Abstract
Strigolactone (SL) plays essential roles in regulating plant growth, development, and stress response. This study was conducted to evaluate the effect of exogenous SL on watermelon resistance against herbicides penoxsulam (PXL) and bensulfuron-methyl (BSM). These herbicides were found to negatively impact watermelon root growth and photosynthetic pigments, probably due to the ultrastructural damage and cell death in leaf and root tissues under herbicide stresses. The activation of SL-related gene expression suggested that the SL pathway may mitigate herbicide toxicity in watermelon. The exogenous SL dose-dependently reversed the PXL- or BSM-induced antioxidant activity, suggesting that SL may participate in maintaining antioxidant enzyme homeostasis under herbicide stresses. The up-regulation of herbicide metabolization and detoxification-related genes (cytochrome P450 and acetolactate synthase) by exogenous SL also in part explained how this phytohormone alleviates herbicide toxicity in watermelon. Our findings will provide valuable information underlying the regulatory effects of SL on herbicide tolerance in Cucurbitaceae crops.
1. Introduction
The use of herbicides in crop rotation, which are essential for controlling weeds and the improvement of crop quality and quantity, often leads to adverse impacts on ecosystems through contamination of soil, water, and the food chain [1]. During the rice-vegetable rotation in China, the negative effects of residual herbicides applied in the rice season restrict the production of vegetables, including watermelon. Penoxsulam (PXL), bensulfuron-methyl (BSM), chylapof-butyl, and penoxifire are herbicides commonly applied to rice fields [2]. PXL and BSM, belonging to the trizolpyrimidine sulfonamide and sulfonylurea families, respectively, were recently identified as negative regulators of watermelon growth and development [3]. PXL and BSM treatments significantly affected the growth of watermelon, squash, and bottle gourd at 10 ppb after one week, suggesting the susceptibility of the cucurbits species to a low concentration of PXL and BSM [3]. They are known to control weeds by inhibiting acetolactate synthase (ALS), an essential enzyme in the biosynthesis of the branched-chain amino acids valine, leucine, and isoleucine [4,5,6,7].
Plant resistance to herbicides is divided mainly into a targeted site and non-targeted site resistance [8]. Targeted site resistance involves changes at the protein, nucleotide sequence, or expression level [9,10]. Recently, some genes related to herbicide resistance and metabolism have been identified, including ATP-binding cassette transporters, oxidases, esterases, hydrolases, peroxidases, glycosyltransferases, glutathione S-transferases, and cytochrome P450 monooxygenases [11]. It was suggested that enhanced herbicide-metabolizing cytochrome P450s (CYPs) might lead to resistance to BSM and PXL [11]. In vitro assays suggested that P450 detoxifies BSM through O-demethylation and converts PXL into 5-OH-penoxsulam by O-dealkylation [12,13]. In the bispyribac-tolerant plant Echinochloa Phyllopogon, the up-regulation of 39 putative P450 genes was associated with herbicide resistance [14]. Moreover, CYP81A12 and CYP81A21 were more actively transcribed in R plants than S plants, and transgenic Arabidopsis, expressing either of the two genes, survived in media containing BSM or PXL [12].
Alteration in various biological processes, including phytohormones and secondary metabolism, was observed in plants exposed to herbicides, even at a concentration below the threshold of evident phenotypic symptoms [15]. The effects of several phytohormones, such as salicylic acid (SA), jasmonic acid (JA), and ethylene, on plant response to herbicides have been reported [16,17,18,19,20]. SA plays a significant role in plant responses to both biotic and abiotic stresses by limiting oxidative damage [21]. While SA and JA are known to regulate plant resistance to herbicides (such as paraquat and imazapic) through the activation of peroxidase and inhibition of catalase activities, ethylene acts by regulating H2O2 [16,22]. Another plant growth hormone-like epibrassinolide (EBL), was used in the detoxification process of heavy metals and herbicides toxicity in plants [15,23]. However, strigolactone (SL) roles in plants exposed to herbicides have not yet been reported.
SL was initially known as a stimulant for the germination of weeds like Striga and Orobanche, as well as a host-detector and hyphal-branched signal for arbuscular mycorrhizal (AM) fungi [24,25]. Recently, SL was identified as a new phytohormone that regulates plant architecture by inhibiting the growth of axillary buds [26,27]. SL has different functions in developing various parts of a plant, e.g., shoot branching and root architecture [28,29]. Moreover, its role in plant response to various abiotic stresses, such as phosphorus and nitrogen deficiency, drought, and salinity, has been identified [30,31,32,33]. SL was found to improve plant resistance to salt stress by regulating the H2O2 signaling molecule [34,35]. The role of SL in plant resistance to drought and salinity stresses is well documented in Arabidopsis, as the SL biosynthesis mutants MAX3 and MAX4 show higher sensitivity, which is also related to delayed ABA-induced stomatal closure [33]. The role of SL in the salt tolerance of lettuce plants was via the positive regulation of photosystem II in the presence of AM fungus [36]. AM symbiosis has been found to help host plants cope with the detrimental effects of high soil salinity via its influence on the plant equilibrium of absorption and compartmentalization of excess ions that positively regulate the cellular osmotic process [37]. Since SL is known to be a stimulator of plant-AM symbiosis and weed germination [24,25], we hypothesize that this phytohormone might play a positive role in crop responses to herbicides via direct or indirect pathways.
This study aims to investigate the effects of SL on watermelon exposed to PXL and BSM. The response of SL biosynthesis and signaling to PXL and BSM exposure suggests SL’s involvement in plant resistance to herbicides. The induction of CYP and ALS genes by exogenous SL implies a possible mechanism for SL to alleviate herbicide toxicity in watermelon. This study will improve our understanding of mechanisms associated with herbicide tolerance and SL’s role in dealing with the ecotoxicological effects of herbicides in rice-watermelon rotation.
2. Material and Methods
2.1. Plant Materials and Growth Conditions
Seeds of the watermelon (Citrullus lanatus) variety XFM (provided by the Laboratory of Germplasm Innovation and Molecular Breeding) were soaked in 1% KNO3 solution in a 55-°C water-bath for 30 min followed by 3 h at room temperature. The clean seeds were germinated at 28–30 °C under darkness for three to four days. They were maintained in a sterilized paper roll after germination in half-strength Hoagland solution in a controlled growth chamber (16 h light at 27 °C and 8 h dark at 24 °C) until the cotyledons became flat. Seedlings were then transferred to full-strength Hoagland solution in 1-L pots, provided with an air stone for oxygen supply, and the solution changed every three days. Once plants reached two true leaves, they were exposed to 10-ppb PXL and BSM herbicides for three days. The 10-ppb concentration was selected based on the screening results obtained from our previous study [3] in the presence and absence of SL (0, 1, and 5 μM). The stock solution of 100-mM GR24 (a racemic mixture, Coolaber, Beijing city, China) was prepared in DMSO (Dimethyl sulfoxide) and diluted into relevant concentrations in Hoagland solution with or without herbicides.
2.2. Morphological Root Measurement
Three replicate seedlings from each treatment were measured for morphological root growth. Total root length and the number of lateral roots were recorded using WinRhizo Pro (S) v. 2009a software (Regent Instruments Inc., Québec, QC, Canada) Samrana, et al., [38].
2.3. Determination of Cellular Respiration
Cellular respiration was determined by the triphenyl tetrazolium chloride (TTC) staining method described by [3]. Three replicate root samples were collected from each treatment, put into a 10-mL tube containing 5 mL of 0.4% TTC, and heated at 37 °C for 1 h. To stop the reaction, 1 M of H2SO4 was added. Root samples were ground with quartz sand in 10 mL of ethyl acetate, and root activity was measured by spectrophotometer at 450 nm (Spectrum SP-752, Shanghai, China).
