Fermentative Potential of Native Yeast Candida famata for Prokupac Grape Must Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Yeasts
2.2. Characterization and Fermentative Performances of Yeast Isolates
2.3. Screening Method for Initial Aroma Production
2.4. Microfermentation of Prokupac Grape Must
2.5. HPLC Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine yeasts for the future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef]
- Ruiz, J.; Ortega, N.; Martín-Santamaría, M.; Acedo, A.; Marquina, D.; Pascual, O.; Rozès, N.; Zamora, F.; Santos, A.; Belda, I. Occurrence and enological properties of two new non-conventional yeasts (Nakazawaea ishiwadae and Lodderomyces elongisporus) in wine fermentations. Int. J. Food Microbiol. 2019, 305. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chua, J.Y.; Huang, D.; Lee, P.R.; Liu, S.Q. Chemical consequences of three commercial strains of Oenococcus oeni co-inoculated with Torulaspora delbrueckii in durian wine fermentation. Food Chem. 2017, 215, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Seguinot, P.; Bloem, A.; Brial, P.; Meudec, E.; Ortiz-Julien, A.; Camarasa, C. Analysing the impact of the nature of the nitrogen source on the formation of volatile compounds to unravel the aroma metabolism of two non-Saccharomyces strains. Int. J. Food Microbiol. 2020, 316. [Google Scholar] [CrossRef] [PubMed]
- Kállai, Z.; Pfliegler, W.P.; Mitercsák, J.; Szendei, G.; Sipiczki, M. Preservation of diversity and oenological properties of wine yeasts during long-term laboratory maintenance: A study of strains of a century-old Tokaj wine yeast collection. Lwt 2019, 101, 789–798. [Google Scholar] [CrossRef]
- Ferrando, N.; Araque, I.; Ortís, A.; Thornes, G.; Bautista-Gallego, J.; Bordons, A.; Reguant, C. Evaluating the effect of using non-Saccharomyces on Oenococcus oeni and wine malolactic fermentation. Food Res. Int. 2020, 138. [Google Scholar] [CrossRef]
- Lappa, I.K.; Kachrimanidou, V.; Pateraki, C.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Indigenous yeasts: Emerging trends and challenges in winemaking. Curr. Opin. Food Sci. 2020, 32, 133–143. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vitic. 2019, 21. [Google Scholar] [CrossRef]
- Maragatham, C.; Panneerselvam, A. Isolation, identification and characterization of wine yeast from rotten papaya fruits for wine production. Adv. Appl. Sci. Res. 2011, 2, 93–98. [Google Scholar]
