Biostimulants Managed Fungal Phytopathogens and Enhanced Activity of Beneficial Microorganisms in Rhizosphere of Scorzonera (Scorzonera hispanica L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Mycological Analysis of Plants
2.3. Microbiological Analysis of Rhizosphere Soil
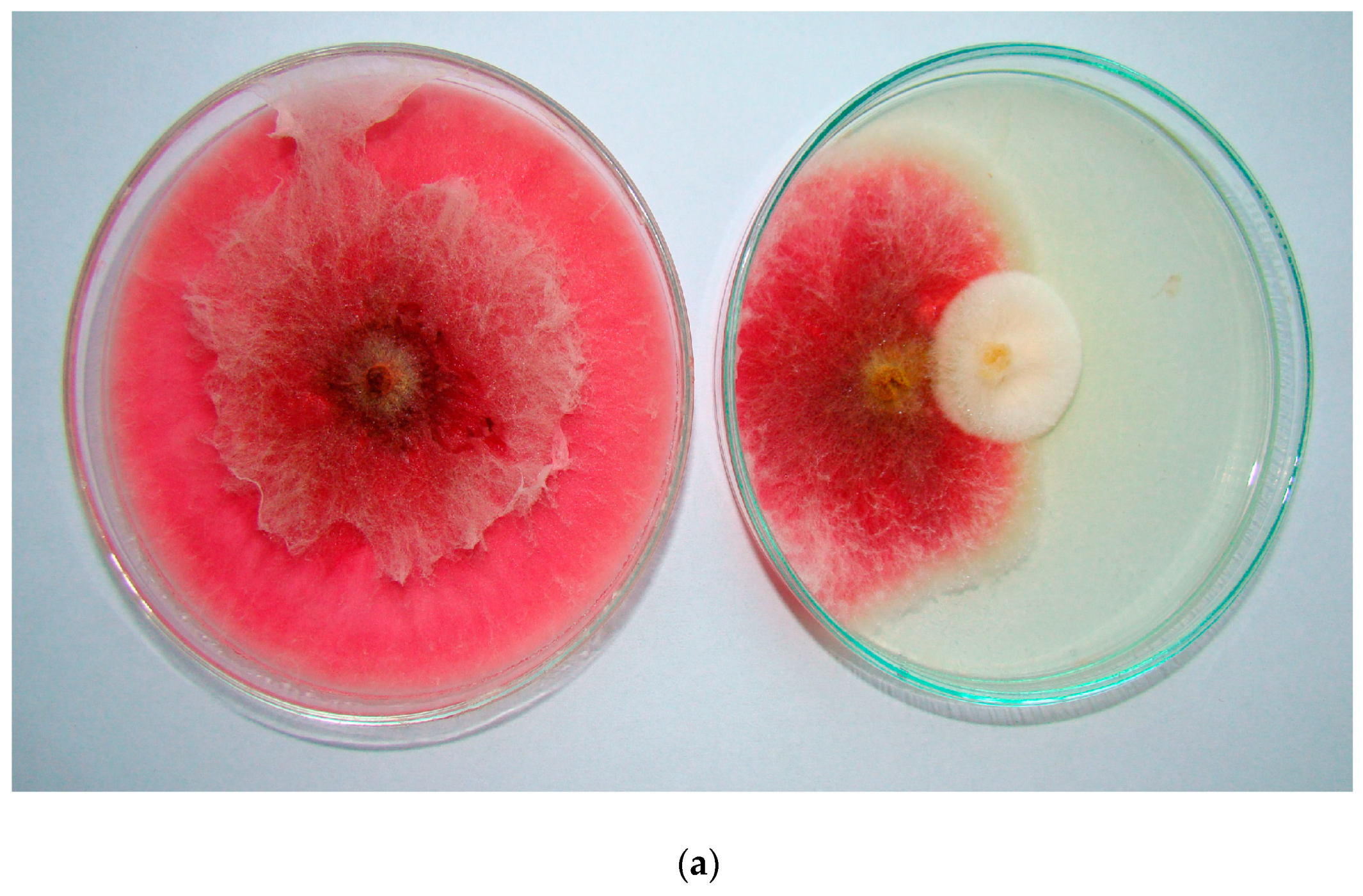
2.4. Antagonistic Activity of Rhizosphere Fungi from Scorzonera Cultivation
2.5. Antagonistic Activity of Pseudomonas sp. and Bacillus sp. Rhizobacteria from Scorzonera Cultivation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayer, Z.; Sasvári, Z.; Szentpéteri, V.; Rétháti, B.P.; Vajna, B.; Posta, K. Effect of Long-Term Cropping Systems on the Diversity of the Soil Bacterial Communities. Agronomy 2019, 9, 878. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef] [Green Version]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Sanderlin, J.S.; Reeves, J.H.; Jenkins, M.B.; Endale, D.M.; Coleman, D.C.; Whitman, W.B. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Sommermann, L.; Geistlinger, J.; Wibberg, D.; Deubel, A.; Zwanzig, J.; Babin, D.; Schlüter, A.; Schellenberg, I. Fungal community profiles in agricultural soils of a long-term field trial under different tillage, fertilization and crop rotation conditions analyzed by high-throughput ITS-amplicon sequencing. PLoS ONE 2018, 13, e0195345. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Patkowska, E.; Mielniczuk, E.; Jamiołkowska, A.; Skwaryło-Bednarz, B.; BłaŻewicz-Woźniak, M. The Influence of Trichoderma harzianum Rifai T-22 and Other Biostimulants on Rhizosphere Beneficial Microorganisms of Carrot. Agronomy 2020, 10, 1637. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Preparaty Biotechniczne i Biologiczne w Ochronie Papryki Słodkiej (Capsicum Annuum L.) Przed Grzybami Chorobotwórczymi i Indukowaniu Reakcji Obronnych Roślin, 1st ed.; Rozprawy Naukowe UP w Lublinie; University of Life Sciences in Lublin: Lublin, Poland, 2013; Volume 379, p. 117. Available online: https://www.up.lublin.pl/5490/ (accessed on 20 February 2021).