2.4. Membrane Integrity
Three root tips were randomly selected from each treatment and used to assess membrane integrity by the protocol of Ali et al. [3], staining 1 cm of root tips in 0.25% Evan’s Blue solution for 30 min at room temperature. The stained samples were washed three times, transferred to a 5-mL tube containing 50:50 SDS-methanol, and heated at 50 °C for 30 min. Finally, the extract solution was centrifuged for 20 min at 4000 rpm, and the absorbance measured at 600 nm.
2.5. Ultrastructure Analysis
Cell ultrastructural analysis was conducted according to the method described by Malangisha et al. [39]. Three replicate root tips and leaves (1 mm2) from control and herbicide treatments with and without SL were collected after three days and steeped overnight in 2.5% glutaraldehyde in 0.1 M PBS (sodium phosphate buffer) at pH 7.4. Samples were washed four times with 0.1 M PBS, then fixed with osmium oxide for 1 h. Fixed samples were washed four times in 0.1 M PBS at 15 min intervals. A series of graded ethanol (50%, 70%, 80%, 90%, 95%, and 100%) was used for progressive dehydration at 15 min intervals. Absolute acetone was then used for further dehydration for 15–20 min. Samples were embedded overnight in Spurr resin then heated at 70 °C for 9 h. Sections (80 nm) were prepared, mounted on copper grids, and examined by electron microscopy (JEOL TEM-1230EX) at the Electron Microscopic Centre Zhejiang University, Hangzhou, China.
2.6. Determination of Photosynthetic Pigments
To measure chlorophyll content, 25 mg of fresh leaves from each treatment were added to 25 mg of magnesium oxide to prevent pheophytin formation and stabilize plant acidity. Methanol (100%, 5 mL) was added, and samples were agitated on a shaker for 2 h in darkness. Samples were then stored overnight in darkness at room temperature before centrifugation in 5-mL tubes for 15 min at 4000 rpm at 25 °C. The supernatant was placed in a 1-cm cuvette, and the absorbance at 470 nm, 653 nm, and 666 nm was recorded by spectrophotometer (Spectrum SP-752, Shanghai, China). Chlorophyll content, i.e., chlorophyll a, b, and carotenoid, was quantified according to the method of Lichtenthaler and Wellburn [40].
2.7. Antioxidant Enzyme Activity
Fresh samples (0.5 g) of control and treated plants were collected to determine antioxidant enzyme activity. Samples were homogenized in 8 mL of 50 mM potassium phosphate buffer pH 7.0 with 1 mM EDTA-Na2 and 0.5% PVP (w/v) then centrifuged at 12,000 rpm at 4 °C for 20 min. The supernatant was collected and stored at −80 °C. The method of Giannopolitis and Ries [41] was used for the superoxide dismutase (SOD, EC 1.15.1.1), based on photoreduction of NBT (nitroblue tetrazolium chloride) formation of purple formazone. One unit of SOD activity is the amount of enzyme required to cause 50% inhibition in the reduction of NBT. Catalase (CAT, EC 1.11.1.6) was determined according to Havir and McHale [42] using the extinction coefficient of 39.4 mM−1 cm−1. One unit of enzyme activity is defined as 1 nmol H2O2 dissociated min−1. The activity of ascorbic acid peroxidase (APX, EC 1.11.1.1) was determined following the method of Nakano and Asada [43]. The oxidation rate of ascorbate was observed at 290 nm at 30 °C. APX activity was expressed as nmol of ascorbate/min/mg protein and was calculated using an extinction coefficient of 2.8 mm−1 cm−1 for ascorbate [43]. Peroxidase (POD) activity was determined by the method of Cakmak et al. [44], the reaction mixture containing 100 μL of enzyme extract, 2.7 mL of 50 mM phosphate buffer pH 6.1, 100 μL of 1.5% guaiacol, and 100 μL of 0.4% H2O2. Enzyme activity was expressed as the absorbance at 470 nm in mM/min/g FW at 25 ± 2 °C.
2.8. Determination of Lipid Peroxidation and Hydrogen Peroxide
Lipid peroxidation, represented by thiobarbituric acid reactive substances (TBARS), was measured by the method of Heath and Packer [45]. A total of 0.5 g fresh samples of roots and leaves were extracted in a 2-mL solution of 0.5% thiobarbituric acid, made in 10% trichloroacetic acid. The extract was heated for 30 min at 95 °C, then cooled on ice and centrifuged for 15 min at 4000 rpm. The supernatant absorbance was measured at 532 nm and 600 nm. TBARS concentration was expressed as nmol/g fresh weight.
Hydrogen peroxide (H2O2) was determined by the method of Sergiev et al. [46]. The reaction mixture comprised 1 mL of enzyme extract, 1 mL of 10 mM potassium phosphate buffer (pH 7.0), and 2 mL of 1 M potassium iodide. Absorbance was measured at 390 nm, and H2O2 concentration was expressed as µmol/g FW. Determinations of total soluble sugar (TSS), total soluble protein (TSP), and proline were carried out according to Bibi et al. [47].
2.9. RNA Extraction and qPCR
Approximately 50 mg of fresh leaf and root sample was used for total RNA extraction by liquid nitrogen and mortar and pestle using the RNAiso reagent (Takara, Nojihigashi, Japan). RNA concentration was determined using the Thermo200 Bioanalyzes Nanodrop (ThermoScientific, Waltham, MA, USA, http://www.thermo.com (accessed on 10 March 2020)). Extracted RNA (1 µg in 20 µL) was used for reverse transcription by applying Revertra Ace qPCR master mix with a gDNA remover kit (Toyobo, Osaka, Japan) and diluting in 200 µL of water. PCR mixture (20 µL) and 2.5 µL of single cDNA were used for qPCR analysis, along with 10 µL of 2 × FastStart Universal SYBR Green Master (Toyobo, Osaka, Japan) and 0.25 µM of forward and reverse primers. Three biological replicates were used for gene expression, and CT values were obtained from the RT-qPCR system StepOne v.2.1 software (Applied Biosystems). ΔCT values were calculated between the gene CT and CIYLS8 CT values, CIYLS8 being used as the internal standard. The primer sequences are given in Table S1.
2.10. Statistical Analyses
An experimental randomized complete block design was applied, and analysis of variance performed using STATIX9 software followed by Tukey’s test for multiple comparisons, p ≤ 0.01 denoting significant differences between groups. All results were plotted as means of three replicates with their corresponding standard deviations.
3. Results
3.1. Effect of SL on Root Architecture, Root Activity, Cell Death, and Chlorophyll Content
The root is an essential organ that translocates nutrients and water to the aerial part of the plant and acts as the first line of defense against stimuli in the soil environment, including herbicides. Under PXL and BSM stress, root growth was restrained and compared with control and synthetic SL (GR24)-treated plants (Figure 1A). Herbicides reduced the number of lateral roots and total root length, while GR24 application alongside herbicides significantly enhanced root growth in a dose-dependent manner compared to herbicide-treated plants (Figure 1B,C). To further investigate SL’s effect on watermelon resistance to herbicides, root activity and cell death in the root tip were examined. Under PXL and BSM stress, root activity reduced while cell death increased compared with controls. The application of 5 μM GR24 restored the herbicide-inhibited root activity close to control levels (Figure 1D). However, cell death in herbicide-only treated plants was significantly higher compared with all other treatments (Figure 1E). These results illustrate the positive effect of SL on root growth and development under herbicide stress.