- Lin, M.M.H.; Boss, P.K.; Walker, M.E.; Sumby, K.M.; Grbin, P.R.; Jiranek, V. Evaluation of indigenous non-Saccharomyces yeasts isolated from a South Australian vineyard for their potential as wine starter cultures. Int. J. Food Microbiol. 2020, 312, 108373. [Google Scholar] [CrossRef] [PubMed]
- González-Alonso, I.; Walker, M.E.; Pascual-Vallejo, M.-E.; Naharro-Carrasco, G.; Jiranek, V. Capturing yeast associated with grapes and spontaneous fermentations of the Negro Saurí minority variety from an experimental vineyard near León. Sci. Rep. 2021, 11, 3748. [Google Scholar] [CrossRef]
- Koulougliotis, D.; Eriotou, E. Isolation and Identification of Endogenous Yeast Strains in Grapes and Must Solids of Mavrodafni Kefalonias and Antioxidant Activity of the Produced Red Wine. Ferment. Technol. 2016, 5, 1000125. [Google Scholar] [CrossRef]
- Fernández-González, M.; Di Stefano, R.; Briones, A. Hydrolysis and transformation of terpene glycosides from muscat must by different yeast species. Food Microbiol. 2003, 20, 35–41. [Google Scholar] [CrossRef]
- Yanai, T.; Sato, M. Isolation and Properties of β-Glucosidase Produced by Debaryomyces hansenii and Its Application in Winemaking. Am. J. Enol. Vitic. 1999, 50, 231–235. [Google Scholar]
- Englezos, V.; Rantsiou, K.; Torchio, F.; Rolle, L.; Gerbi, V.; Cocolin, L. Exploitation of the non-Saccharomyces yeast Starmerella bacillaris (synonym Candida zemplinina) in wine fermentation: Physiological and molecular characterizations. Int. J. Food Microbiol. 2015, 199, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gientka, I.; Bzducha-Wróbel, A.; Stasiak-Różańska, L.; Bednarska, A.A.; Błażejak, S. The exopolysaccharides biosynthesis by Candida yeast depends on carbon sources. Electron. J. Biotechnol. 2016, 22, 31–37. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Bäumlisberger, M.; Moellecken, U.; König, H.; Claus, H. The Potential of the Yeast Debaryomyces hansenii H525 to Degrade Biogenic Amines in Food. Microorganisms 2015, 3, 839–850. [Google Scholar] [CrossRef]
- Malićanin, M.; Danilović, B.; Cvetković, D.; Stamenković-Stojanović, S.; Nikolić, N.; Lazić, M.; Karabegović, I. Modulation of Aroma and Sensory Properties of Prokupac Wines by a Bacillus-based Preparation Applied to Grapes Prior to Harvest. S. Afr. J. Enol. Vitic. 2020, 41, 158–167. [Google Scholar] [CrossRef]
- Pantelić, M.; Dabić Zagorac, D.; Gašić, U.; Jović, S.; Bešlić, Z.; Todić, S.; Natić, M. Phenolic profiles of Serbian autochthonous variety ‘Prokupac’ and monovarietal international wines from the Central Serbia wine region. Nat. Prod. Res. 2018, 32, 2356–2359. [Google Scholar] [CrossRef]