- Patkowska, E. The effect of biopreparations on the formation of rhizosphere microorganism populations of soybean (Glycine max (L.) Merrill). Acta Sci. Pol. Hortorum Cultus 2005, 4, 89–99. [Google Scholar]
- Lupatini, M.; Korthals, G.W.; De Hollander, M.; Janssens, T.K.S.; Kuramae, E.E. Soil Microbiome Is More Heterogeneous in Organic Than in Conventional Farming System. Front. Microbiol. 2017, 7, 2064. [Google Scholar] [CrossRef] [Green Version]
- Konopiński, M. Wpływ Zróżnicowanych Systemów Uprawy na Kształtowanie Warunków Wzrostu, Plonowanie i Wartość Biologiczną Skorzonery (Scorzonera Hispanica L.), 1st ed.; Rozprawy Naukowe Akademii Rolniczej w Lublinie, University of Life Sciences in Lublin: Lublin, Poland, 2003; Volume 271, p. 93. Available online: https://publikacje.up.lublin.pl (accessed on 20 February 2021).
- Petkova, N. Characterization of Inulin from Black Salsify (Scorzonera Hispanica L.) for Food and Pharmaceutical Purposes. Asian J. Pharm. Clin. Res. 2018, 11, 221–225. [Google Scholar] [CrossRef]
- Unal, O.; Göktürk, R.S. A new species of Scorzonera L. (Asteraceae) from south Anatolia, Turkey. Bot. J. Linn. Soc. 2003, 142, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Erden, Y.; Kırbağ, S.; Yılmaz, Ö. Phytochemical Composition and Antioxidant Activity of Some Scorzonera Species. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2012, 83, 271–276. [Google Scholar] [CrossRef]
- Causey, J.L.; Feirtag, J.M.; Gallaher, D.D.; Tungland, B.C.; Slavin, J.L. Effects of dietary inulin on serum lipids, blood glucose and the gastrointestinal environment in hypercholesterolemic men. Nutr. Res. 2000, 20, 191–201. [Google Scholar] [CrossRef]
- Konopiński, M. Influence of Intercrop Plants and Varied Tillage on Yields and Nutritional Value of Scorzonera (Scorzonera Hispanica L.) roots. Acta Sci. Pol. Hortorum Cultus 2011, 10, 49–59. Available online: http://www.hortorumcultus.actapol.net/volume10/issue1/10_1_49.pdf (accessed on 20 February 2021).
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental Factors Affecting the Mineralization of Crop Residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Patkowska, E.; Konopiński, M. Harmfulness of Soil-Borne Fungi towards Root Chicory (Cichorium intybus L. var. sativum Bisch.) Cultivated with the Use of Cover Crops. Acta Sci. Pol. Hortorum Cultus 2013, 12, 3–18. Available online: http://www.hortorumcultus.actapol.net/volume12/issue4/12_4_3.pdf (accessed on 20 February 2021).
- Patkowska, E.; Konopiński, M. Cover Crops and Soil-Borne Fungi Dangerous towards the Cultivation of Salsify (Tragopogon porrifolius var. sativus (Gaterau) Br.). Acta Sci. Pol. Hortorum Cultus 2011, 10, 167–181. Available online: http://www.acta.media.pl (accessed on 20 February 2021).
- Loerakker, W.M. A rare leaf spot disease of Scorzonera hispanica, caused by Alternaria scorzonerae (Aderhold) Comb. Nov. Eur. J. Plant Pathol. 1984, 90, 35–39. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Thines, M. Host Jumps and Radiation, Not Co-Divergence Drives Diversification of Obligate Pathogens. A Case Study in Downy Mildews and Asteraceae. PLoS ONE 2015, 10, e0133655. [Google Scholar] [CrossRef] [PubMed]
- Patkowska, E.; Konopiński, M. Pathogenicity of selected soil-borne microorganisms for scorzonera seedlings (Scorzonera hispanica L.). Folia Hortic. 2008, 20, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Patkowska, E.; Konopiński, M. Fungi threatening scorzonera (Scorzonera hispanica L.) cultivation using plant mulches. Acta Sci. Pol. Hortorum Cultus 2013, 12, 215–225. [Google Scholar]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.M.D.; Babar, M.D.A. Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PLoS ONE 2020, 15, e0231426. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General Principles to Justify Plant Biostimulant Claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-Treated Seedlings under Sustainable Agriculture: A Global Perspective Facing Climate Change. Agronomy 2020, 11, 14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Le Mire, G.; Nguyen, M.L.; Fassotte, B.; du Jardin, P.; Verheggen, F.; Delaplace, P.; Jijakli, M.H. Implementing plant biostimulants and biocontrol strategies in the agroecological management of cultivated ecosystems. A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 299–313. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Seema, K.; Mehta, K.; Singh, N. Studies on the Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Growth, Physiological Parameters, Yield and Fruit Quality of Strawberry cv. Chandler. J. Pharmacog. Phytochem. 2018, 7, 383–387. Available online: https://www.phytojournal.com (accessed on 20 February 2021).