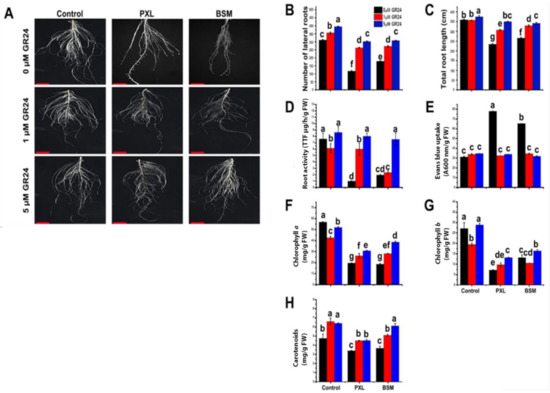
Figure 1.
Effect of herbicides with and without SL on root growth and chlorophyll pigments accumulation. (A) Root phenotype, (B) numbers of lateral roots, (C) total root length, (D) root activity, (E) Evans’ blue uptake, (F) chlorophyll a content, (G) chlorophyll b content, and (H) carotenoids content under herbicide stress with and without SL. Different letter shows a significant difference (p < 0.01). Values are the mean of three biological replicates.
The effect of SL on chlorophyll pigment content under PXL and BSM stress was evaluated. Both herbicides reduced chlorophyll a, chlorophyll b, and carotenoid contents, indicating an impairment of photosynthetic function compared with controls (Figure 1F,G). The dose-dependent application of GR24 reduced PXL and BSM toxicity in terms of chlorophyll a and b contents. On the other hand, GR24 combined with BSM increased chlorophyll a dose-dependently, while both chlorophyll a and b contents were higher under BSM with 5 μM of GR24. Interestingly, all GR24 applications with or without herbicides induced significant carotenoid accumulation (Figure 1H), implying that exogenous SL may promote herbicide tolerance via the enhancement of watermelon pigments.
3.2. Effect of SL on Watermelon Antioxidant Activity under Herbicide Stress
Herbicide treatment induced significant antioxidant enzyme activity in both roots and leaves. APX activity in roots and leaves was significantly increased compared with controls. APX activity in plants treated with GR24 with and without herbicides was reduced compared with PXL (17–27%) and BSM (25–44%); however, there was no difference compared with controls in both roots and leaves (Figure 2A,B). In herbicide-treated plants, CAT activity was significantly enhanced in both roots and leaves. In the roots of PXL-treated plants, CAT activity increased by 331% and by 287% after BSM treatment compared with untreated plants (Figure 2C). In leaves, CAT activity was enhanced by 358% (PXL treatment) and 123% (BSM treatment) (Figure 2D). CAT activity was higher in plants treated with PXL than with BSM. In the presence of GR24 (1 and 5 μM), CAT activity in PXL- and BSM-treated plants showed 56–81% and 31–50% reductions, respectively, compared with herbicide-only treatments.
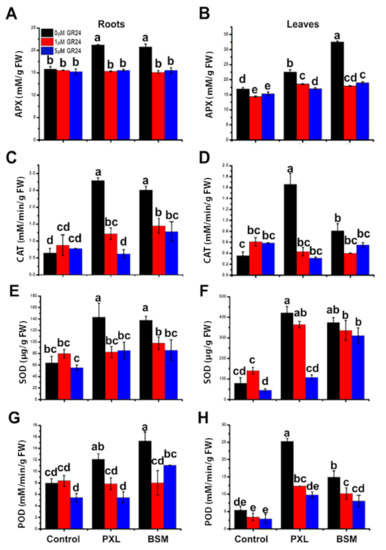
Figure 2.
Effect of different concentrations of SL with and without herbicides on roots and leaves antioxidant activities. (A,B) APX, (C,D) CAT, (E,F) SOD, and (G,H) POD in roots and leaves, respectively. Different letter shows a significant difference (p < 0.01) from each other. Data is the mean of three independent replicates. ANOVA was conducted separately for roots and leaves.
SOD activity increased by 124% and 115% in roots, and 436% and 376% in leaves under PXL and BSM treatments, respectively (Figure 2F). This implies higher dismutation activity in leaves than in roots and higher activity under PXL treatment than BSM. When GR24 was combined with herbicides, SOD activity was reduced, particularly in roots compared with leaves. Furthermore, 5 μM of GR24 treatment alone reduced SOD activity by 13% and 14% compared with control roots and leaves, respectively (Figure 2E,F).
POD activity increased under PXL (by 52% and 361%) and BSM (by 91% and 172%) in both roots and leaves, respectively, compared with controls (Figure 2G,H). Combined SL (1 μM of GR24) application and herbicides restored POD activity to that seen in control roots. SL (1 μM and 5 μM) combined with herbicides reduced POD activity in leaves by 31–61% compared with herbicide-only treated plants. POD activity in roots decreased by 32% with 5 μM of GR24 without herbicides. In leaves, both 1 μM and 5 μM GR24 significantly reduced POD activity (by 37% and 47%) than controls (Figure 2G,H). These results show that herbicide treatment induced APX, CAT, SOD, and POD activities (Figure 2). This effect was, in most cases, mitigated, and enzyme activities restored to control levels by GR24 application, indicating that SL alleviated herbicide toxicity in watermelon by regulating antioxidant activities.
3.3. Effect of SL on H2O2 Accumulation, TBARS, and Metabolites under Herbicide Stress
H2O2 accumulation and lipid peroxidation were investigated to confirm SL’s effect of mitigating oxidative damage. H2O2 in roots increased significantly in response to PXL (175%) and BSM (131%) (Figure 3A). However, there was no significant change in leaves under any treatment except for BSM and BSM with 5 μM of GR24, which showed significant decreases compared with 1 μM of GR24. The combined application of GR24 (1 μM and 5 μM) and PXL reduced H2O2 by 9% and 52%, respectively. Moreover, the combination of BSM and GR24 (1 μM and 5 μM) reduced H2O2 by 15% and 71%, respectively. The toxic effects of herbicides were mitigated by a dose-dependent GR24 application by reducing H2O2 concentrations (Figure 3A,B).
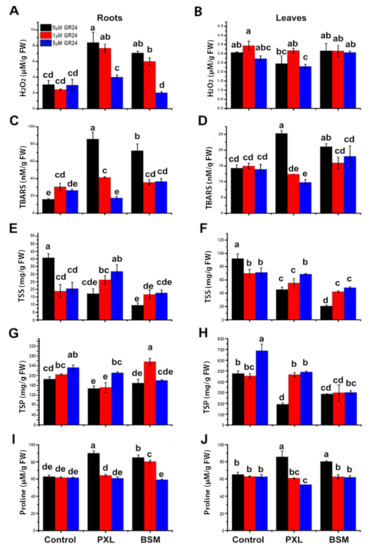
Figure 3.
Effect of SL on H2O2, TBARS content, and biochemical activities under herbicides stress. (A,B) H2O2 content, (C,D) TBARS content, (E,F) TSS content, (G,H) TSP content, and (I,J) proline content in watermelon roots and leaves, respectively, after 3 days of treatments. Values are the mean of three replicates. Different letter shows significant difference (p < 0.01) from each other.
TBARS were determined in different parts of the watermelon seedlings to assess the degree of lipid peroxidation. TBARS significantly increased under both herbicide treatments. The increase was greater in roots (435% and 351%) than leaves (77% and 48%) under PXL and BSM exposure, respectively. The combined application of SL and PXL reduced the TBARS dose dependently in both roots and leaves. GR24 (1 μM and 5 μM) with BSM caused a 50% reduction in TBARS in roots, while in leaves, this reduction was higher with 1 μM of GR24 and BSM than with BSM alone (Figure 3C,D). These data demonstrate that herbicide stress increased the concentration of TBARS while exogenous GR24 reduced it. This is consistent with the effects of herbicide and SL treatments on antioxidant activities and H2O2 concentrations (Figure 2 and Figure 3).
