Application of Dynamic Controlled Atmosphere Technologies to Reduce Incidence of Physiological Disorders and Maintain Quality of ‘Granny Smith’ Apples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Supply, Treatments and Storage
- RLOS + ULO: Cycles of RLOS (0.5% O2 for 10 d) followed ULO (0.9% O2 and 0.8% CO2 for 21 d and 0.5% O2 for 7 d);
- RLOS + CA: Cycles of RLOS (0.5% O2 for 10 d) followed by CA (1.5% O2 and 1% CO2 for 21 d and 0.5% O2 for 7 d);
- DCA-CF: Storage at 0.6% O2 and 0.8% CO2;
- RA: Storage at ≈21% O2 and 90–95% RH.
2.2. Assessment of Quality and Analysis
2.2.1. Physiological Disorders
Superficial Scald
Coreflush
2.2.2. Physicochemical Properties
Firmness
Total Soluble Solids and Titratable Acidity
Background Color
2.2.3. Headspace Volatile Analysis
2.2.4. Biochemical Analysis
Total Phenolic Content
Radical Scavenging Activity
2.2.5. Statistical Analysis
3. Results
3.1. Physiological Disorders
3.1.1. Superficial Scald
3.1.2. Coreflush
3.2. Physicochemical Properties
3.2.1. Flesh Firmness
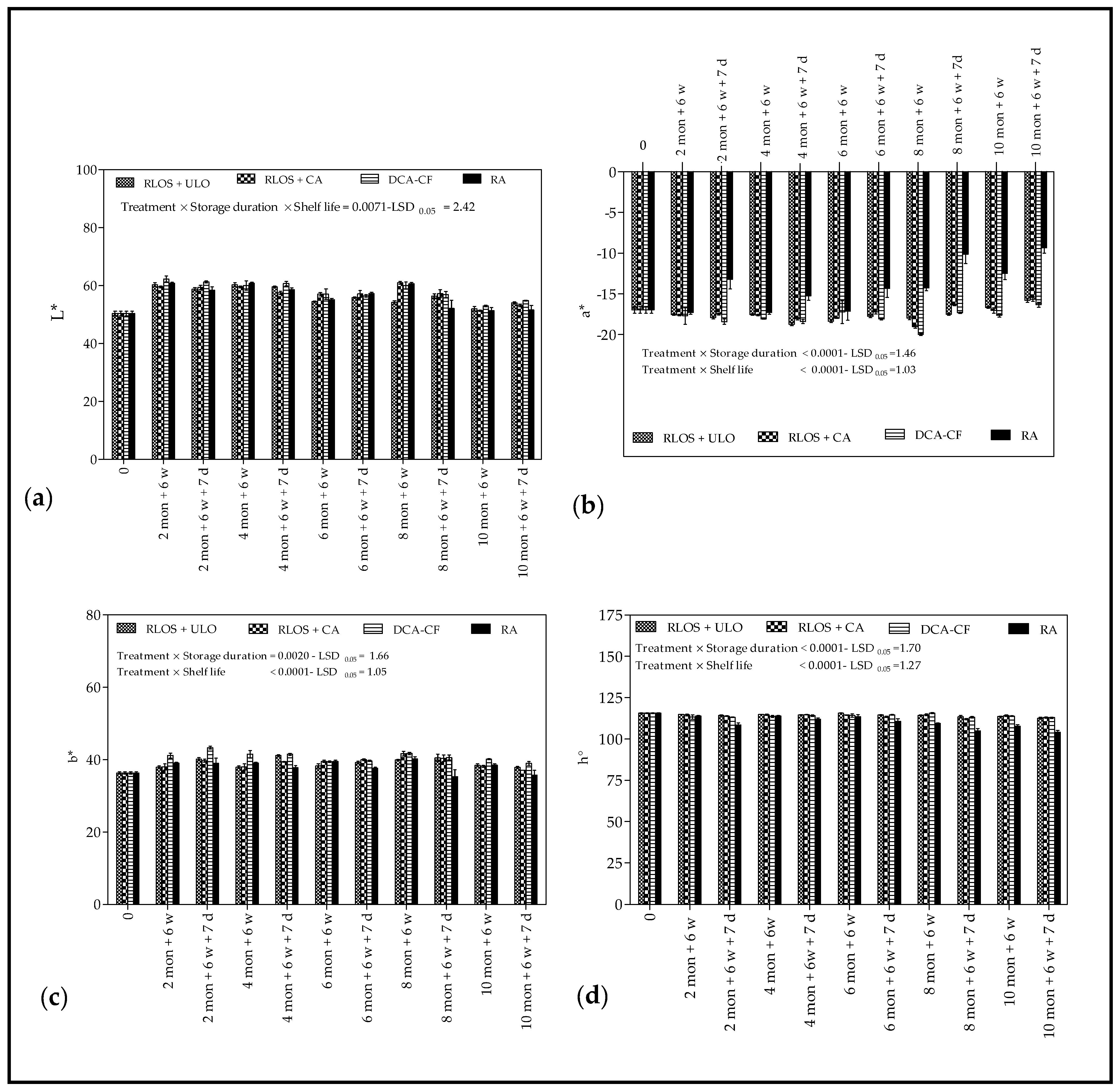
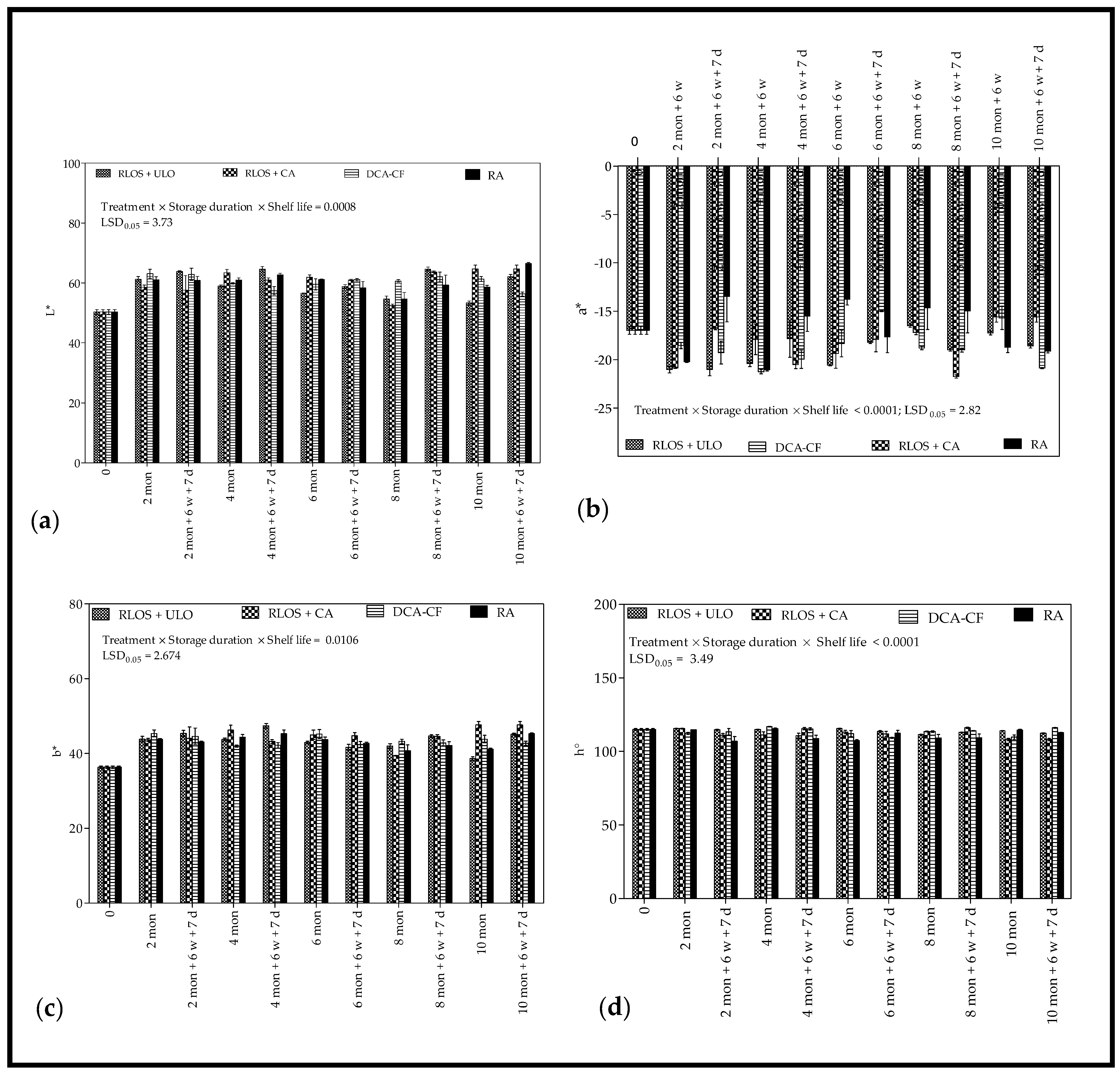
3.2.2. Background Color
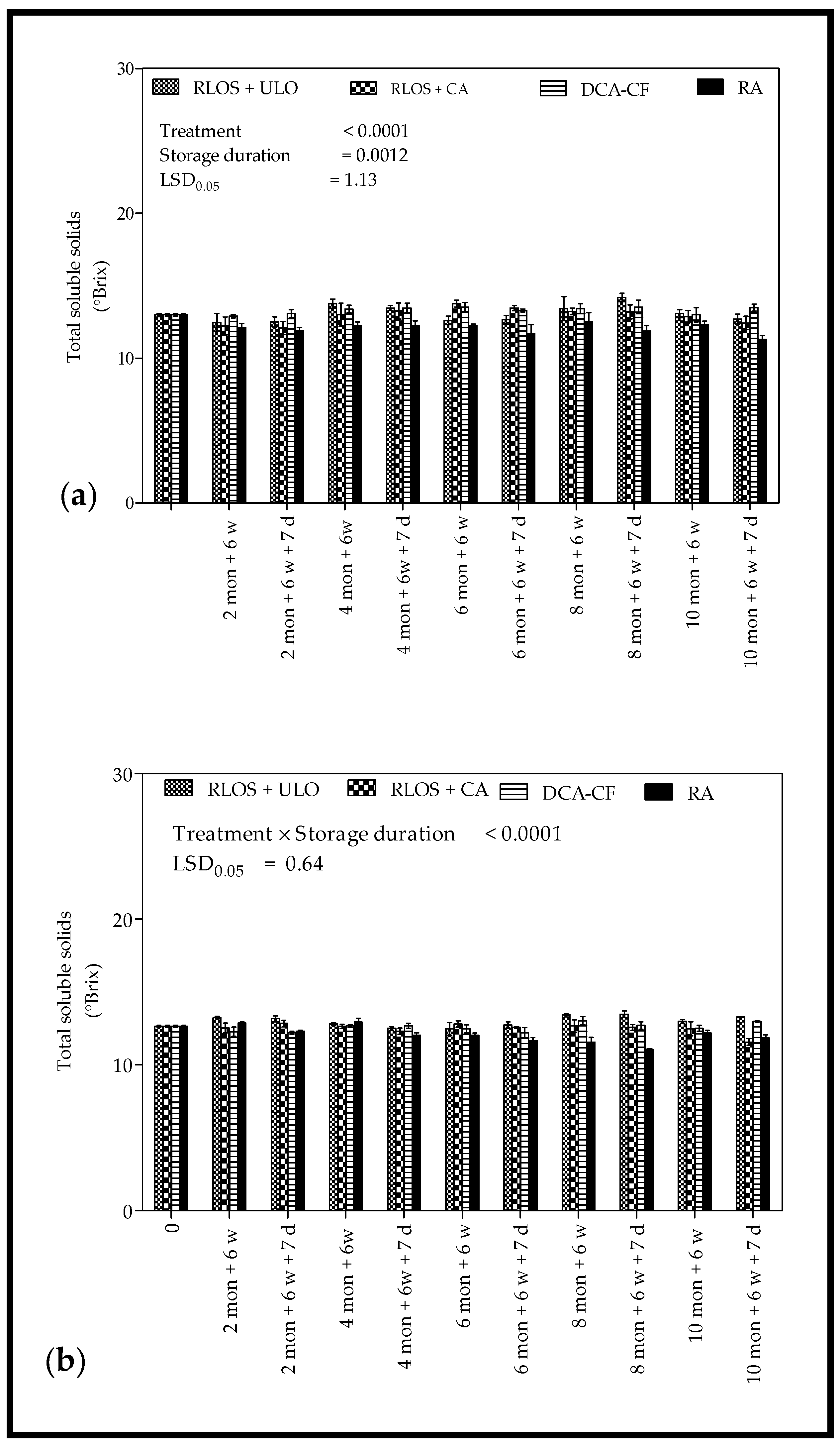
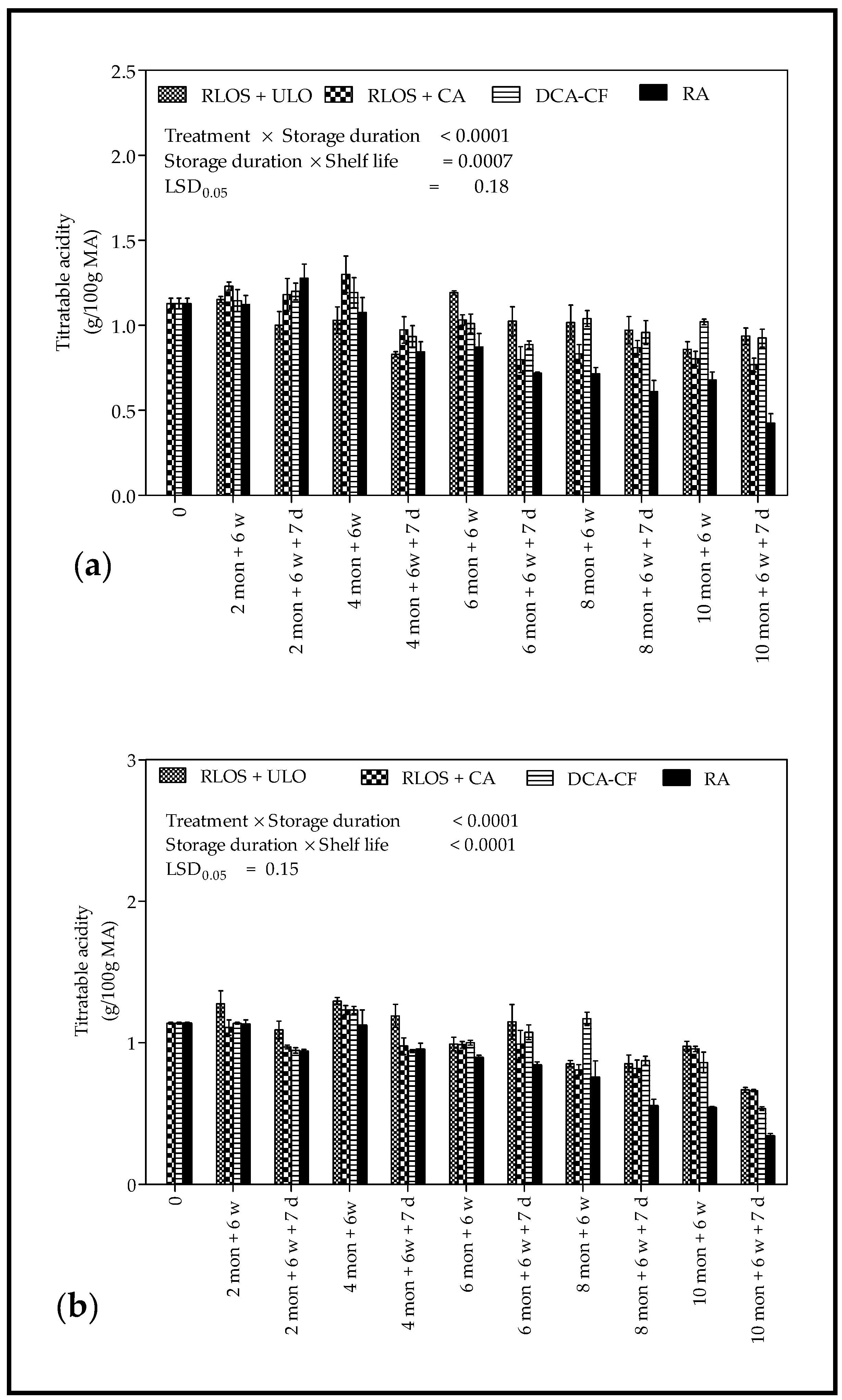
3.2.3. Total Soluble Solids and Titratable Acidity
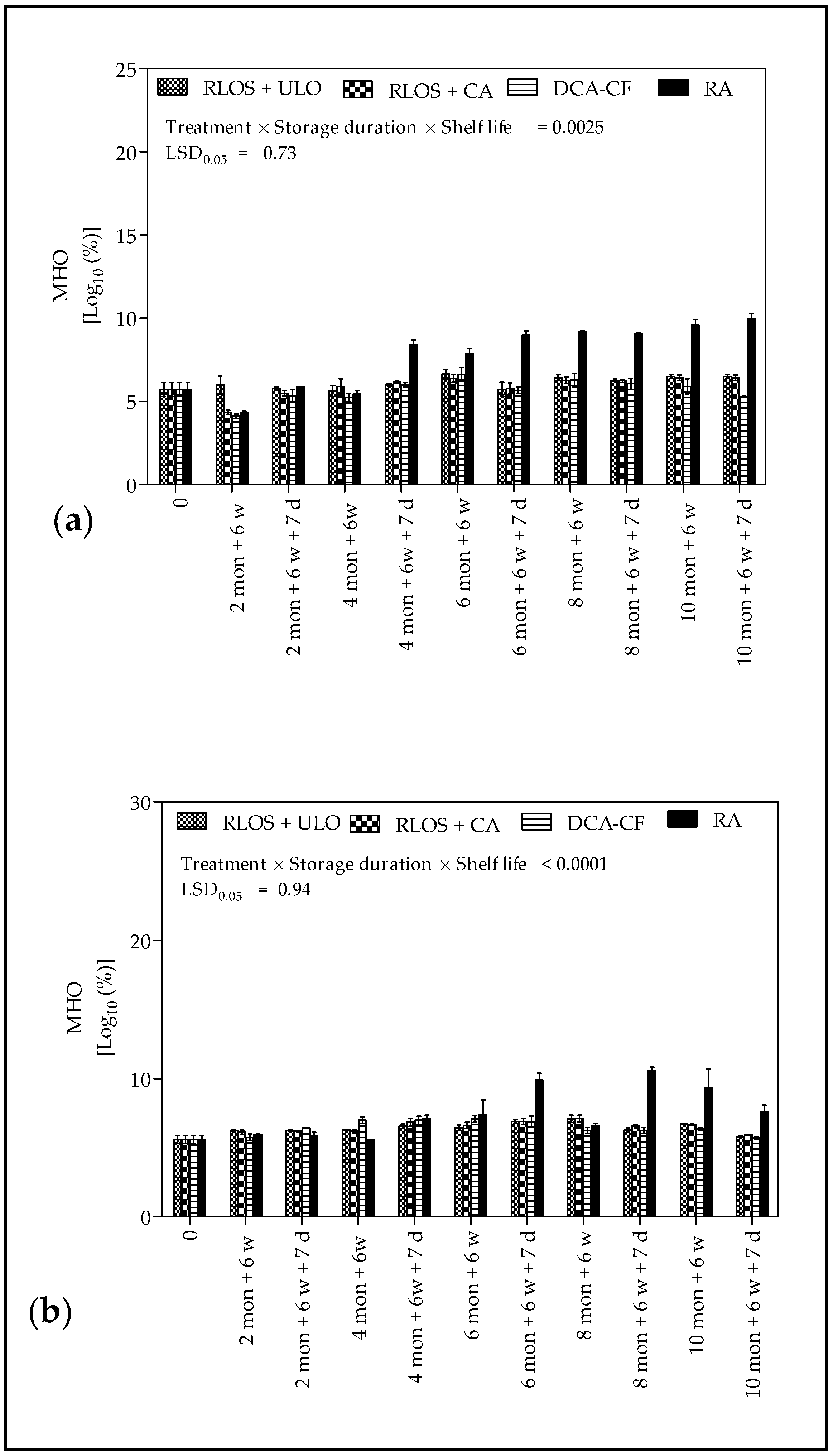
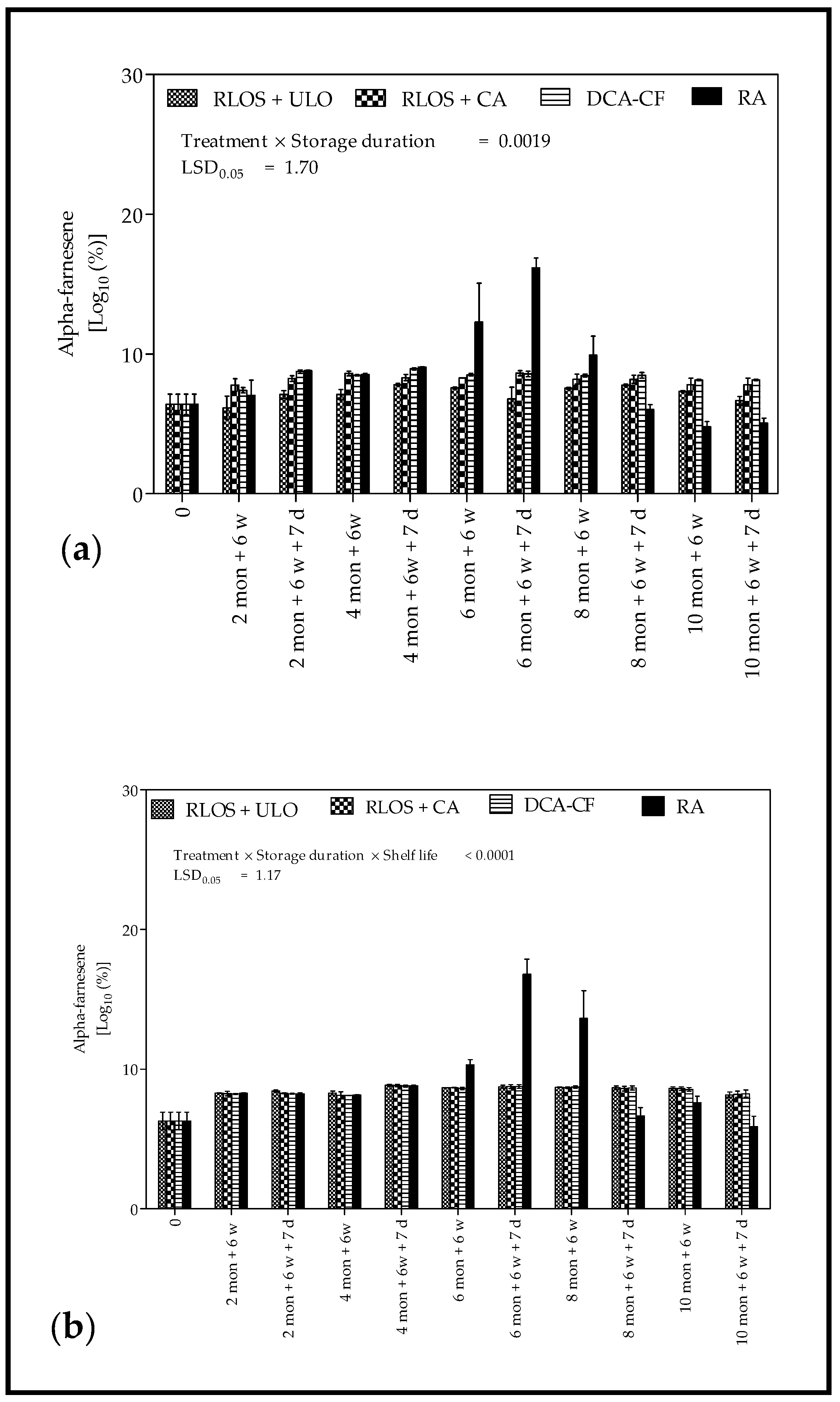
3.3. Headspace Volatile Analysis
3.4. Biochemical Analysis
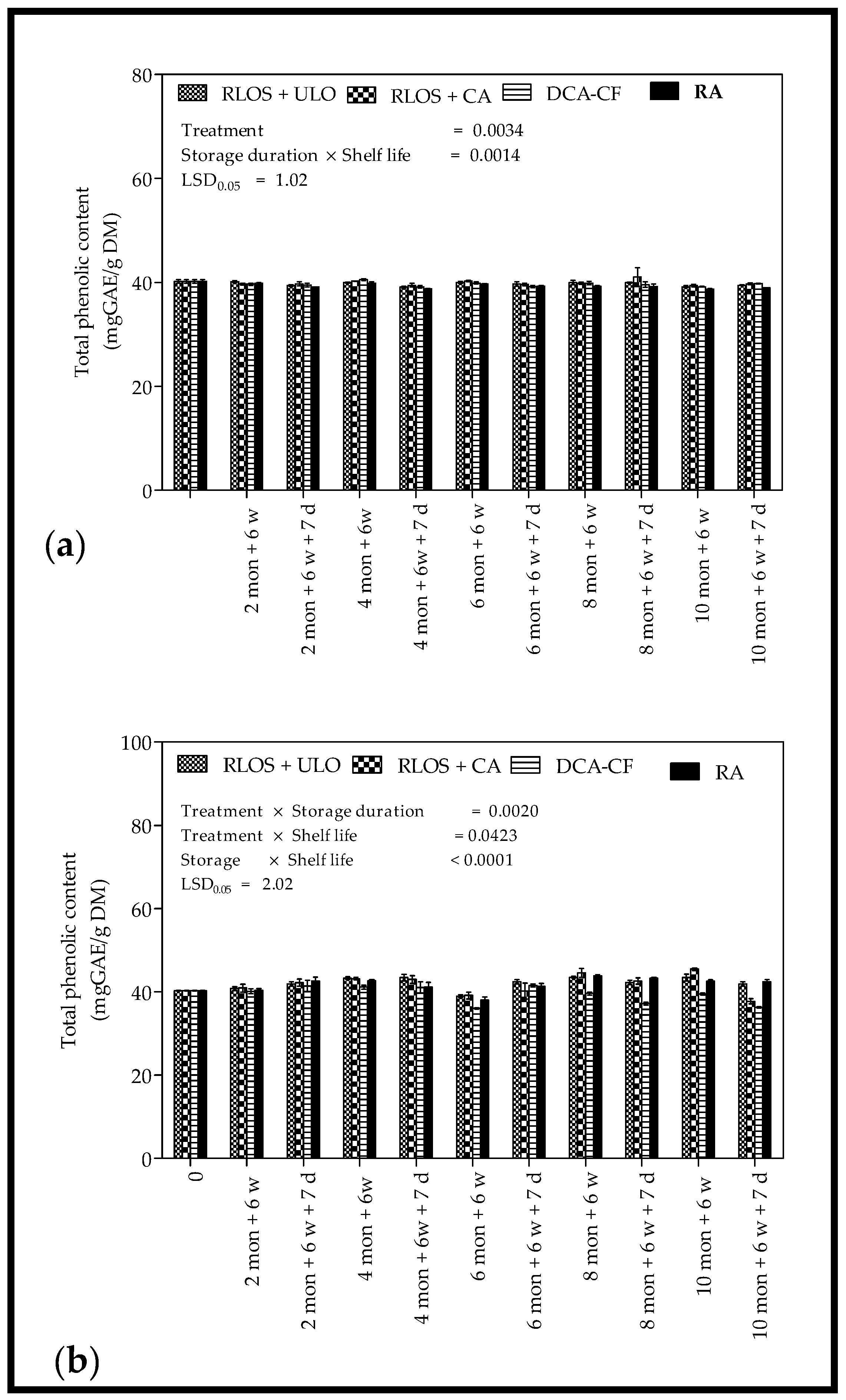
3.4.1. Total Phenolic Content
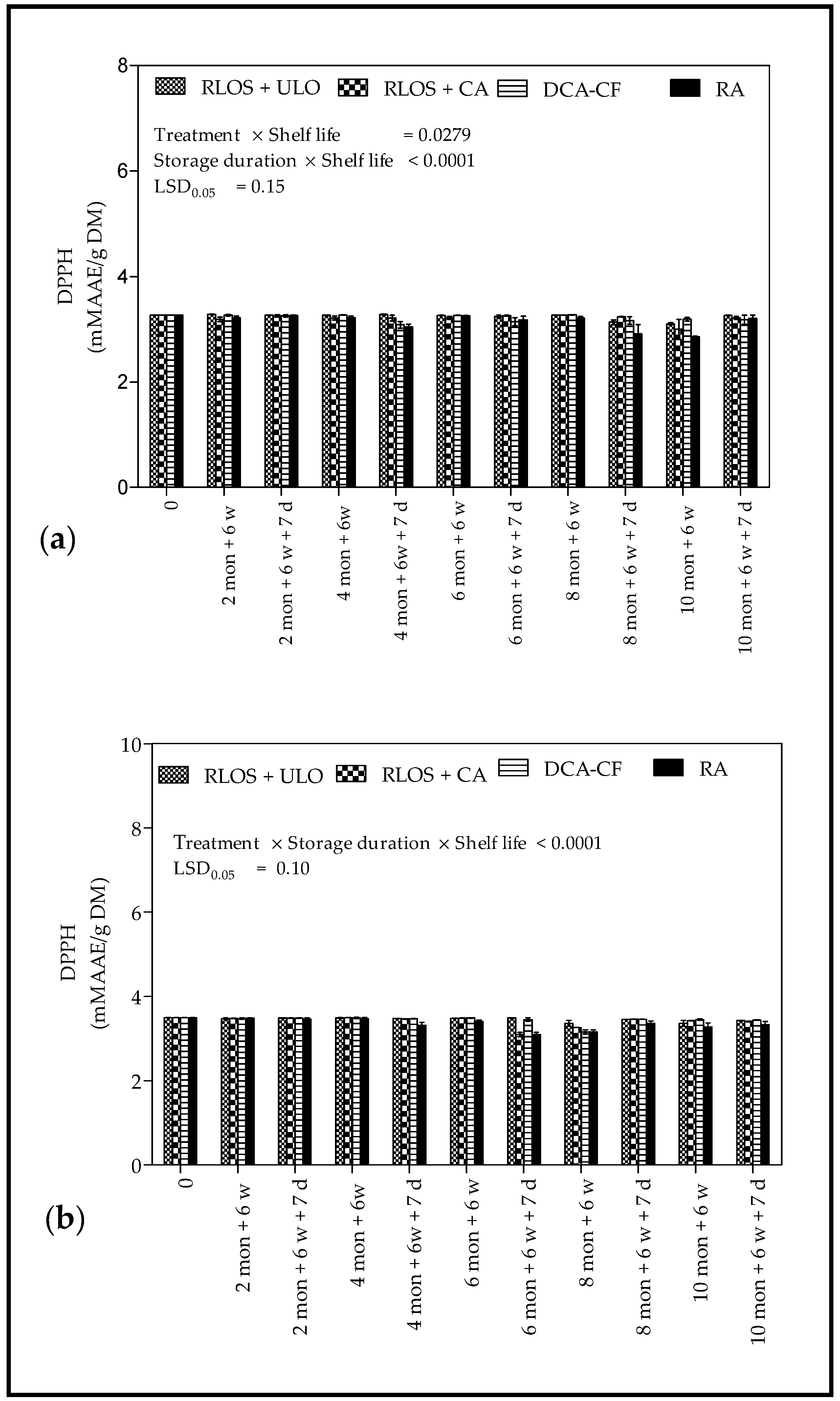