- Gaiero, J.R.; McCall, C.A.; Thompson, K.A.; Day, N.J.; Best, A.S.; Dunfield, K.E. Inside the root microbiome: Bacterial root endophytes and plant growth promotion. Am. J. Bot. 2013, 100, 1738–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robledo-Buriticá, J.; Aristizábal-Loaiza, J.C.; Ceballos-Aguirre, N.; Cabra-Cendales, T. Influence of plant growth-promoting rhizobacteria (PGPR) on blackberry (Rubus glaucus Benth. cv. Thornless) growth under semi-cover and field conditions. Acta Agron. 2018, 67, 258–263. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Usuki, F.; Narisawa, K. A mutualistic symbiosis between a dark septate endophytic fungus, Heteroconium chaetospira, and a nonmycorrhizal plant, Chinese cabbage. Mycologia 2007, 99, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Jamiołkowska, A.; Skwaryło-Bednarz, B.; Patkowska, E.; Buczkowska, H.; Gałązka, A.; Grządziel, J.; Kopacki, M. Effect of Mycorrhizal Inoculation and Irrigation on Biological Properties of Sweet Pepper Rhizosphere in Organic Field Cultivation. Agronomy 2020, 10, 1693. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Belachew, S.T.; Yoon, E.; Chun, S.C. Expression of β-1,3-glucanase (GLU) and phenylalanine ammonia-lyase (PAL) genes and their enzymes in tomato plants induced after treatment with Bacillus subtilis CBR05 against Xanthomonas campestris pv. vesicatoria. J. Gen. Plant Pathol. 2016, 83, 7–13. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Abbo, A.Z.; Idris, M.O.; Hammad, A.M. The Antifungal Effects of Four Tomato Rhizosphere Bacillus spp. against Alternaria alternata. Inter. J. Sci. Res. 2014, 3, 1324–1328. [Google Scholar]
- Ma, G.-Z. Purification and characterization of chitinase from Gliocladium catenulatum strain HL-1-1. Afr. J. Microbiol. Res. 2012, 6, 4377–4383. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Chet, I.; Harman, G.E.; Baker, R. Trichoderma hamatum: Its hyphal interactions with Rhizoctonia solani and Pythium spp. Microb. Ecol. 1981, 7, 29–38. [Google Scholar] [CrossRef]
- Sivan, A.; Chet, I. Degradation of Fungal Cell Walls by Lytic Enzymes of Trichoderma harzianum. Microbiology 1989, 135, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Sonkar, P.; Pritam, M. Dynamics of Trichoderma spp. against Fusarium wilt based on in vitro and in silico. J. Biopest. 2020, 13, 97–102. [Google Scholar]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef] [Green Version]
- Jamiołkowska, A.; Hetman, B. Mechanizm działania preparatów biologicznych stosowanych w ochronie roślin przed patogenami. Ann. UMCS Sectio E Agric. 2016, 71, 13–29. [Google Scholar]
- Reuveni, M.; Sanches, E.; Barbier, M. Curative and Suppressive Activities of Essential Tea Tree Oil against Fungal Plant Pathogens. Agronomy 2020, 10, 609. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Rudbäck, J.; Bergström, M.A.; Börje, A.; Nilsson, U.; Karlberg, A.-T. α-Terpinene, an Antioxidant in Tea Tree Oil, Autoxidizes Rapidly to Skin Allergens on Air Exposure. Chem. Res. Toxicol. 2012, 25, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Terzi, V.; Morcia, C.; Faccioli, P.; Valè, G.; Tacconi, G.; Malnati, M. In vitro antifungal activity of the tea tree (Melaleuca alternifolia) essential oil and its major components against plant pathogens. Lett. Appl. Microbiol. 2007, 44, 613–618. [Google Scholar] [CrossRef]
- KhangLE, T.; HuongNguyen, T.; TienLE, T. Antifungal Activity of Tea Tree Essential Oils (Melaleuca Alternifolia) Against Phytopathogenic Fungi. Int. J. Adv. Res. 2019, 7, 1239–1248. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Céliz, G.; Daz, M.; Audisio, M. Antibacterial activity of naringin derivatives against pathogenic strains. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Pięta, D.; Patkowska, E.; Pastucha, A. Oddziaływanie biopreparatów na wzrost i rozwój niektórych grzybów chorobotwórczych dla roślin motylkowatych. Acta Sci. Pol. Hortorum Cultus 2004, 3, 117–177. [Google Scholar]
- Kurzawińska, H.; Mazur, S. The application of some biotechnical preparations in potato protection against Phytophthora infestans. Phytopathology 2012, 63, 31–37. [Google Scholar]
- Orlikowski, L.B.; Skrzypczak, C. Biocides in the control of soil-borne and leaf pathogens. Hortic. Veget. Grow. 2003, 22, 426–433. [Google Scholar]
- Patkowska, E. Effect of Miedzian 50 WP and grapefruit extract on the healthiness and communities of soil microorganisms of pea (Pisum sativum L.). Acta Sci. Pol. Hortorum Cultus 2014, 13, 23–33. [Google Scholar]
- Patkowska, E. Effectiveness of grapefruit extract and Pythium oligandrum in the control of bean and peas pathogens. J. Plant Protect. Res. 2006, 46, 15–28. [Google Scholar]
- Patkowska, E.; Krawiec, M. Yielding and healthiness of pea cv. ‘Sześciotygodniowy TOR’ after applying biotechnical preparations. Acta Sci. Pol. Hortorum Cultus 2016, 15, 143–156. [Google Scholar]
- Czaban, J.; Gajda, A.; Wróblewska, B. The mobility of bacteria from rhizosphere and different zones of winter wheat roots. Pol. J. Environ. Stud. 2007, 16, 301–308. [Google Scholar]
- Patkowska, E. Effect of bio-products on bean yield and bacterial and fungal communities in the rhizosphere and non-rhizosphere. Pol. J. Environ. Stud. 2009, 18, 255–263. [Google Scholar]
- Jamiołkowska, A.; Skwaryło-Bednarz, B.; Patkowska, E. Morphological Identity and Population Structure of Hemibiotrophic Fungus Colletotrichum Coccodes Colonizing Pepper Plants. Acta Sci. Pol. Hortorum Cultus 2018, 17, 181–192. [Google Scholar] [CrossRef]
- Patkowska, E.; Błażewicz-Woźniak, M. The microorganisms communities in the soil under the cultivation of carrot (Daucus carota L.). Acta Sci. Pol. Hortorum Cultus 2014, 13, 103–115. [Google Scholar]
- Patkowska, E. The Effect of Biopreparations on the Healthiness of Soybean Cultivated in a Growth Chamber Experiment. Electr. J. Pol. Agric. Univer. Hortic. 2005, 8, 8. Available online: http://www.ejpau.media.pl/volume8/issue4/art.-08.html (accessed on 20 February 2021).