Under herbicide stress, the total soluble sugars (TSS) content significantly decreased in both roots and leaves. SL combined with herbicides enhanced TSS accumulation in a dose-dependent manner in both roots and leaves compared with herbicide-only treated plants. SL (1 μM and 5 μM of GR24) combined with PXL led to 53% and 84% enhancement of TSS in roots and 22% and 52% in leaves. With BSM and SL treatment, TSS increased in both roots and leaves (73–133%) over BSM-only treatment (Figure 3E,F). Total soluble protein (TSP) content was also investigated. Under both herbicides, TSP significantly decreased in leaves but non-significantly in roots. PXL combined with SL (5 μM of GR24) significantly enhanced TSP in roots (43%) and leaves (142–156%) dose-dependently compared with the PXL-only treatment. The application of SL (1 μM and 5 μM GR24) alongside BSM enhanced TSP in roots compared with BSM alone, however, no difference was observed in leaves (Figure 3G,H). Proline content significantly increased under PXL (43% and 31%) and BSM (35% and 22%) in roots and leaves, respectively, compared with controls. However, SL (with and without herbicides) restored proline content to control levels in both roots and leaves (Figure 3I,J). This antagonistic interaction between herbicides and GR24 further confirmed SL’s role in watermelon resistance to herbicide stress.
3.4. Effect of SL and Herbicide on Watermelon Cell Ultrastructure
Ultrastructural analysis of roots and leaves was performed to assess the role of SL in mitigating herbicide-induced cellular injury. Under normal (herbicide-free) conditions, cells in the root tips exhibited oval mitochondria, small vacuoles, and well-developed nuclei, nucleoli, and nuclear membranes (Figure 4A). The application of PXL and BSM caused significant cellular damage, including swollen mitochondria, visible Golgi bodies, and starch granules in plastids, and a few giant vacuoles pushing the smaller nuclei into the corners of the cell. Thickened cell walls, large intercellular spaces, and irregular nuclear shapes were also observed (Figure 4B,C). No subcellular organ damage was observed in roots after treatment with GR24 (Figure 4D,G). Simultaneously, the mitochondrial swelling and vacuole expansion caused by PXL and BSM were alleviated by 1 µM of GR24 (Figure 4E,F) and restored almost to control levels by 5 µM of GR24 (Figure 4H,I).
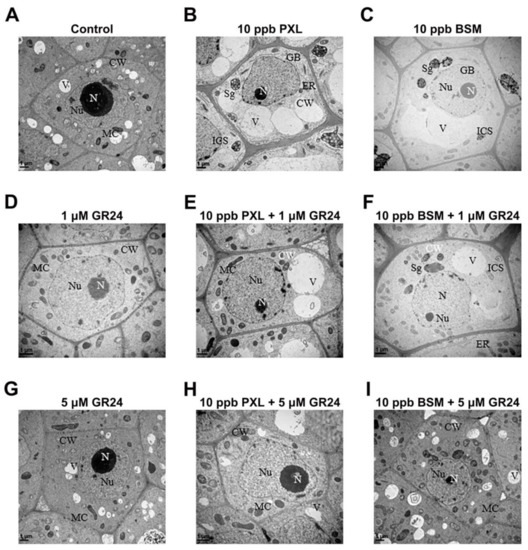
Figure 4.
Effect of SL on the ultrastructure of root tips under herbicide treatments after three days. (A) Control, (B) PXL, (C) BSM, (D) 1 μM GR24, (E) PXL + 1 μM GR24, (F) BSM + 1 μM GR24, (G) 5 μM GR24, (H) PXL + 5 μM GR24, and (I) BSM + 5 μM GR24. CW (cell wall), V (vacuole), MC (mitochondria), N (nucleus), Nu (nucleolus), ICS (intercellular spaces), Sg (starch granules), GB (golgi bodies), and ER (endoplasmic reticulum).
Cells of control leaves exhibited well-developed chloroplasts with properly arranged thylakoids, a few plastoglobuli and starch granules, and thin cell walls (Figure S1A). Subcellular damage, including smaller chloroplasts, more starch granules, and plasmolysis, was observed in PXL- and BSM-treated leaves (Figure S1B,C). Mitochondria were hardly observed in the cells of PXL-treated leaves (Figure S1B), while thick cell walls and irregular mitochondria were observed after BSM treatment (Figure S1C). The organelles of GR24-treated leaves were similar to the controls (Figure S1D,G). Co-treatment with 1-μM GR24 and herbicide increased chloroplast and thylakoid numbers and normalized mitochondria shapes (Figure S1E,F). Furthermore, the addition of 5 μM of GR24 almost completely restored chloroplast and thylakoid conditions to control levels in herbicide-treated leaf cells (Figure S1H,I).
3.5. Response of SL-Related Genes to Herbicide Stress
To further elucidate the role of SL in plant response to herbicide stress, the expression of SL biosynthesis, signal transduction, and downstream genes were investigated in roots and leaves. BSM exposure increased expression of MORE AXILLARY GROWTH1 (MAX1, an SL biosynthesis gene) but inhibited DWARF10 (D10, also an SL biosynthesis gene) in both roots and leaves (Figure 5). However, the PXL treatment significantly enhanced the expression of three SL biosynthesis genes, D27 (DWARF27), MAX1, and IPA1 (IDEAL PLANT ARCHITECTURE1), in leaves and slightly increased the MAX1 expression in roots (Figure 5). These results suggest that the herbicide stress affects SL biosynthesis, probably via MAX1 expression. The qPCR investigation of SL signal transduction and downstream genes was also carried out. Both DWARF14 (D14) and MORE AXILLARY GROWTH2 (MAX2), crucial genes in SL signal reception, were up-regulated in the roots of PXL- and BSM- treated watermelon. BRANCHED1 (BRC1) expression, a known TCP gene that acts downstream of SL signaling, was induced by BSM and PXL in roots and leaves. The SUPPRESSOR OF MAX2-LIKE6 (SMXL6) expression in leaves was also induced by both herbicides (Figure 5). Thus, the SL pathway was stimulated by both herbicides.
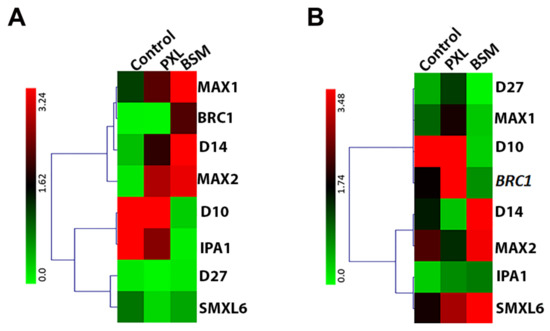
Figure 5.
Response of SL-related genes to herbicide stresses in watermelon after three days of treatments. The expression level of SL biosynthesis, signal transduction, and downstream genes in (A) roots, and (B) leaves under different herbicide stress for three days.