- Lakićević, S.; Popović, T.; Matijašević, S.; Ćirković, B.; Lazić, M.; Popović-Đorđević, J. Chemical evaluation of autochthonous variety “Prokupac” red wine with the addition of selected aromatic herbs. Ann. Univ. Craiova Agric. Mont. Cadastre Ser. 2019, 49, 87–97. [Google Scholar]
- del Mónaco, S.M.; Rodríguez, M.E.; Lopes, C.A. Pichia kudriavzevii as a representative yeast of North Patagonian winemaking terroir. Int. J. Food Microbiol. 2016, 230, 31–39. [Google Scholar] [CrossRef]
- Riesute, R.; Salomskiene, J.; Moreno, D.S.; Gustiene, S. Effect of yeasts on food quality and safety and possibilities of their inhibition. Trends Food Sci. Technol. 2021, 108, 1–10. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef]
- Schiavone, M.; Sieczkowski, N.; Castex, M.; Dague, E.; Marie François, J. Effects of the strain background and autolysis process on the composition and biophysical properties of the cell wall from two different industrial yeasts. FEMS Yeast Res. 2015, 15, 12. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Fleet, G.H.; Rogers, P.L. Composition of the cell walls of several yeast species. Appl. Microbiol. Biotechnol. 1998, 50, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, S.F.; Matese, A.; Mancin, M.; Primicerio, J.; Palliotti, A. An open-source and low-cost monitoring system for precision enology. Sensors 2014, 14, 23388–23397. [Google Scholar] [CrossRef] [PubMed]
- Santesteban, L.G. Precision viticulture and advanced analytics. A short review. Food Chem. 2019, 279, 58–62. [Google Scholar] [CrossRef] [PubMed]
- OIV Resolution, 370-2012. Guidelines for the Characterization of Wine Yeasts of the Genus Saccharomyces Isolated from Vitivinicultural Environments; International Organisation of Vine and Wine: Paris, France, 2012. [Google Scholar]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- Ganga, M.A.; Martínez, C. Effect of wine yeast monoculture practice on the biodiversity of non-Saccharomyces yeasts. J. Appl. Microbiol. 2004, 96, 76–83. [Google Scholar] [CrossRef]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Iris, L.; Antonio, M.; Antonia, B.M.; Antonio, S.L.J. Isolation, selection, and identification techniques for non-Saccharomyces yeasts of oenological interest. In Biotechnological Progress and Beverage Consumption, Volume 19: The Science of Beverages; Academic Press: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318. [Google Scholar] [CrossRef] [PubMed]