- Pięta, D.; Patkowska, E.; Pastucha, A. The protective effect of biopreparations applied as the dressing for common bean (Phaseolus vulgaris L.) and pea (Pisum sativum L.). Acta Sci. Pol. Hortorum Cultus 2005, 4, 59–67. [Google Scholar]
- Ferrigo, D.; Raiola, A.; Picollo, E.; Scopel, C.; Causin, R. Trichoderma harzianum T22 induces in maize systemic resistance against Fusarium verticillioides. J. Plant. Pathol. 2014, 96, 133–142. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Bio/Technol. 2019, 18, 597–616. [Google Scholar] [CrossRef] [Green Version]
- Wojtkowiak-Gębarowska, E. Mechanizmy zwalczania fitopatogenów glebowych przez grzyby z rodzaju Trichoderma. Post. Mikrobiol. 2006, 45, 261–273. [Google Scholar]
- Strakowska, J.; Błaszczyk, L.; Chełkowski, J. The significance of cellulolytic enzymes produced by Trichoderma in opportunistic lifestyle of this fungus. J. Basic Microbiol. 2014, 54, S2–S13. [Google Scholar] [CrossRef]
- Poole, P.R.; Ward, B.G.; Whitaker, G. The effects of topical treatments with 6-pentyl-2-pyrone and structural analogues on stem end postharvest rots in kiwifruit due to Botrytis cinerea. J. Sci. Food Agric. 1998, 77, 81–86. [Google Scholar] [CrossRef]
- Tarus, P.; Lang’At-Thoruwa, C.; Wanyonyi, A.; Chhabra, S. Bioactive metabolites from Trichoderma harzianum and Trichoderma longibrachiatum. Bull. Chem. Soc. Ethiop. 2004, 17, 185–190. [Google Scholar] [CrossRef] [Green Version]
- El-Hasan, A.; Buchenauer, H. Actions of 6-Pentyl-alpha-pyrone in Controlling Seedling Blight Incited by Fusarium moniliforme and Inducing Defence Responses in Maize. J. Phytopathol. 2009, 157, 697–707. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.; Marra, R.; Barbetti, M.; Li, H.; Woo, S.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Harel, Y.M.; Kolton, M.; Elad, Y.; Rav-david, D.; Cytryn, E.; Borenstein, M.; Shulchani, R.; Graber, E.R. Induced systemic resistance in strawberry (Fragaria ananassa) to powdery mildew using various control agents. IOBC/Wprs. Bull. 2011, 71, 47–51. [Google Scholar]
- Sas-Paszt, L.; Sumorok, B.; Derkowska, E.; Trzciński, P.; Lisek, A.; Grzyb, Z.S.; Sitarek, M.; Przybył, M.; Frąc, M. Effect of microbiologically enriched fertilizers on the vegetative growth of strawberry plants under field conditions in the first year of plantation. J. Res. Appl. Agric. Engin. 2019, 64, 29–37. [Google Scholar]
- Esitken, A.; Yildiz, H.E.; Ercisli, S.; Donmez, M.F.; Turan, M.; Gunes, A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci. Hortic. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- De Silva, A.; Patterson, K.; Rothrock, C.; Moore, J. Growth Promotion of Highbush Blueberry by Fungal and Bacterial Inoculants. HortScience 2000, 35, 1228–1230. [Google Scholar] [CrossRef] [Green Version]
- Smolińska, U.; Kowalska, B. Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum—A review. J. Plant Pathol. 2018, 100, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Chang, M.P.P.; Gao, M.L.L.; Wang, X. The Endophytic Fungus Albifimbria verrucaria from Wild Grape as an Antagonist of Botrytis cinerea and Other Grape Pathogens. Phytopathology 2020, 110, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jha, D.K. Trichothecium roseum: A potential agent for the biological control of soybean rust. Ind. Phytopathol. 2002, 55, 232–234. [Google Scholar]
- Hinarejos, E.; Castellano, M.; Rodrigo, I.; Bellés, J.M.; Conejero, V.; López-Gresa, M.P.; Lisón, P. Bacillus subtilis IAB/BS03 as a potential biological control agent. Eur. J. Plant Pathol. 2016, 146, 597–608. [Google Scholar] [CrossRef]
- Goel, A.; Sindhu, S.; Dadarwal, K. Pigment Diverse Mutants of Pseudomonas sp.: Inhibition of Fungal Growth and Stimulation of Growth of Cicer arietinum. Biol. Plant. 2000, 43, 563–569. [Google Scholar] [CrossRef]