3.6. Effect of SL on the Expression of Antioxidant-Related CYP450 and ALS Genes
The expression of genes encoding antioxidant enzymes was investigated in herbicide-treated plants with and without SL application. The expression in roots of most antioxidant genes, including SOD (Mn), SOD2 (Fe), SOD1-2 (Zn), CAT2, and APX2, was induced by both herbicides. However, the GR24 application significantly antagonized this herbicide effect on the expression of SOD1-2 (Zn), CAT2, and APX2 and restored PXL-induced SOD (Mn) and BSM-induced SOD2 (Fe) expression. CAT1 and APX1 genes’ expression was also down-regulated by the GR24 treatment with or without herbicide stress (Figure 6A). Notably, the herbicides and SL exhibited different regulatory effects on antioxidant-related gene expression in leaves compared with roots. The expression of almost all examined genes was inhibited by PXL and/or BSM treatment, while all except APX2 were up-regulated by GR24 (Figure 6B). These results indicate that SL acts antagonistically against PXL and BSM in regulating the expression of antioxidant-related genes.
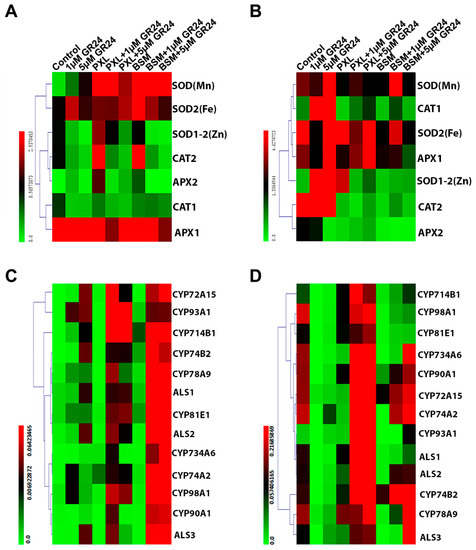
Figure 6.
Effect of SL on the expression level of antioxidant-related, CYP, and ALS genes under herbicide stresses after 4 days of treatments. The expression level of (A,B) antioxidant-related genes and (C,D) CYP and ALS genes in roots and leaves, respectively, under different treatments for three days.
To elucidate the effects of SL on herbicide metabolization and detoxification, CYP and ALS expressions were investigated (Figure 6C,D). In the absence of herbicides, the expression of these genes in roots was enhanced by exogenous GR24. PXL and BSM slightly induced some CYP genes, indicating the activation of the metabolization pathway under herbicide stress. Furthermore, the effect of SL on the CYP and ALS genes was significantly enhanced in roots when applied under herbicide stress (Figure 6C). On the other hand, while a similar synergistic enhancement was observed in leaves under combined SL and herbicide treatment, the SL application alone down-regulated the expression of most of the genes investigated (Figure 6D). These results indicate that exogenous SL may mitigate herbicide toxicity in watermelon via CYP and ALS expression induction.
4. Discussion
Herbicides are agrochemicals used to combat weeds. Their use results in environmental pollution and harmful effects on target and non-target plants by reducing their growth and development [1,48]. Herbicide residues in the soil after the rice-growing season can significantly affect the production of subsequently cultivated crops, such as watermelon. In response to herbicide toxicity, plants activate their antioxidant enzyme defense system. When antioxidant defense capacity is compromised, various exogenous signaling compounds can be applied to improve plant resistance [22,49,50].
Plant root growth and development are primarily related to cell elongation, differentiation, and division in the root apex, which are partially regulated by SL [51,52]. However, the role of SL in plant growth and development under herbicide stress is not well understood. The present study indicates that PXL and BSM negatively affect both total root length and lateral root formation. However, the application of SL to plants exposed to either herbicide restores root growth to control levels (Figure 1). In Arabidopsis, the root length of several SL-biosynthesis mutants (MAX1, MAX2, MAX3, and MAX4) was shortened, but exogenous GR24 restored the phenotype in mutants MAX1, MAX3, and MAX4. In agreement with the current findings, SL also regulated root growth under other abiotic stresses [33].
SL also affects lateral root formation through auxin efflux by regulating PIN proteins [53,54]. In rice and Arabidopsis, SL biosynthesis genes such as CAROTENOID CLEAVAGE DIOXYGENASE8 (CCD8) and MAX1 were highly expressed in the vascular tissues of roots [55]. The application of auxin also up-regulated CCD8 in the cortical region of root tips [56,57]. The root elongation zone is a site of complex auxin biosynthesis and polar auxin transport [58,59,60,61], and SL regulates root growth by influencing auxin efflux in this zone [62]. We recently demonstrated that auxin plays a positive role in watermelon exposed to both PXL and BSM [3]. SL’s alleviation of PXL and BSM-induced damage in watermelon (Figure 1) suggests a possible interaction of SL and auxin during herbicide stress.
The accumulation of reactive oxygen species (ROS) negatively impacts the cell’s tolerance of abiotic stresses and causes systemic damage to the photosynthetic pigments, including chlorophyll [63,64,65]. It is not surprising that higher H2O2 content (Figure 3B) coincides with lower chlorophyll content in PXL- and BSM-treated watermelon leaves compared with herbicide and SL treatment (Figure 1F). The increase in photosynthetic pigments following the GR24 application indicates that SL might positively impact the photosynthetic system in watermelon under herbicide stress. A similar finding was reported by Ling et al. [66] and Ma et al. [67]—SL improved plant growth by protecting the photosynthetic apparatus against oxidative stress from salinity in Oryza sativa and Brassica napus.
The primary indicators of plant response to abiotic stresses are an imbalance between ROS and enzymatic antioxidants (SOD, POD, CAT, and APX) and macromolecular impacts, including lipid peroxidation and protein and nucleic acid damage [68,69]. Due to its stability and ability to penetrate membranes, H2O2 acts as both a signaling molecule at low concentrations and a toxic molecule at high concentrations under stress conditions [70]. TBARS concentration is also considered a classic symptom of stress arising from the lipid peroxidation of cell membranes [71]. In this study, both PXL and BSM increased TBARS concentration, highlighting the significance of lipid peroxidation and oxidative stress under exposure to herbicides. Conversely, this herbicide-induced TBARS increase was dose-dependently mitigated by the SL application (Figure 3C,D), demonstrating the ability of SL to prevent oxidative stress in watermelon seedlings. The antioxidant enzyme system plays a dynamic role in ROS scavenging [72,73,74]. SOD, POD, and CAT activities in watermelon roots and leaves were induced by PXL and BSM exposure but were restored by SL application (Figure 2). Lower SOD activation under abiotic stress may indicate a healthy balance in ROS generation [73]. SL reduced H2O2 accumulation in watermelon under herbicide stress and limited activation of antioxidant enzymes and their gene expressions (Figure 2, Figure 3, and Figure 6). This study collectively shows that SL reduces herbicide toxicity in watermelon seedlings by promoting homeostasis between ROS production and antioxidant enzymatic defenses.
The application of exogenous phytohormones has been considered a strategy for enhancing plant resistance to stress by regulating various physiological and biochemical disorders [19,75,76,77,78,79]. The application of jasmonic acid was reported to alleviate herbicide toxicity in tobacco plants [18]. The current study found that the application of GR24 significantly relieved cellular damage caused by PXL and BSM exposure in both roots and leaves of watermelon seedlings (Figure 4 and Figure S1). Similar effects were observed in arbuscular mycorrhizal symbiosis (in which SL signaling is essential), demonstrating the restoration of cell membrane stability under environmental stress [25]. Our data show that PXL and BSM are phytotoxic to watermelon by increasing H2O2, TBARS, and cell death and disturbing antioxidant enzyme activities while, exogenous SL can significantly improve the cellular condition in this plant (Figure 2 and Figure 3).