3.4.2. Radical Scavenging Activity
3.5. Correlation Analysis
4. Discussion
4.1. Physiological Disorders
4.1.1. Superficial Scald
4.1.2. Coreflush
4.2. Physicochemical Properties
4.2.1. Flesh Firmness
4.2.2. Background Color
4.2.3. Total Soluble Solids and Titratable Acidity
4.3. Headspace Volatile Analysis
4.4. Biochemical Analysis
4.4.1. Total Phenolic Content
4.4.2. Radical Scavenging Activity
4.5. Correlation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bordonaba, J.G.; Matthieu-Hurtiger, V.; Westercamp, P.; Coureau, C.; Dupille, E.; Larrigaudière, C. Dynamic changes in conjugated trienols during storage may be employed to predict superficial scald in ‘Granny Smith’ apples. LWT Food Sci. Technol. 2013, 54, 535–541. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Vries, F.; van der Merwe, K.; Crouch, E.; Opara, U.L. Repeated application of dynamic controlled atmospheres reduced superficial scald incidence in ‘Granny Smith’ apples. Sci. Hortic. 2017, 220, 168–175. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Opara, U.L. Recent developments on dynamic controlled atmosphere storage of apples—A review. Food Packag. Shelf Life 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Lurie, S.; Watkins, C.B. Superficial scald, its etiology and control. Postharvest Biol. Technol. 2012, 65, 44–60. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Vries, F.; van der Merwe, K.; Crouch, E.; Opara, U.L. Classification of ‘Granny Smith’ apples with different levels of superficial