- Srivastava, R.; Shalini, R. Antifungal activity of Pseudomonas fluorescens against different plant pathogenic fungi. Int. J. Microbiol. 2008, 7, 2789–2796. [Google Scholar]
- Chlebek, D.; Pinski, A.; Żur, J.; Michalska, J.; Hupert-Kocurek, K. Genome Mining and Evaluation of the Biocontrol Potential of Pseudomonas fluorescens BRZ63, a New Endophyte of Oilseed Rape (Brassica napus L.) against Fungal Pathogens. Int. J. Mol. Sci. 2020, 21, 8740. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Cheng, S.; Wang, H.; Yu, D.; Mungai, C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 2013, 114, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Reuveni, M.; Neifeld, D.; Dayan, D.; Kotzer, Y. BM-608—A Novel Organic Product Based on Essential Tea Tree Oil for the Control of Fungal Diseases in Tomato. Acta Hortic. 2009, 808, 129–132. [Google Scholar] [CrossRef]
- Woedtke, T.; Schluter, B.; Pflegel, P.; Lindequist, U.; Julich, W.D. Aspects of the antimicrobial efficacy of grapefruit seed extract and its selection to preservative substances contained. Pharmazie 1999, 54, 452–456. [Google Scholar] [PubMed]








| Experimental Treatment | Field Stand Per 1 m2 | |||
|---|---|---|---|---|
| 2014 | 2015 | 2016 | Mean | |
| Biosept Active | 38.5 b | 47.0 b | 46.5 b | 44.0 b |
| Timorex Gold 24 EC | 36.0 b | 44.5 b | 43.0 b | 41.2 b |
| Trianum P | 48.0 a | 52.5 a | 56.0 a | 52.2 a |
| Zaprawa Nasienna T 75 DS/WS | 40.5 b | 50.0 a | 49.0 a | 46.5 b |
| Control | 30.0 c | 38.5 b | 28.5 c | 32.3 c |
| Fungi | Number of Isolates/Experimental Treatment | |||||
|---|---|---|---|---|---|---|
| I * | II | III | IV | V | Total (%) | |
| Acremonium rutilum W. Gams | 5 | 7 | 3 | 4 | 9 | 28 (2.2) |
| Alternaria alternata (Fr.) Keissler | 17 | 21 | 8 | 11 | 27 | 84 (6.6) |
| Alternaria scorzonerae (Aderh.) Loer. | 22 | 28 | 10 | 15 | 36 | 111 (8.6) |
| Botrytis cinerea Pers. | 6 | 9 | 2 | 4 | 11 | 32 (2.5) |
| Cladosporium herbarum (Pers.) Link | 7 | 9 | 3 | 5 | 12 | 36 (2.8) |
| Cylindrocarpon didymum (Harting) Wollenw. | 10 | 12 | 4 | 7 | 16 | 49 (3.8) |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert | 2 | 2 | 3 | - | - | 7 (0.5) |
| Fusarium culmorum (W.G.Sm.) Sacc. | 23 | 29 | 14 | 18 | 36 | 120 (9.3) |
| Fusarium oxysporum Schl. | 46 | 54 | 32 | 38 | 74 | 244 (18.9) |
| Fusarium graminearum Schwabe | 7 | 9 | 4 | 5 | 10 | 35 (2.7) |
| Neocosmospora solani (Mart.) L. Lombard and Crous | 16 | 21 | 6 | 10 | 27 | 80 (6.3) |
| Hyalocylindrophora rosea (Petch) Réblová and W. Gams | 9 | 11 | 4 | 6 | 14 | 44 (3.4) |
| Penicillium janczewskii K.W. Zaleski | 12 | 14 | 6 | 8 | 18 | 58 (4.3) |
| Penicillium simplicissimum (Oudem.) Thom | 7 | 9 | 3 | 5 | 10 | 34 (2.6) |
| Penicillium verrucosum Dierckx | 6 | 8 | 4 | 5 | 10 | 33 (2.6) |
| Rhizoctonia solani J.G. Kühn | 37 | 42 | 27 | 31 | 57 | 194 (15.1) |
| Talaromyces flavus (Klöcker) Stolk and Samson | 12 | 15 | 6 | 9 | 19 | 61 (4.8) |
| Trichoderma sp. | 12 | 12 | 15 | - | - | 39 (3.0) |
| Total isolates | 256 | 312 | 154 | 181 | 386 | 1289 (100) |
| Fungi | Number of Isolates/Experimental Treatment | |||||
|---|---|---|---|---|---|---|
| I * | II | III | IV | V | Total (%) | |
| Alternaria alternata (Fr.) Keissler | 9 | 11 | 5 | 7 | 13 | 45 (2.7) |
| Alternaria scorzonerae (Aderh.) Loer. | 26 | 32 | 13 | 21 | 40 | 132 (7.0) |
| Aureobasidium pullulans (de Bary and Löwenthal) G. Arnaud | 6 | 8 | 2 | 4 | 10 | 30 (1.8) |
| Botrytis cinerea Pers. | 8 | 11 | 3 | 4 | 16 | 42 (2.5) |
| Cladosporium cladosporioides (Fresen.) G.A. de Vries | 11 | 15 | 3 | 6 | 21 | 56 (3.4) |
| Cylindrocarpon didymum (Harting) Wollenw. | 7 | 10 | 3 | 5 | 12 | 37 (2.2) |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert | 4 | 3 | 5 | - | - | 12 (0.7) |