In Medicago truncatula, plants deficient in nitrogen and phosphorous, ROS accumulation coincided with activated NADPH and SL biosynthetic gene expression [80]. In the present study, herbicides stimulated H2O2 accumulation and SL biosynthesis and signaling (Figure 3 and Figure 5), suggesting that SL and antioxidant pathways may work synergistically in the development of herbicide resistance. The application of exogenous GR24 reduced H2O2 (Figure 5) and increased root activity, cell viability, and photosynthetic pigments (Figure 1). It may be possible that the accumulation of SL under stress eliminates ROS or promotes the accumulation of downstream osmolytes and antioxidants to maintain cell permeability [81].
Proline is also known to play a vital role in plants exposed to abiotic stress, adjusting osmotic pressure and stabilizing cellular homeostasis. For instance, increased proline content in maize was reported to respond to oxidative stress caused by atrazine herbicides [47]. Increased proline was also associated with the response of Vicia faba to metosulam exposure [45]. These studies are in line with the current demonstration of increased proline content as a response of watermelon seedlings to herbicide exposure. The application of exogenous SL significantly suppressed proline accumulation (Figure 3I,J), further demonstrating SL’s antagonistic mode of action.
In this study, the induced expression of D14 and MAX2 under both PXL and BSM suggested the onset of the SL signaling response of watermelon to herbicide stress (Figure 5). In Arabidopsis and rice, the proteins D14, MAX2, SUPPRESSOR OF MAX2 LIKE (SMXL), and their orthologs form a complex required for SL perception and degradation of SMXL6 through the ubiquitination proteolysis system, which plays a central role in SL signaling. The degradation of SMXL6 is necessary for SL biosynthesis and the expression of BRC1, TCP DOMAIN PROTEIN1, and PRODUCTION OF ANTHOCYANIN PIGMENT1 genes [82,83]. BRC1 was up-regulated in BSM-treated roots and PXL-treated leaves (Figure 5). On the other hand, Mashiguchi et al. [84] reported that GR24 induced theexpression of BRC1. These findings imply that herbicides activate the SL response in watermelon, directly or indirectly enhancing resistance to such stresses.
In the present study, the up-regulation of CYP genes under exposure to SL together with herbicides (Figure 6C,D) implies that SL may alleviate PXL and BSM toxicity by enhancing herbicide metabolism or degradation pathways. Similarly, the up-regulation of several CYP genes has been reported in E. phyllopogon treated with bispyribac herbicides [12] and in A. aequalis after mesosulfuron-methyl and fenoxaprop-P-ethyl treatments [85,86], suggesting that CYP genes are likely to play essential roles in plant resistance to herbicides [87,88,89]. SL-induced ALS gene expression was also observed in watermelon roots in the absence of herbicides (Figure 6C), implying that SL may also enhance herbicide resistance by antagonizing the herbicide effect on acetolactate synthase. Further study is needed to elucidate the molecular mechanism of this SL action in herbicide resistance.
In summary, herbicides induce proline, H2O2, and antioxidant enzymes while the negative regulator (SMXL6) of SL biosynthesis (D14 and MAX2) is suppressed by herbicide exposure, negatively affecting the expression of CYP and ALS genes. However, the application of exogenous GR24 alleviates all the side effects of PXL and BSM exposure. Consequently, SL treatment enhances root growth and development in herbicide-treated watermelon seedlings (Figure 7).
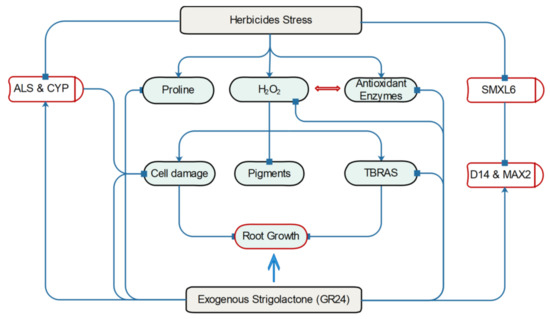
Figure 7.
The schematic presentation of the alleviatory role of SL against herbicide stress in watermelon. Herbicides induce proline, H2O2, and antioxidant enzymes and suppress the negative regulator (SMXL6) of strigolactone biosynthesis (D14 and MAX2). This effect of herbicides negatively affects the expression of CYP and ALS genes. The exogenous application of GR24 restores the negative effects of PXL and BSM and enhances the root growth and development in herbicide-treated watermelon.
5. Conclusions
The present research shows that PXL and BSM herbicides induced oxidative stress and thus inhibited the growth and development of watermelon. Furthermore, these herbicides destroyed the plant cell’s ultra-structure and disturbed the cell’s normal function. We found that the application of SL alleviated herbicide toxicity in watermelon by regulating the antioxidant enzyme defense system, probably via influencing CYP and ALS genes. The exogenous SL dose-dependently reversed the PXL- or BSM-induced antioxidant activity, suggesting that SL may participate in maintaining antioxidant enzyme homeostasis under herbicide stresses. The activation of SL-related gene expression indicated a possible implication of the SL pathway in the detoxification process of herbicide toxicity in watermelon. Understanding PXL and BSM resistance in cucurbit crops and the role of SL in abiotic stress resistance is enhanced.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agriculture11050419/s1, Table S1: Primer sequence used for qPCR, gene ID, and melting temperature. Figure S1: Effect of SL on ultrastructure of leaves under herbicides treatments after three days.
Author Contributions
Conceptualization, A.A. and Z.H.; Data curation, A.A., C.L., J.K., and Z.H.; Formal analysis, A.A., H.Y., C.L., C.W., Y.Y., and A.M.; Funding acquisition, A.A., J.Y., and M.Z.; Investigation, A.A. and J.Y.; Methodology, G.K.M., H.Y., C.L., A.M., and Z.H.; Resources, C.W. and Y.Y.; Software, A.A., G.K.M., A.M., J.K., and Z.H.; Supervision, J.Y., Z.H., and M.Z.; Validation, J.Y., Z.H., and M.Z.; Visualization, A.A.; Writing—original draft, A.A.; Writing—review & editing, G.K.M., A.M., J.K., Z.H., and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Plan of China (2019YFD1000303, 2019YFD100904, and 2018YFD0201300), the Earmarked Fund for Modern Agro-Industry Technology Research System of China (CARS-25), the National Natural Science Foundation of China (31501782, 31672175), and the Key Science and Technology Program of Zhejiang Province (2016C02051-4-1).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Boutin, C.; Strandberg, B.; Carpenter, D.; Mathiassen, S.K.; Thomas, P. Herbicide impact on non-target plant reproduction: What are the toxicological and ecological implications? Environ. Pollut. 2014, 185, 295–306. [Google Scholar] [CrossRef]
- Pacanoski, Z.; Glatkova, G. The use of herbicides for weed control in direct wet-seeded rice (Oryza sativa L.) in rice production regions in the Republic of Macedonia. Plant Prot. Sci. 2009, 45, 113–118. [Google Scholar] [CrossRef]
- Ali, A.; Xue, Q.; Chen, S.; Ren, Y.; Fu, Q.; Shao, W.; Yang, Y.; Shen, L.; Wang, J.; Lin, Y. Herbicides act as restrictive factors in rice-watermelon rotation. Sci. Hortic. 2020, 261, 108974. [Google Scholar] [CrossRef]
- Whitcomb, C.E. An introduction to ALS-inhibiting herbicides. Toxicol. Ind. Health 1999, 15, 232–240. [Google Scholar] [CrossRef]
- Roberts, D.W.; Knuteson, J.A.; Jackson, R. The dissipation of penoxsulam in flooded rice fields. Pesticides in air, plant, soil & water systems. In Proceedings of the XII Symposium Pesticide Chemistry, Piacenza, Italy, 4–6 June 2003; pp. 349–357. [Google Scholar]
