]
- Gallo, A.; Stea, G.; Battilani, P.; Logrieco, A.F.; Perrone, G. Molecular characterisation of an Aspergillus flavus population isolated from maize during the first outbreak of aflatoxin contamination in Italy. Phytopathol. Mediterr. 2012, 51, 198–206. [Google Scholar] [CrossRef]
- Ortega-Beltran, A.; Cotty, P.J. Frequent shifts in Aspergillus flavus populations associated with maize production in Sonora, Mexico. Phytopathology 2018, 108, 412–420. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Giorni, P.; Pietri, A.; Battilani, P. Aspergillus flavus and Fusarium verticillioides interaction: Modelling the impact on mycotoxin production. Front. Microbiol. 2019, 10, 2653. [Google Scholar] [CrossRef]
- Palencia, E.R.; Hinton, D.M.; Bacon, C.W. The black Aspergillus species of maize and peanuts and their potential for mycotoxin production. Toxins 2010, 2, 399–416. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium species and their associated mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Humana Press: Totowa, NJ, USA, 2017; pp. 51–106. ISBN 9781493967056. [Google Scholar]
- Broders, K.D.; Lipps, P.E.; Paul, P.A.; Dorrance, A.E. Evaluation of Fusarium graminearum associated with corn and soybean seed and seedling disease in Ohio. Plant Dis. 2007, 91, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Pfordt, A.; Romero, L.R.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Impact of environmental conditions and agronomic practices on the prevalence of Fusarium species associated with ear- and stalk rot in maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef]
- Gupta, R.C.; Mostrom, M.S.; Evans, T.J. Zearalenone. In Veterinary Toxicology: Basic and Clinical Principles, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1055–1063. ISBN 9780128114117. [Google Scholar]
- Reddy, K.R.N.; Salleh, B. Co-occurrence of moulds and mycotoxins in corn grains used for animal feeds in Malaysia. J. Anim. Vet. Adv. 2011, 10, 668–673. [Google Scholar] [CrossRef]
- Soleimany, F.; Jinap, S.; Abas, F. Determination of mycotoxins in cereals by liquid chromatography tandem mass spectrometry. Food Chem. 2012, 130, 1055–1060. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Validation of the procedure for the simultaneous determination of aflatoxins, ochratoxin A and zearalenone in cereals using HPLC-FLD. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, N.A.I.M.; Perumal, N. Mycotoxins production by Fusarium and Aspergillus species isolated from cornmeal. Int. J. Agric. Biol. 2015, 17, 440–448. [Google Scholar] [CrossRef]
- Widiastuti, R.; Maryam, R.; Blaney, B.J.; Stoltz, D.R. Corn as a source of mycotoxins in Indonesian poultry feeds and the effectiveness of visual examination methods for detecting contamination. Mycopathologia 1998, 102, 45–49. [Google Scholar] [CrossRef]
- Widiastuti, R.; Maryam, R.; Blaney, B.J.; Stoltz, D.R. Cyclopiazonic acid in combination with aflatoxins, zearalenone and ochratoxin A in Indonesian corn. Mycopathologia 1988, 104, 153–156. [Google Scholar] [CrossRef]