| Fusarium culmorum (W.G.Sm.) Sacc. | 27 | 32 | 17 | 22 | 38 | 136 (8.2) |
| Fusarium oxysporum Schl. | 59 | 71 | 41 | 49 | 92 | 312 (18.8) |
| Neocosmospora solani (Mart.) L. Lombard and Crous | 9 | 11 | 4 | 6 | 15 | 45 (2.7) |
| Penicillium aurantiogriseum Dierckx | 15 | 18 | 8 | 11 | 25 | 77 (4.7) |
| Penicillium canescens Sopp | 11 | 13 | 5 | 8 | 16 | 53 (3.2) |
| Rhizoctonia solani J.G. Kühn | 38 | 46 | 24 | 31 | 60 | 199 (11.9) |
| Rhizopus stolonifer (Ehrenb.) Vuill., | 6 | 10 | 1 | 2 | 14 | 33 (2.0) |
| Sclerotinia sclerotiorum (Lib.) de Bary | 68 | 79 | 50 | 57 | 102 | 356 (21.4) |
| Trichoderma sp. | 15 | 13 | 15 | - | - | 43 (2.5) |
| Trichothecium roseum (Pers.) Link | 10 | 15 | 4 | 6 | 21 | 56 (3.4) |
| Total isolates | 329 | 398 | 203 | 239 | 495 | 1664 (100) |
| Experimental Treatment | Total CFU of Fungi (103/g Soil DW) | Total CFU of Bacteria (106/g Soil DW) | CFU of Pseudomonas sp. (106/g Soil DW) | CFU of Bacillus sp. (106/g Soil DW) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | Mean | 2014 | 2015 | 2016 | Mean | 2014 | 2015 | 2016 | Mean | 2014 | 2015 | 2016 | Mean | |
| Biosept Active | 6.38 b | 5.92 b | 6.64 b | 6.31 b | 5.93 b | 4.26 b | 5.30 b | 5.16 b | 1.16 b | 1.90 a | 1.86 b | 1.64 bc | 2.14 b | 2.00 b | 3.06 b | 2.40 b |
| Timorex Gold 24 EC | 6.84 b | 6.04 b | 6.92 b | 6.60 b | 5.22 b | 4.12 b | 5.12 b | 4.82 b | 1.12 b | 1.82 a | 1.55 b | 1.50 c | 1.95 b | 1.88 b | 2.85 b | 2.22 b |
| Trianum P | 4.25 c | 3.16 c | 4.06 c | 3.82 c | 8.02 a | 7.45 a | 8.32 a | 7.93 a | 2.94 a | 2.46 a | 3.00 a | 2.80 a | 4.15 a | 3.92 a | 4.91 a | 4.32 a |
| Zaprawa Nasienna T 75 DS/WS | 4.34 c | 3.28 c | 4.15 c | 3.92 c | 8.14 a | 7.68 a | 8.54 a | 8.12 a | 3.05 a | 2.66 a | 3.14 a | 2.95 a | 4.38 a | 3.96 a | 5.32 a | 4.55 a |
| Control | 9.55 a | 8.82 a | 9.76 a | 9.38 a | 2.68 c | 2.05 c | 2.35 c | 2.36 c | 0.94 b | 0.14 b | 0,50 c | 0.53d | 0.62 c | 0.28 c | 0.53 c | 0.48 c |
| Fungi | Number of Isolates/Experimental Treatment | |||||
|---|---|---|---|---|---|---|
| I * | II | III | IV | V | Total (%) | |
| Acremonium rutilum W. Gams | 1 | 3 | 3 | 2 | 5 | 14 (1.4) |
| Albifimbria verrucaria (Alb. and Schwein.) L. Lombard and Crous | 7 | 13 | 16 | 4 | 1 | 41 (4.3) |
| Alternaria alternata (Fr.) Keissler | 1 | 2 | 1 | - | 3 | 7 (0.7) |
| Alternaria scorzonerae (Aderh.) Loer. | 3 | 4 | 1 | 3 | 7 | 18 (1.9) |
| Arthrinium phaeospermum (Corda) M.B. Ellis | - | - | - | - | 4 | 4 (0.4) |
| Aspergillus fumigatus Fresen. | 2 | 4 | - | - | 8 | 14 (1.4) |
| Botrytis cinerea Pers. | 1 | 2 | - | - | 5 | 8 (0.8) |
| Chaetomium piluliferum J. Daniels | 6 | 10 | 3 | 4 | 15 | 38 (4.0) |
| Cladosporium cladosporioides (Fresen.) G.A. de Vries | - | 2 | - | - | 5 | 7 (0.7) |
| Cladosporium herbarum (Pers.) Link | 7 | 11 | 2 | 4 | 16 | 40 (4.2) |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert | 4 | 12 | 16 | 3 | 1 | 36 (3.8) |
| Dipodascus geotrichum (E.E. Butler and L.J. Petersen) Arx | 1 | 3 | - | - | 7 | 11 (1.2) |
| Fusarium avenaceum (Fr.) Sacc. | - | 2 | - | - | 4 | 6 (0.6) |
| Fusarium culmorum (W.G.Sm.) Sacc. | 8 | 9 | 4 | 6 | 12 | 39 (4.1) |
| Fusarium graminearum Schwabe | - | 1 | - | - | 3 | 4 (0.4) |
| Fusarium oxysporum Schl. | 37 | 43 | 25 | 31 | 66 | 202 (21.0) |
| Mucor hiemalis Wehmer | - | 5 | - | 4 | 14 | 23 (2.4) |
| Neocosmospora solani (Mart.) L. Lombard and Crous | 1 | 4 | - | 1 | 9 | 15 (1.6) |
| Penicillium chermesinum Biourge | 6 | 14 | 11 | 8 | 8 | 47 (4.9) |
| Penicillium decumbens Thom | 5 | 15 | 11 | 6 | 3 | 40 (4.2) |
| Penicillium simplicissimum (Oudem.) Thom | - | 4 | 3 | - | 7 | 14 (1.4) |
| Rhizoctonia solani J.G. Kühn | 22 | 28 | 12 | 16 | 41 | 119 (12.4) |