- Lemos Junior, W.J.F.; Binati, R.L.; Bersani, N.; Torriani, S. Investigating the glutathione accumulation by non-conventional wine yeasts in optimized growth conditions and multi-starter fermentations. LWT 2021, 142, 110990. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and identification of organic acids in wine samples. Problems and challenges. TrAC Trends Anal. Chem. 2019, 120. [Google Scholar] [CrossRef]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic Acid Metabolism and the Impact of Fermentation Practices on Wine Acidity: A Review. J. Enol. Vitic 2018, 39, 1–15. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Formation of amino acid derivatives in white and red wines during fermentation: Effects of non-Saccharomyces yeasts and Oenococcus Oeni. Food Chem. 2021, 343. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated with non- Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef]
- Sirén, H.; Sirén, K.; Sirén, J. Evaluation of organic and inorganic compounds levels of red wines processed from Pinot Noir grapes. Anal. Chem. Res. 2015, 3, 26–36. [Google Scholar] [CrossRef]
- Magyar, I.; Nyitrai-Sárdy, D.; Leskó, A.; Pomázi, A.; Kállay, M. Anaerobic organic acid metabolism of Candida zemplinina in comparison with Saccharomyces wine yeasts. Int. J. Food Microbiol. 2014, 178, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- van Breda, V.; Jolly, N.; van Wyk, J. Characterisation of commercial and natural Torulaspora delbrueckii wine yeast strains. Int. J. Food Microbiol. 2013, 163, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Nieuwoudt, H.H.; Prior, B.A.; Pretorius, L.S.; Bauer, F.F. Glycerol in South African Table Wines: An Assessment of its Relationship to Wine Quality. S. Afr. J. Enol. Vitic. 2017, 23, 22–30. [Google Scholar] [CrossRef]
- Chidi, B.S.; Rossouw, D.; Buica, A.S.; Bauer, F.F. Determining the Impact of Industrial Wine Yeast Strains on Organic Acid Production Under White and Red Wine-like Fermentation Conditions. S. Afr. J. Enol. Vitic. 2015, 36, 316–327. [Google Scholar] [CrossRef]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Eberlin, M.N.; Sawaya, A.C.H.F.; Marcos, S.; Eberlin, N.; Helena, A.C.; Sawaya, F.; Lena, F.; Silva, N.; Schmidt, E.M.; Audio, C.; et al. Quantitation of organic acids in wine and grapes by direct infusion electrospray ionization mass spectrometry. Anal. Methods 2015, 7, 53–62. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The influence of non-Saccharomyces species on wine fermentation quality parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Csernus, O.; Pomázi, A.; Magyar, I. Isolation, characterisation, and selection of wine yeast strains in Etyek-Buda wine district, Hungary. Acta Aliment. 2014, 43, 489–500. [Google Scholar] [CrossRef]