scald severity based on targeted metabolites and discriminant analysis. J. Appl. Bot. Food Qual. 2016, 89, 49–55. [Google Scholar]
- Rudell, D.R.; Mattheis, J.P.; Hertog, M.L. Metabolomic change precedes apple superficial scald symptoms. J. Agric. Food Chem. 2009, 57, 8459–8466. [Google Scholar] [CrossRef]
- Barden, C.L.; Bramlage, W.J. Relationships of antioxidants in apple peel to changes in α-farnesene and conjugated trienes during storage, and to superficial scald development after storage. Postharvest Biol. Technol. 1994, 4, 23–33. [Google Scholar] [CrossRef]
- Golding, J.B.; McGlasson, W.B.; Wyllie, S.G. Relationship between production of ethylene and α-farnesene in apples, and how it is influenced by the timing of diphenylamine treatment. Postharvest Biol. Technol. 2001, 21, 225–2533. [Google Scholar] [CrossRef]
- Mditshwa, A.; Vries, F.; van der Merwe, K.; Crouch, E.; Opara, U.L. Antioxidant content and phytochemical properties of apple ‘Granny Smith’ at different harvest times. S. Afr. J. Plant Soil 2015, 32, 221–226. [Google Scholar] [CrossRef]
- Zanella, A.; Stürz, S. Replacing DPA postharvest treatment by strategical application of novel storage technologies controls scald in 1/10th of EU’s apples producing area. Acta Hortic. 2013, 1012, 419–426. [Google Scholar] [CrossRef]
- Liu, Y.B. Ultralow oxygen treatment for postharvest control of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), on iceberg lettuce. II. Effects of pre-treatment storage on lettuce quality. Postharvest Biol. Technol. 2008, 49, 135–139. [Google Scholar] [CrossRef]