- Ali, N.; Sardjono; Yamashita, A.; Yoshizawa, T. Natural co-occurrence of aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol, nivalenol and zearalenone) in corn from Indonesia. Food Addit. Contam. 1998, 15, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Yamashita, A.; Chokethaworn, N. Occurrence of fumonisins and aflatoxins in corn from Thailand. Food Addit. Contam. 1996, 13, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Tansakul, N.; Jala, P.; Laopiem, S.; Tangmunkhong, P.; Limsuwan, S. Co-occurrence of five Fusarium toxins in corn-dried distiller’s grains with solubles in Thailand and comparison of ELISA and LC-MS/MS for fumonisin analysis. Mycotoxin Res. 2013, 29, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Thieu, N.Q.; Ogle, B.; Pettersson, H. Screening of aflatoxins and zearalenone in feedstuffs and complete feeds for pigs in Southern Vietnam. Trop. Anim. Health Prod. 2008, 40, 77–83. [Google Scholar] [CrossRef]
- Huong, B.T.M.; Do, T.T.; Madsen, H.; Brimer, L.; Dalsgaard, A. Aflatoxins and fumonisins in rice and maize staple cereals in Northern Vietnam and dietary exposure in different ethnic groups. Food Control 2016, 70, 191–200. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Conte, G.; Fontanelli, M.; Galli, F.; Cotrozzi, L.; Pagni, L.; Pellegrini, E. Mycotoxins in feed and food and the role of ozone in their detoxification and degradation: An update. Toxins 2020, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.J. Good agricultural and harvest practices to reduce mycotoxin contamination in wheat in temperate countries. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; Wiley Blackwell: New Delhi, India, 2014; pp. 209–219. ISBN 9781118832790. [Google Scholar]
| Month | Kampong Dadong | Rhu Tapai | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Rainfall (mm) | Relative Humidity (%) | Temperature (°C) | Rainfall (mm) | Relative Humidity (%) | |
| March | 27.3 | 173.6 | 79.6 | 27.7 | 147.8 | 79.1 |
| April | 28.2 | 33.2 | 79.4 | 28.4 | 32.2 | 81.1 |
| May | 28.4 | 204.4 | 79.8 | 28.6 | 152.6 | 81.0 |
| June | 27.9 | 180.2 | 81.0 | 28.0 | 77.4 | 82.1 |
| July | 27.9 | 203.4 | 81.2 | 28.0 | 132.0 | 82.7 |
| August | 27.7 | 164.2 | 81.4 | 27.5 | 206.2 | 83.4 |
| Tmean (°C) | 27.9 | 28.0 | ||||
| Trange | 1.1 | 1.1 | ||||
| CR (mm) | 959.0 | 748.2 | ||||
| RHmean (%) | 80.4 | 81.6 | ||||
| RHrange | 2 | 4.3 | ||||
| No. | Fungi | Sequence Similarity (%) | GenBank Accession No. | Isolate Code |
|---|---|---|---|---|
| 1. | Aspergillus aculeatus | 99.82 | MW542998 | Aa3TD |
| 2. | Aspergillus flavus | 100 | MW542999 | Af2SR, Af1KD, Af3SD, Af5TD, Af6KR, Af7KR, Af4SR |
| 3. | Aspergillus niger | 100 | MW543000 | An1KD, An3KD, An4KD, An2KR, An5KR |
| 4. | Bjerkandera adusta | 100 | MW543001 | Ba42DD |
| 5. | Curvularia sp. | 99 | MW543002 | C13SR |