- Magyar, I.; Tóth, T. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 94–100. [Google Scholar] [CrossRef] [PubMed]
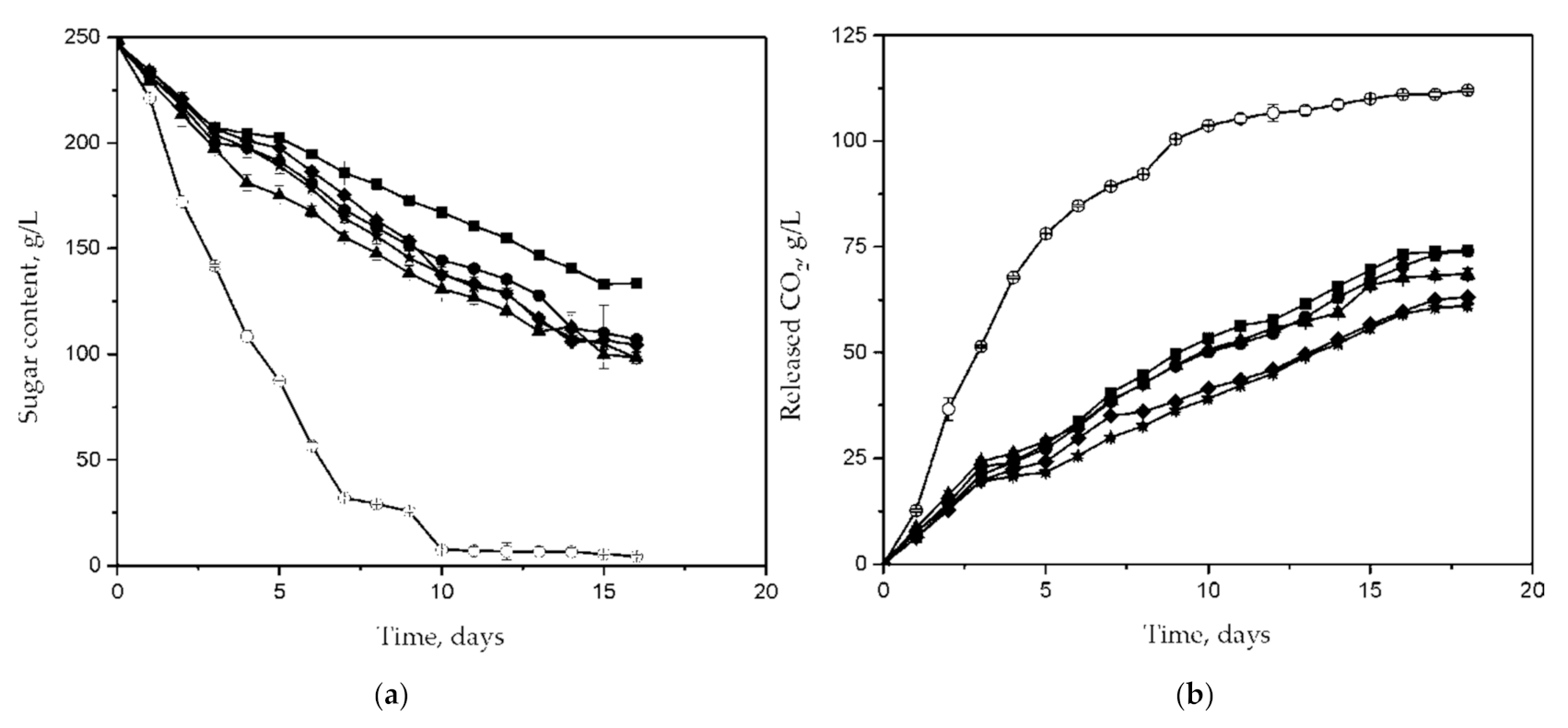
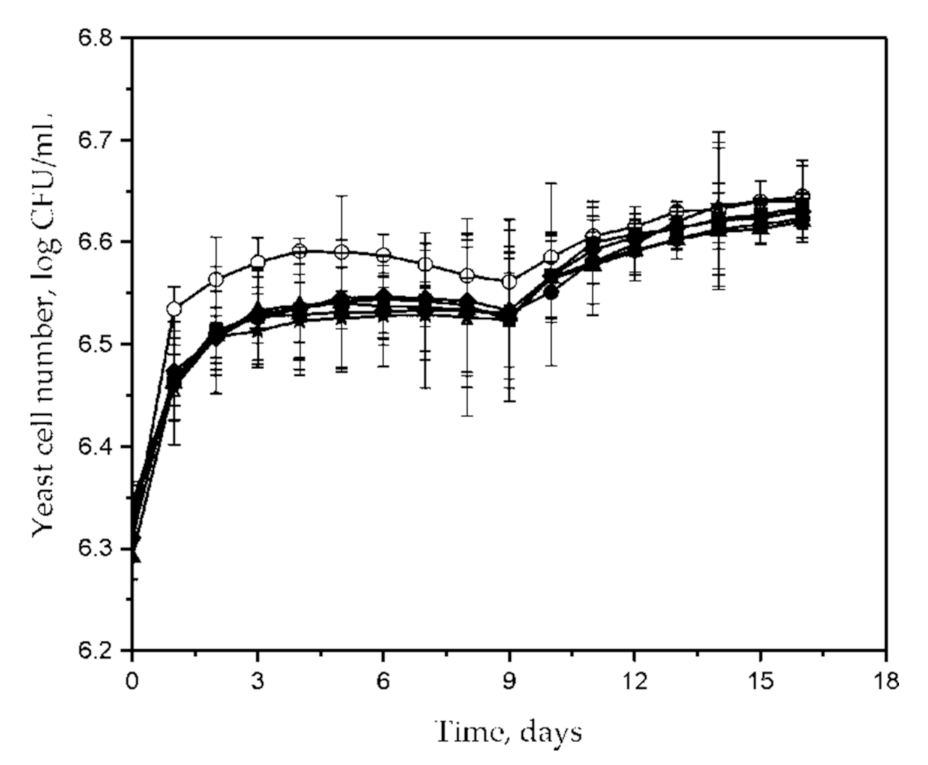
| Characteristics and Fermentative Performances | C. famata Isolate | S. cerevisiae ICV D254 | |||||
|---|---|---|---|---|---|---|---|
| WB-1 | WB-2 | WB-4 | WB-17 | W-5 | |||
| A620 | 1.020 ± 0.00 a | 0.902 ± 0.024 b | 1.004 ± 0.003 ac | 0.876 ± 0.004 b | 1.016 ± 0.031 a | 1.101 ± 0.070 ad | |
| CO2 production | + | + | + | + | + | + | |
| Growth at different temperature | 4 °C | + | + | + | + | + | + |
| 10 °C | + | + | + | + | + | + | |
| 15 °C | + | + | + | + | + | + | |
| 20 °C | + | + | + | + | + | + | |
| Tolerance to ethanol | 3% vol. | 100% | 97% | 99% | 100% | 100% | 100% |
| 5% vol. | 81% | 89% | 86% | 88% | 80% | 100% | |
| 7% vol. | 65% | 64% | 63% | 62% | 52% | 96% | |
| 9% vol. | 31% | 29% | 25% | 37% | 21% | 88% | |
| 11% vol. | 12% | 4% | 13% | 15% | 4% | 78% | |
| 13% vol. | 6% | - | 6% | 8% | 2% | 67% | |
| 15% vol. | - | - | - | - | - | 47% | |
| Tolerance to SO2 | 50 ppm | + | + | + | + | + | + |
| 100 ppm | + | + | + | + | + | + | |
| 200 ppm | + | + | + | + | + | + | |
| 300 ppm | + | + | + | + | + | + | |
| Fermentative vigor 1 | 2.66 ± 0.05 a | 2.44 ± 0.08 b | 2.24 ± 0.02 c | 2.51 ± 0.06 b | 2.17 ± 0.06 c | 5.18 ± 0.05 d | |
| Fermentative power 2 | 7.41 ± 0.09 a | 6.51 ± 0.22 b | 6.07 ± 0.00 c | 7.36 ± 0.10 a | 6.62 ± 0.10 b | 11.22 ± 0.07 d | |
| Aroma production 3 | P++ | P+ | U+ | U+ | P++ | P+++ | |
| Compound | Prokupac Grape Must | C. famata Isolate | S. cerevisiae ICV D254 | ||||
|---|---|---|---|---|---|---|---|
| WB-1 | WB-2 | WB-4 | WB-17 | W-5 | |||
| Tartaric acid, g/L | 5.85 ± 0.29 a | 5.09 ± 0.06 b | 5.17 ± 0.03 b | 5.51 ± 0.02 c | 5.35 ± 0.06 d | 5.57 ± 0.07 a | 4.80 ± 0.08 e |
| Malic acid, g/L | 3.23 ± 0.01 a | 2.86 ± 0.05 b | 3.37 ± 0.06 c | 4.56 ± 0.06 d | 3.22 ± 0.06 a | 3.03 ± 0.06 e | 1.11 ± 0.04 f |
| Lactic acid, g/L | n.d | 1.84 ± 0.08 a | 0.98 ± 0.02 b | 1.49 ± 0.01 c | 1.17 ± 0.03d | 1.71 ± 0.06 e | 1.64 ± 0.02 e |
| Succinic acid, g/L | n.d | 0.62 ± 0.02 a | 0.91 ± 0.04 b | 0.56 ± 0.03 a | 0.85 ± 0.01 c | 0.81 ± 0.02 c | n.d d |
| Acetic acid, g/L | n.d | n.d | n.d | n.d | n.d | n.d | 0.57 ± 0.02 |
| Glucose, g/L | 120.85 ± 3.24 a | 25.94 ± 1.57 b | 31.74 ± 3.11 c,f | 51.22 ± 2.28 d | 28.83 ± 1.51 b,f | 28.82 ± 0.26 b,f | 0.20 ± 0.11 e |
| Fructose, g/L | 115.43 ± 2.78 a | 64.31 ± 1.74 b | 62.92 ± 2.35 b | 79.73 ± 2.61 c | 61.57 ± 1.50 b | 62.11 ± 0.29 b | 2.52 ± 0.09 d |