| Rhizopus stolonifer (Ehrenb.) Vuill. | 10 | 14 | 4 | 6 | 20 | 54 (5.6) |
| Sarocladium kiliense (Grütz) Summerb. | - | 1 | - | - | 3 | 4 (0.4) |
| Sclerotinia sclerotiorum (Lib.) de Bary | 8 | 11 | 4 | 6 | 15 | 44 (4.6) |
| Talaromyces flavus (Klöcker) Stolk and Samson | - | 3 | - | 1 | 7 | 11 (1.1) |
| Torula herbarum (Pers.) Link | - | - | - | - | 3 | 3 (0.3) |
| Trichoderma sp. | 11 | 25 | 34 | 8 | 2 | 80 (8.4) |
| Trichothecium roseum (Pers.) Link | 3 | 5 | 7 | 1 | 1 | 17 (1.8) |
| Total isolates | 144 | 250 | 157 | 114 | 295 | 960 (100) |
| Fungi | Average Number of Isolates (2014–2016) | Alternaria scorzonerae | Fusarium culmorum | Fusarium oxysporum | Rhizoctonia solani | ||||
|---|---|---|---|---|---|---|---|---|---|
| IBE* | GBE** | IBE* | GBE** | IBE* | GBE** | IBE* | GBE** | ||
| Biosept Active | |||||||||
| Acremonium rutilum W. Gams | 1 | +4 | +4 | +3 | +3 | +2 | +2 | +2 | +2 |
| Albifimbria verrucaria (Alb. and Schwein.) L. Lombard and Crous | 7 | +6 | +42 | +4 | +28 | +3 | +21 | +4 | +28 |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert | 4 | +7 | +28 | +5 | +20 | +4 | +16 | +4 | +16 |
| Penicillium chermesinum Biourge | 6 | +2 | +12 | +1 | +6 | +1 | +6 | +2 | +12 |
| Penicillium decumbens Thom | 5 | +1 | +5 | +1 | +5 | +1 | +5 | +1 | +5 |
| Trichoderma sp. | 11 | +8 | +88 | +8 | +88 | +8 | +88 | +8 | +88 |
| Trichothecium roseum (Pers.) Link | 3 | +7 | +21 | +6 | +18 | +4 | +12 | +5 | +15 |
| Number of isolates | 37 | ||||||||
| SBE*** | +200 | +168 | +150 | +276 | |||||
| Timorex Gold 24 EC | |||||||||
| Acremonium rutilum W. Gams | 3 | +4 | +12 | +3 | +12 | +2 | +6 | +2 | +6 |
| Albifimbria verrucaria (Alb. and Schwein.) L. Lombard and Crous | 13 | +6 | +78 | +4 | +52 | +3 | +39 | +4 | +52 |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert | 12 | +7 | +84 | +5 | +60 | +4 | +48 | +4 | +48 |
| Penicillium chermesinum Biourge | 14 | +2 | +28 | +1 | +14 | +1 | +14 | +2 | +28 |
| Penicillium decumbens Thom | 15 | +1 | +15 | +1 | +15 | +1 | +15 | +1 | +15 |
| Penicillium simplicissimum (Oudem.) Thom | 4 | +3 | +12 | +1 | +4 | +1 | +4 | +1 | +4 |
| Talaromyces flavus (Klöcker) Stolk and Samson | 3 | +2 | +6 | +2 | +6 | +1 | +3 | +1 | +3 |
| Trichoderma sp. | 25 | +8 | +200 | +8 | +200 | +8 | +200 | +8 | +200 |
| Trichothecium roseum (Pers.) Link | 5 | +7 | +35 | +6 | +30 | +4 | +20 | +5 | +25 |
| Number of isolates | 94 | ||||||||
| SBE*** | +470 | +393 | +349 | +381 | |||||
| Trianum P | |||||||||
| Acremonium rutilum W. Gams | 3 | +4 | +12 | +3 | +9 | +2 | +6 | +2 | +6 |
| Albifimbria verrucaria (Alb. and Schwein.) L. Lombard and Crous | 16 | +6 | +96 | +4 | +64 | +3 | +48 | +4 | +64 |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert | 16 | +7 | +112 | +5 | +80 | +4 | +64 | +4 | +64 |
| Penicillium chermesinum Biourge | 11 | +2 | +22 | +1 | +11 | +1 | +11 | +2 | +22 |
| Penicillium decumbens Thom | 11 | +1 | +11 | +1 | +11 | +1 | +11 | +1 | +11 |
| Penicillium simplicissimum (Oudem.) Thom | 3 | +3 | +9 | +1 | +3 | +1 | +3 | +1 | +3 |
| Trichoderma sp. | 34 | +8 | +272 | +8 | +272 | +8 | +272 | +8 | +272 |
| Trichothecium roseum (Pers.) Link | 7 | +7 | +49 | +6 | +42 | +4 | +28 | +5 | +35 |
| Number of isolates | 101 | ||||||||
| SBE*** | +583 | +492 | +443 | +477 | |||||
| Zaprawa Nasienna T 75 DS/WS | |||||||||
| Acremonium rutilum W. Gams | 2 | +4 | +8 | +3 | +6 | +2 | +4 | +2 | +4 |
| Albifimbria verrucaria (Alb. and Schwein.) L. Lombard and Crous | 4 | +6 | +24 | +4 | +16 | +3 | +12 | +4 | +16 |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert | 3 | +7 | +21 | +5 | +15 | +4 | +12 | +4 | +12 |
| Penicillium chermesinum Biourge | 8 | +2 | +16 | +1 | +8 | +1 | +8 | +2 | +16 |