| 6. | Fusarium incarnatum | 100 | MW543003 | Fi110KD, Fi53TD, Fi56TR |
| 7. | Fusarium longipes | 99.44 | MW543004 | Fl57TD |
| 8. | Fusarium proliferatum | 100 | MW543005 | Fp9DD |
| 9. | Fusarium verticillioides | 100 | MW543006 | Fv9TD, Fv79DD, Fv9DD, Fv70DD, Fv21KD, Fv25KD, Fv32KD, Fv90SD, Fv93SD, Fv106SD, Fv16TD, Fv3DR, Fv4DR, Fv21DR, Fv69DR, Fv25KR, Fv26KR, Fv58SR, Fv89SR, Fv90SR, Fv92SR, Fv99SR, Fv111SR, Fv22TR |
| 10. | Lasiodiplodia theobromae | 100 | MW543007 | Lt107DR, Lt73DD, Lt12KD Lt13SD Lt15SD, Lt21SD, Lt14TD, Lt16TD, Lt12KR, Lt55KR, Lt100SR, Lt101SR, Lt102SR, Lt5TR, Lt15TR, Lt55TR |
| 11. | Neosartorya fischeri | 100 | MW543008 | Nf94SR, Nf43DD, Nf72DD, Nf9SD, Nf13SD, Nf14SD, Nf16SD, Nf17SD, Nf20SD, Nf49SD, Nf105SD, Nf47DR, Nf82SR, Nf84SR, Nf88SR, Nf95SR, Nf98SR |
| 12. | Penicillium sp. | 100 | MW543009 | Pd8TR, Pd8KR |
| 13. | Penicillium charlesii | 100 | MW543010 | Pc30KD |
| 14. | Penicillium citreonigrum | 100 | MW543011 | Pc74SD, Pc104SD, Pc108SD |
| 15. | Penicillium daleae | 100 | MW543012 | Pd66SR, Pd76DD, Pd60SR |
| 16. | Penicillium janthinellum | 99.64 | MW543013 | Pj81SR, Pj27SD, Pj46SD, Pj86SD, Pj104DR, Pj37SR, Pj68SR, Pj75SR, Pj87SR, Pj90SR, Pj91SR |
| 17. | Penicillium sp. | 97.01 | MW543014 | P83SR, P102DR, P59SR, P93SR |
| 18. | Penicillium polonicum | 99.6 | MW543015 | Pp51SD, Pp52SD, Pp93SD, Pp112SD, Pp36KR |
| 19. | Penicillium rubens | 100 | MW543016 | Pr85SR, Pr80DD, Pr32TD, Pr34TD, Pr50TD, Pr108DR, |
| Pr67SR, Pr91SR | ||||
| 20. | Phomopsis sp. | 99.46 | MW543017 | P47TD |
| 21. | Pyrrhoderma noxium | 99.24 | MW543018 | Pn45SR |
| 22. | Schizophyllum commune | 99.20 | MW543019 | Sc40DD, Sc29DD |
| 23. | Talaromyces islandicus | 99.13 | MW543020 | Ti38KD |
| 24. | Talaromyces radicus | 99.49 | MW543021 | Tr35KR |
| 25. | Talaromyces purpureogenus | 100 | MW543022 | Tp71DD, Tp94TD, Tp65SR |
| 26. | Trametes cubensis | 100 | MW543023 | Tc28DD |
| 27. | Trichoderma asperelloides | 100 | MW543024 | Ta39KR |
| 28. | Trichoderma asperellum | 100 | MW543025 | Ta31KR |
| 29. | Trichoderma harzianum | 99.67 | MW543026 | Th33SR, Th20SR, Th24KD, Th36SR |
| 30. | Trichoderma yunnanense | 99.83 | MW543027 | Ty34KD |
| Species | Kampong Dadong | Total (n = 12) | Rhu Tapai | Total (n = 12) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Debris | Kernel | Soil | Tassel | Debris | Kernel | Soil | Tassel | |||
| Ascomycota | ||||||||||
| A. aculeatus | 0/3 | 0/3 | 0/3 | 2/3 | 2/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| A. flavus | 0/3 | 1/3 | 1/3 | 3/3 | 5/12 | 0/3 | 3/3 | 1/3 | 2/3 | 6/12 |
| A. niger | 0/3 | 3/3 | 0/3 | 3/3 | 6/12 | 0/3 | 3/3 | 0/3 | 2/3 | 5/12 |
| Curvularia sp. | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 | 0/3 | 0/3 | 1/3 | 0/3 | 1/12 |
| F. incarnatum | 0/3 | 2/3 | 0/3 | 1/3 | 3/12 | 0/3 | 0/3 | 0/3 | 3/3 | 3/12 |
| F. longipes | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 | 0/3 | 0/3 | 0/3 | 3/3 | 3/12 |