- Yadav, D.B.; Yadav, A.; Punia, S. Efficacy of penoxsulam against weeds in transplanted rice. Indian J. Weed Sci. 2008, 40, 142–146. [Google Scholar]
- Janaki, P.; Nithya, C.; Kalaiyarasi, D.; Sakthivel, N.; Prabhakaram, N.; Chinnusamy, C. Residue of bensulfuron methyl in soil and rice following its pre-and post-emergence application. Plant Soil Environ. 2016, 62, 428–434. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S. Metabolism-based herbicide resistance and cross-resistance in crop weeds: A threat to herbicide sustainability and global crop production. Plant Physiol. 2014, 166, 1106–1118. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Ngo, T.D.; Malone, J.M.; Boutsalis, P.; Gill, G.; Preston, C. EPSPS gene amplification conferring resistance to glyphosate in windmill grass (Chloris truncata) in Australia. Pest. Manag. Sci. 2018, 74, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Délye, C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest. Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, S.; Endo, M.; Saika, H.; Okuno, J.; Nakamura, N.; Yokoyama, M.; Watanabe, H.; Toki, S.; Uchino, A.; Inamura, T. Cytochrome P450 CYP81A12 and CYP81A21 are associated with resistance to two acetolactate synthase inhibitors in Echinochloa phyllopogon. Plant Physiol. 2014, 165, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Werck-Reichhart, D.; Hehn, A.; Didierjean, L. Cytochromes P450 for engineering herbicide tolerance. Trends Plant Sci. 2000, 5, 116–123. [Google Scholar] [CrossRef]
- Iwakami, S.; Uchino, A.; Kataoka, Y.; Shibaike, H.; Watanabe, H.; Inamura, T. Cytochrome P450 genes induced by bispyribac-sodium treatment in a multiple-herbicide-resistant biotype of Echinochloa phyllopogon. Pest. Manag. Sci. 2014, 70, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Ganugi, P.; Miras-Moreno, B.; Garcia-Perez, P.; Lucini, L.; Trevisan, M. Concealed metabolic reprogramming induced by different herbicides in tomato. Plant Sci. 2021, 303, 110727. [Google Scholar] [CrossRef]
- Ananieva, E.A.; Alexieva, V.S.; Popova, L.P. Treatment with salicylic acid decreases the effects of paraquat on photosynthesis. J. Plant Physiol. 2002, 159, 685–693. [Google Scholar] [CrossRef]
- Porheidar Ghafarbi, S.; Rahimian Mashhadi, H.; Alizadeh, H.; Hassannejad, S. Effect of Different Herbicides and Salicylic Acid Treatment on the Photosynthetic Efficiency of Corn Cultivars Using Chlorophyll a Fluorescence Transient Curve Analysis. J. Plant Physiol. Breed. 2017, 7, 31–40. [Google Scholar]
- Kaya, A.; Doganlar, Z.B. Exogenous jasmonic acid induces stress tolerance in tobacco (Nicotiana tabacum) exposed to imazapic. Ecotoxicol. Environ. Saf. 2016, 124, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, R.; Wu, G.L.; Zhu, H.M.; Yang, H. Salicylic acid reduces napropamide toxicity by preventing its accumulation in rapeseed (Brassica napus L.). Arch. Environ. Contam. Toxicol. 2010, 59, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.C.; Alam, S.; Murr, D. Ethylene biosynthesis following foliar application of picloram to biotypes of wild mustard (Sinapis arvensis L.) susceptible or resistant to auxinic herbicides. Pestic. Biochem. Physiol. 1993, 47, 36–43. [Google Scholar] [CrossRef]
- Radwan, D.; Mohamed, A.; Fayez, K.; Abdelrahman, A. Oxidative stress caused by Basagran® herbicide is altered by salicylic acid treatments in peanut plants. Heliyon 2019, 5, e01791. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.u.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.-I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.-C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef]
- Gomez-Roldan, M.V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hanada, A.; Umehara, M.; Akiyama, K.; Magome, H.; Yamaguchi, S.; Kamiya, Y.; Arite, T.; Kyozuka, J.; Yoshida, S.; Shirasu, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar]
- Domagalska, M.A.; Leyser, O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Delaux, P.-M.; Resnick, N.; Mayzlish-Gati, E.; Wininger, S.; Bhattacharya, C.; Séjalon-Delmas, N.; Combier, J.-P.; Bécard, G.; Beeckman, T. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 2011, 233, 209–216. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Koltai, H. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiol. 2014, 166, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.; Tahmasebi-Sarvestani, Z.; Emam, Y.; Mokhtassi-Bidgoli, A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017, 119, 59–69. [Google Scholar] [CrossRef]
- Beltrano, J.; Ruscitti, M.; Arango, M.; Ronco, M. Effects of arbuscular mycorrhiza inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and P levels. J. Soil Sci. Plant Nutr. 2013, 13, 123–141. [Google Scholar] [CrossRef]
- Saeed, W.; Naseem, S.; Ali, Z. Strigolactones Biosynthesis and Their Role in Abiotic Stress Resilience in Plants: A Critical Review. Front. Plant Sci. 2017, 8, 1487. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J.-K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Ren, C.; Li, R.; Xie, Z.; Wang, J. Hydrogen peroxide and strigolactones signaling are involved in alleviation of salt stress induced by arbuscular mycorrhizal fungus in Sesbania cannabina seedlings. J. Plant Growth Regul. 2017, 36, 734–742. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef] [PubMed]
- Samrana, S.; Ali, A.; Muhammad, U.; Azizullah, A.; Ali, H.; Khan, M.; Naz, S.; Khan, M.D.; Zhu, S.; Chen, J. Physiological, ultrastructural, biochemical, and molecular responses of glandless cotton to hexavalent chromium (Cr6+) exposure. Environ. Pollut. 2020, 266, 115394. [Google Scholar] [CrossRef]
- Malangisha, G.K.; Yang, Y.; Moustafa-Farag, M.; Fu, Q.; Shao, W.; Wang, J.; Shen, L.; Huai, Y.; Lv, X.; Shi, P.; et al. Subcellular distribution of aluminum associated with differential cell ultra-structure, mineral uptake, and antioxidant enzymes in root of two different Al+3-resistance watermelon cultivars. Plant Physiol. Biochem. 2020, 155, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1985, 11, 591–592. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Sergiev, I.; Alexieva, V.; Karanov, E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt. Rend. Acad. Bulg. Sci. 1997, 51, 121–124. [Google Scholar]
- Bibi, S.; Khan, S.; Taimur, N.; Daud, M.K.; Azizullah, A. Responses of morphological, physiological, and biochemical characteristics of maize (Zea mays L.) seedlings to atrazine stress. Environ. Monit. Assess. 2019, 191, 1–14. [Google Scholar] [CrossRef]
- Gar’kova, A.; Rusyaeva, M.; Nushtaeva, O.; Aroslankina, Y.N.; Lukatkin, A. Treatment with the herbicide granstar induces oxidative stress in cereal leaves. Russ. J. Plant Physiol. 2011, 58, 1074. [Google Scholar] [CrossRef]
- Piotrowska, A.; Bajguz, A.; Godlewska-Żyłkiewicz, B.; Czerpak, R.; Kamińska, M. Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae). Environ. Exp. Bot. 2009, 66, 507–513. [Google Scholar] [CrossRef]
- Kadioglu, A.; Kadioglu, A.; Saruhan, N.; Saruhan, N.; Sağlam, A.; Sağlam, A.; Terzi, R.; Terzi, R.; Acet, T.; Acet, T. Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regul. 2011, 64, 27–37. [Google Scholar] [CrossRef]
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 2016, 7, 434. [Google Scholar] [CrossRef]