| Penicillium decumbens Thom | 6 | +1 | +6 | +1 | +6 | +1 | +6 | +1 | +6 |
| Talaromyces flavus (Klöcker) Stolk and Samson | 1 | +2 | +2 | +2 | +2 | +1 | +1 | +1 | +1 |
| Trichoderma sp. | 8 | +8 | +64 | +8 | +64 | +8 | +64 | +8 | +64 |
| Trichothecium roseum (Pers.) Link | 1 | +7 | +7 | +6 | +6 | +4 | +4 | +5 | +5 |
| Number of isolates | 33 | ||||||||
| SBE*** | +148 | +123 | +111 | +124 | |||||
| Control | |||||||||
| Acremonium rutilum W. Gams | 5 | +4 | +20 | +3 | +15 | +2 | +10 | +2 | +10 |
| Albifimbria verrucaria (Alb. and Schwein.) L. Lombard and Crous | 1 | +6 | +6 | +4 | +4 | +3 | +3 | +4 | +4 |
| Clonostachys rosea (Link) Schroers, Samuels, Seifert | 1 | +7 | +7 | +5 | +5 | +4 | +4 | +4 | +4 |
| Penicillium chermesinum Biourge | 8 | +2 | +16 | +1 | +8 | +1 | +8 | +2 | +16 |
| Penicillium decumbens Thom | 3 | +1 | +3 | +1 | +3 | +1 | +3 | +1 | +3 |
| Penicillium simplicissimum (Oudem.) Thom | 7 | +3 | +21 | +1 | +7 | +1 | +7 | +1 | +7 |
| Talaromyces flavus (Klöcker) Stolk and Samson | 7 | +2 | +14 | +2 | +14 | +1 | +7 | +1 | +7 |
| Trichoderma sp. | 2 | +8 | +16 | +8 | +16 | +8 | +16 | +8 | +16 |
| Trichothecium roseum (Pers.) Link | 1 | +7 | +7 | +6 | +6 | +4 | +4 | +5 | +5 |
| Number of isolates | 35 | ||||||||
| SBE*** | +110 | +78 | +62 | +72 | |||||
| Genus of Bacteria | Number of Antagonistic Isolates (2014–2016) | Alternaria scorzonerae | Fusarium culmorum | Fusarium oxysporum | Rhizoctonia solani | Total Antagonistic Activity | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| A* | B* | A | B | A | B | A | B | |||
| Biosept Active | ||||||||||
| Pseudomonas sp. | 21 | 4 | 84 | 3 | 63 | 3 | 63 | 3 | 63 | 273 |
| Bacillus sp. | 11 | 3 | 33 | 2 | 22 | 2 | 22 | 3 | 33 | 110 |
| Total antagonistic activity | 117 | 85 | 85 | 96 | 383 | |||||
| Timorex Gold 24 EC | ||||||||||
| Pseudomonas sp. | 25 | 3 | 75 | 2 | 50 | 2 | 50 | 3 | 75 | 250 |
| Bacillus sp. | 20 | 2 | 40 | 2 | 40 | 2 | 40 | 2 | 40 | 160 |
| Total antagonistic activity | 115 | 90 | 90 | 115 | 410 | |||||
| Trianum P | ||||||||||
| Pseudomonas sp. | 31 | 5 | 155 | 4 | 124 | 3 | 93 | 4 | 124 | 496 |
| Bacillus sp. | 19 | 4 | 76 | 3 | 57 | 2 | 38 | 3 | 57 | 228 |
| Total antagonistic activity | 231 | 181 | 131 | 181 | 724 | |||||
| Zaprawa Nasienna T 75 DS/WS | ||||||||||
| Pseudomonas sp. | 17 | 2 | 34 | 2 | 34 | 1 | 17 | 2 | 34 | 119 |
| Bacillus sp. | 9 | 2 | 18 | 1 | 9 | 1 | 9 | 1 | 9 | 45 |
| Total antagonistic activity | 52 | 43 | 26 | 43 | 164 | |||||
| Control | ||||||||||
| Pseudomonas sp. | 13 | 2 | 26 | 2 | 26 | 1 | 13 | 1 | 13 | 78 |
| Bacillus sp. | 5 | 2 | 10 | 2 | 10 | 1 | 10 | 1 | 10 | 40 |
| Total antagonistic activity | 36 | 36 | 23 | 23 | 118 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patkowska, E. Biostimulants Managed Fungal Phytopathogens and Enhanced Activity of Beneficial Microorganisms in Rhizosphere of Scorzonera (Scorzonera hispanica L.). Agriculture 2021, 11, 347. https://doi.org/10.3390/agriculture11040347
Patkowska E. Biostimulants Managed Fungal Phytopathogens and Enhanced Activity of Beneficial Microorganisms in Rhizosphere of Scorzonera (Scorzonera hispanica L.). Agriculture. 2021; 11(4):347. https://doi.org/10.3390/agriculture11040347
Chicago/Turabian StylePatkowska, Elżbieta. 2021. "Biostimulants Managed Fungal Phytopathogens and Enhanced Activity of Beneficial Microorganisms in Rhizosphere of Scorzonera (Scorzonera hispanica L.)" Agriculture 11, no. 4: 347. https://doi.org/10.3390/agriculture11040347
APA StylePatkowska, E. (2021). Biostimulants Managed Fungal Phytopathogens and Enhanced Activity of Beneficial Microorganisms in Rhizosphere of Scorzonera (Scorzonera hispanica L.). Agriculture, 11(4), 347. https://doi.org/10.3390/agriculture11040347