| F. proliferatum | 1/3 | 0/3 | 0/3 | 0/3 | 1/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| F. verticillioides | 2/3 | 3/3 | 2/3 | 3/3 | 10/12 | 2/3 | 3/3 | 3/3 | 2/3 | 10/12 |
| L. theobromae | 1/3 | 3/3 | 2/3 | 3/3 | 9/12 | 1/3 | 3/3 | 2/3 | 3/3 | 9/12 |
| N. fischeri | 2/3 | 0/3 | 3/3 | 0/3 | 5/12 | 1/3 | 0/3 | 3/3 | 0/3 | 4/12 |
| Penicillium sp. | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 | 1/3 | 0/3 | 3/3 | 0/3 | 4/12 |
| Penicillium sp. | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 | 0/3 | 3/3 | 0/3 | 1/3 | 4/12 |
| P. charlesii | 0/3 | 3/3 | 0/3 | 0/3 | 3/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| P. citreonigrum | 0/3 | 0/3 | 2/3 | 0/3 | 2/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| P. daleae | 1/3 | 0/3 | 0/3 | 0/3 | 1/12 | 0/3 | 0/3 | 1/3 | 0/3 | 1/12 |
| P. janthinellum | 0/3 | 0/3 | 2/3 | 0/3 | 2/12 | 1/3 | 0/3 | 3/3 | 0/3 | 4/12 |
| P. polonicum | 0/3 | 0/3 | 3/3 | 0/3 | 3/12 | 0/3 | 3/3 | 0/3 | 0/3 | 3/12 |
| P. rubens | 1/3 | 0/3 | 0/3 | 3/3 | 4/12 | 1/3 | 0/3 | 3/3 | 0/3 | 4/12 |
| Phomopsis sp. | 0/3 | 2/3 | 0/3 | 0/3 | 2/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| T. islandicus | 0/3 | 1/3 | 0/3 | 0/3 | 1/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| T. radicus | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 | 0/3 | 1/3 | 0/3 | 0/3 | 1/12 |
| T. purpureogenus | 1/3 | 0/3 | 0/3 | 3/3 | 2/12 | 0/3 | 0/3 | 1/3 | 0/3 | 1/12 |
| T. asperelloides | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 | 0/3 | 1/3 | 0/3 | 0/3 | 1/12 |
| T. asperellum | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 | 0/3 | 1/3 | 0/3 | 0/3 | 1/12 |
| T. harzianum | 0/3 | 1/3 | 0/3 | 0/3 | 1/12 | 0/3 | 0/3 | 2/3 | 0/3 | 2/12 |
| T. yunnanense | 0/3 | 1/3 | 0/3 | 0/3 | 1/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| Basidiomycota | ||||||||||
| B. adusta | 1/3 | 0/3 | 0/3 | 0/3 | 1/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| P. noxium | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 | 0/3 | 0/3 | 1/3 | 0/3 | 1/12 |
| S. commune | 1/3 | 0/3 | 0/3 | 0/3 | 1/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| T. cubensis | 1/3 | 0/3 | 0/3 | 0/3 | 1/12 | 0/3 | 0/3 | 0/3 | 0/3 | 0/12 |
| Species | Farm | Sample | Mycotoxin (µg/g) | ||
|---|---|---|---|---|---|
| AFB1 | AFB2 | OTA | |||
| A. flavus | KD | Kernel | n.d. | n.d. | - |
| A. flavus | KD | Soil | 0.08 ± 0.002 | n.d. | - |
| A. flavus | KD | Tassel | n.d. | n.d. | - |
| A. flavus | RT | Kernel | 0.13 ± 0.01 | n.d. | - |
| A. flavus | RT | Kernel | 0.24 ± 0.04 | 0.01 ± 0.0003 | - |
| A. flavus | RT | Soil | n.d. | n.d. | - |
| A. flavus | RT | Tassel | n.d. | n.d. | - |
| A. niger | KD | Kernel | - | - | 32.60 ± 2.40 |
| A. niger | KD | Kernel | - | - | 23.92 ± 0.31 |
| A. niger | KD | Tassel | - | - | 32.48 ± 5.81 |
| A. niger | RT | Kernel | - | - | 21.87 ± 6.13 |
| A. niger | RT | Tassel | - | - | 4.08 ± 0.43 |
| Species | Farm | Sample | Mycotoxin (µg/g) | ||||