- Cheng, X.; Ruyter-Spira, C.; Bouwmeester, H. The interaction between strigolactones and other plant hormones in the regulation of plant development. Front. Plant Sci. 2013, 4, 199. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I. Lateral root initiation: One step at a time. New Phytol. 2012, 193, 867–873. [Google Scholar] [CrossRef]
- Koltai, H. Receptors, repressors, PINs: A playground for strigolactone signaling. Trends Plant Sci. 2014, 19, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Arite, T.; Iwata, H.; Ohshima, K.; Maekawa, M.; Nakajima, M.; Kojima, M.; Sakakibara, H.; Kyozuka, J. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007, 51, 1019–1029. [Google Scholar] [CrossRef]
- Sorefan, K.; Booker, J.; Haurogné, K.; Goussot, M.; Bainbridge, K.; Foo, E.; Chatfield, S.; Ward, S.; Beveridge, C.; Rameau, C. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003, 17, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, K.; Sorefan, K.; Ward, S.; Leyser, O. Hormonally controlled expression of the Arabidopsis MAX4 shoot branching regulatory gene. Plant J. 2005, 44, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- De Smet, I.; Tetsumura, T.; De Rybel, B.; dit Frey, N.F.; Laplaze, L.; Casimiro, I.; Swarup, R.; Naudts, M.; Vanneste, S.; Audenaert, D. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 2007, 134, 681–690. [Google Scholar] [CrossRef]
- Moreno-Risueno, M.A.; Van Norman, J.M.; Moreno, A.; Zhang, J.; Ahnert, S.E.; Benfey, P.N. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 2010, 329, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.; Shinohara, N.; Sieberer, T.; Williamson, L.; George, G.; Hepworth, J.; Müller, D.; Domagalska, M.A.; Leyser, O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 2010, 137, 2905–2913. [Google Scholar] [CrossRef]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crops Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Sen, G.; Eryilmaz, I.E.; Ozakca, D. The effect of aluminium-stress and exogenous spermidine on chlorophyll degradation, glutathione reductase activity and the photosystem II D1 protein gene (psbA) transcript level in lichen Xanthoria parietina. Phytochemistry 2014, 98, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Sivaci, A.; Kaya, A.; Duman, S. Effects of ascorbic acid on some physiological changes of pepino (Solanum muricatum Ait.) under chilling stress. Acta Biol. Hung. 2014, 65, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Su, Q.; Jiang, H.; Cui, J.; He, X.; Wu, Z.; Zhang, Z.; Liu, J.; Zhao, Y. Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings. Sci. Rep. 2020, 10, 1–8. [Google Scholar]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.; Zhang, C. Strigolactones improve plant growth, photosynthesis, and alleviate oxidative stress under salinity in rapeseed (Brassica napus L.) by regulating gene expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Kucukoduk, M. Upregulation of antioxidant enzymes by exogenous gallic acid contributes to the amelioration in Oryza sativa roots exposed to salt and osmotic stress. Environ. Sci. Pollut. Res. 2015, 22, 1487–1498. [Google Scholar] [CrossRef]
- Pazmiño, D.M.; Romero-Puertas, M.C.; Sandalio, L.M. Insights into the toxicity mechanism of and cell response to the herbicide 2, 4-D in plants. Plant Signal. Behav. 2012, 7, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 2016, 7, 570. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, A.; Feng, Z. Amelioration of chilling stress by triadimefon in cucumber seedlings. Plant Growth Regul. 2003, 39, 277–283. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Fecht-Christoffers, M.M.; Maier, P.; Horst, W.J. Apoplastic peroxidases and ascorbate are involved in manganese toxicity and tolerance of Vigna unguiculata. Physiol. Plant. 2003, 117, 237–244. [Google Scholar] [CrossRef]
- Wang, H.; Shan, X.; Wen, B.; Owens, G.; Fang, J.; Zhang, S.-Z. Effect of indole-3-acetic acid on lead accumulation in maize (Zea mays L.) seedlings and the relevant antioxidant response. Environ. Exp. Bot. 2007, 61, 246–253. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Duan, B.; Korpelainen, H.; Li, C. Effect of drought and ABA on growth, photosynthesis and antioxidant system of Cotinus coggygria seedlings under two different light conditions. Environ. Exp. Bot. 2011, 71, 107–113. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Lu, J.; Li, L. Salicylic acid involved in the regulation of nutrient elements uptake and oxidative stress in Vallisneria natans (Lour.) Hara under Pb stress. Chemosphere 2011, 84, 136–142. [Google Scholar] [CrossRef]
- Liang, L.; Lu, Y.L.; Yang, H. Toxicology of isoproturon to the food crop wheat as affected by salicylic acid. Environ. Sci. Pollut. Res. 2012, 19, 2044–2054. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Bonneau, L.; Huguet, S.; Wipf, D.; Pauly, N.; Truong, H.N. Combined phosphate and nitrogen limitation generates a nutrient stress transcriptome favorable for arbuscular mycorrhizal symbiosis in Medicago truncatula. New Phytol. 2013, 199, 188–202. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Strigolactones: Multi-level regulation of biosynthesis and diverse responses in plant abiotic stresses. Acta Physiol. Plant. 2018, 40, 86. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Yu, H.; Guo, H.; Lin, T.; Kou, L.; Wang, A.; Shao, N.; Ma, H.; Xiong, G. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 2020, 1–5. [Google Scholar] [CrossRef]
- Zhao, L.-H.; Zhou, X.E.; Yi, W.; Wu, Z.; Liu, Y.; Kang, Y.; Hou, L.; De Waal, P.W.; Li, S.; Jiang, Y. Destabilization of strigolactone receptor DWARF14 by binding of ligand and E3-ligase signaling effector DWARF3. Cell Res. 2015, 25, 1219–1236. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Sasaki, E.; Shimada, Y.; Nagae, M.; Ueno, K.; Nakano, T.; Yoneyama, K.; Suzuki, Y.; Asami, T. Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci. Biotech. Biochem. 2009, 73, 2460–2465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, W.; Bai, S.; Guo, W.; Yuan, G.; Wang, F.; Liu, W.; Wang, J. Transcriptome profiling to identify genes involved in mesosulfuron-methyl resistance in Alopecurus aequalis. Front. Plant Sci. 2017, 8, 1391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yan, Y.; Ge, L.a.; Zhu, B.; Liu, W.; Wang, J. Target site mutations and cytochrome P450s confer resistance to fenoxaprop- P -ethyl and mesosulfuron-methyl in Alopecurus aequalis. Pest. Manag. Sci. 2019, 75, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, Y.; Li, J.; Fang, J.; Liu, T.; Dong, L. Transcriptome profiling to identify cytochrome P450 genes involved in penoxsulam resistance in Echinochloa glabrescens. Pestic. Biochem. Physiol. 2019, 158, 112–120. [Google Scholar] [CrossRef]
- Yasuor, H.; Osuna, M.D.; Ortiz, A.; Saldaín, N.s.E.; Eckert, J.W.; Fischer, A.J. Mechanism of Resistance to Penoxsulam in Late Watergrass [Echinochloa phyllopogon (Stapf) Koss.]. J. Agric. Food Chem. 2009, 57, 3653–3660. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Wang, X.; Ren, T. Expression of a wheat cytochrome P450 monooxygenase cDNA in yeast catalyzes the metabolism of sulfonylurea herbicides. Pestic. Biochem. Physiol. 2006, 85, 1–6. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).