| Glycerol, g/L | n.d | 4.98 ± 0.21 a | 3.94 ± 0.25 b | 3.53 ± 0.11 b | 3.48 ± 0.22 b | 4.70 ± 0.14 a | 2.42 ± 0.11 c |
| Ethanol, % vol. | n.d | 6.42 ± 0.26 a | 6.12 ± 0.28 a | 5.93 ± 0.18 b,d | 6.52 ± 0.33 a | 5.71 ± 0.05 a,d | 11.03 ± 0.45 c |
| Parameter | Prokupac Wine Sample | ||
|---|---|---|---|
| CF 1 | CFSC 2 | SC 3 | |
| Ethanol, % vol. | 11.3 ± 0.07 a | 12.6 ± 0.02 b | 13.0 ± 0.08 c |
| Reducing sugar, g/L | 2.25 ± 0.06 a | 2.21 ± 0.05 a | 1.39 ± 0.02 b |
| Total acidity (as tartaric acid), g/L | 6.53 ± 0.06 a | 6.55 ± 0.07 a | 6.48 ± 0.09 a |
| Volatile acidity (as acetic acid,) g/L | 0.30 ± 0.015 a | 0.32 ± 0.015 b | 0.43 ± 0.02 c |
| HPLC analysis | |||
| Tartaric acid, g/L | 4.62 ± 0.12 a | 5.47 ± 0.18 b | 6.03 ± 0.01 c |
| Malic acid, g/L | 1.32 ± 0.02 a | 1.52 ± 0.01 b | 3.02 ± 0.09 c |
| Lactic acid, g/L | 0.67 ± 0.00 a | 0.27 ± 0.02 b | 0.29 ± 0.01 b |
| Succinic acid, g/L | 0.75 ± 0.02 a | 0.53 ± 0.05 b | 0.37 ± 0.01 c |
| Acetic acid, g/L | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.33 ± 0.01 b |
| Glucose, g/L | 0.19 ± 0.00 a | 0.14 ± 0.00 b | 0.12 ± 0.00 c |
| Fructose, g/L | 1.56 ± 0.00 a | 1.79 ± 0.02 b | 1.60 ± 0.00 c |
| Glycerol, g/L | 7.12 ± 0.13 a | 7.45 ± 0.23 a | 5.76 ± 0.09 b |
| Ethanol, % vol. | 11.63 ± 0.08 a | 12.05 ± 0.16 b | 13.00 ± 0.42 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mančić, S.; Danilović, B.; Malićanin, M.; Stamenković Stojanović, S.; Nikolić, N.; Lazić, M.; Karabegović, I. Fermentative Potential of Native Yeast Candida famata for Prokupac Grape Must Fermentation. Agriculture 2021, 11, 358. https://doi.org/10.3390/agriculture11040358
Mančić S, Danilović B, Malićanin M, Stamenković Stojanović S, Nikolić N, Lazić M, Karabegović I. Fermentative Potential of Native Yeast Candida famata for Prokupac Grape Must Fermentation. Agriculture. 2021; 11(4):358. https://doi.org/10.3390/agriculture11040358
Chicago/Turabian StyleMančić, Stojan, Bojana Danilović, Marko Malićanin, Sandra Stamenković Stojanović, Nada Nikolić, Miodrag Lazić, and Ivana Karabegović. 2021. "Fermentative Potential of Native Yeast Candida famata for Prokupac Grape Must Fermentation" Agriculture 11, no. 4: 358. https://doi.org/10.3390/agriculture11040358
APA StyleMančić, S., Danilović, B., Malićanin, M., Stamenković Stojanović, S., Nikolić, N., Lazić, M., & Karabegović, I. (2021). Fermentative Potential of Native Yeast Candida famata for Prokupac Grape Must Fermentation. Agriculture, 11(4), 358. https://doi.org/10.3390/agriculture11040358