|---|---|---|---|---|---|---|---|
| FB1 | FB2 | H-T2 | T-2 | ZEN | |||
| F. incarnatum | KD | Tassel | n.d. | n.d. | n.d. | n.d. | 68,550.30 ± 16,523.75 |
| F. incarnatum | KD | Kernel | n.d. | n.d. | 631.82 ± 74.77 | 434.07 ± 171.36 | 29,717.31 ± 3265.67 |
| F. incarnatum | RT | Tassel | n.d. | n.d. | n.d. | n.d. | 36,116.14 ± 4745.50 |
| F. proliferatum | KD | Debris | 6727.7 ± 1089.80 | 2021.70 ± 326.06 | n.d. | n.d. | n.d. |
| F. verticillioides | KD | Debris | 7536.12 ± 1293.65 | 1009.07 ± 186.21 | n.d. | n.d. | n.d. |
| F. verticillioides | KD | Debris | 3894.67 ± 3444.56 | 451.55 ± 379.01 | n.d. | n.d. | n.d. |
| F. verticillioides | KD | Kernel | 9507.97 ± 1992.10 | 2657.21 ± 485.01 | n.d. | n.d. | n.d. |
| F. verticillioides | KD | Kernel | 8863.63 ± 269.95 | 2030.13 ± 42.69 | n.d. | n.d. | n.d. |
| F. verticillioides | KD | Kernel | 1384.79 ± 332.01 | 496.49 ± 65.76 | n.d. | n.d. | n.d. |
| F. verticillioides | KD | Soil | n.d. | n.d. | n.d. | n.d. | n.d. |
| F. verticillioides | KD | Tassel | 7775.18 ± 1496.84 | 1848.78 ± 326.99 | n.d. | n.d. | n.d. |
| F. verticillioides | KD | Tassel | 5705.14 ± 823.33 | 756.18 ± 102.55 | n.d. | n.d. | n.d. |
| F. verticillioides | RT | Debris | 1118.18 ± 87.13 | 646.61 ± 134.54 | n.d. | n.d. | n.d. |
| F. verticillioides | RT | Kernel | 4471.13 ± 787.72 | 1908.59 ± 292.91 | n.d. | n.d. | n.d. |
| F. verticillioides | RT | Soil | 4109.80 ± 1666.75 | 914.40 ± 168.17 | n.d. | n.d. | n.d. |
| F. verticillioides | RT | Soil | 10,087.77 ± 1190.38 | 1624.13 ± 194.24 | n.d. | n.d. | n.d. |
| F. verticillioides | RT | Soil | 3125.12 ± 391.60 | 608.03 ± 47.61 | n.d. | n.d. | 2601.86 ± 466.02 |
| F. verticillioides | RT | Tassel | 2512.25 ± 753.22 | 298.91 ± 52.40 | n.d. | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazid, S.N.E.; Ng, W.J.; Selamat, J.; Ismail, S.I.; Samsudin, N.I.P. Diversity and Toxigenicity of Mycobiota in Grain Corn: A Case Study at Pioneer Grain Corn Plantations in Terengganu, Malaysia. Agriculture 2021, 11, 237. https://doi.org/10.3390/agriculture11030237
Yazid SNE, Ng WJ, Selamat J, Ismail SI, Samsudin NIP. Diversity and Toxigenicity of Mycobiota in Grain Corn: A Case Study at Pioneer Grain Corn Plantations in Terengganu, Malaysia. Agriculture. 2021; 11(3):237. https://doi.org/10.3390/agriculture11030237
Chicago/Turabian StyleYazid, Siti Nur Ezzati, Wan Jing Ng, Jinap Selamat, Siti Izera Ismail, and Nik Iskandar Putra Samsudin. 2021. "Diversity and Toxigenicity of Mycobiota in Grain Corn: A Case Study at Pioneer Grain Corn Plantations in Terengganu, Malaysia" Agriculture 11, no. 3: 237. https://doi.org/10.3390/agriculture11030237
APA StyleYazid, S. N. E., Ng, W. J., Selamat, J., Ismail, S. I., & Samsudin, N. I. P. (2021). Diversity and Toxigenicity of Mycobiota in Grain Corn: A Case Study at Pioneer Grain Corn Plantations in Terengganu, Malaysia. Agriculture, 11(3), 237. https://doi.org/10.3390/agriculture11030237