- Juhņeviča-Radenkova, K.; Radenkovs, V. Influence of 1-Methylcyclopropene and ULO conditions on sensory characteristics of apple fruit grown in Latvia. J. Hortic. Res. 2016, 24, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dilley, D.R. Initial low oxygen stress controls superficial scald of apples. Postharvest Biol. Technol. 2000, 18, 201–213. [Google Scholar] [CrossRef]
- Ramokonyane, T.M. Effects of Dynamic Controlled Atmosphere and Initial Low Oxygen Stress on Superficial Scald of ‘Granny Smith’ Apples and ‘Packham’s Triumph’ Pears. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, March 2016. Available online: https://scholar.sun.ac.za/handle/10019.1/98327 (accessed on 20 January 2021).
- Ghahramani, F.; Scott, K.J. Oxygen stress of ‘Granny Smith’ apples in relation to superficial scald, ethanol and alpha-farnesene, and conjugated trienes. Aust. J. Agric. Res. 1998, 49, 207–210. [Google Scholar] [CrossRef]
- Chervin, C.; Brouard, L.; Frémondière, G.; Westercamp, P.; Thieffry, N.; Larrigaudiere, C. Superficial scald versus ethanol vapours: A dose response. Superficial scald versus ethanol vapours: A dose response. Acta Hortic. 2003, 600, 117–121. [Google Scholar] [CrossRef]
- Wang, Z.; Dilley, D.R. Initial low oxygen stress (ILOS) controls scald of apples without using postharvest chemical treatments. Acta Hortic. 2001, 553, 261–266. [Google Scholar] [CrossRef]
- Thewes, F.R.; Both, V.; Brackmann, A.; Weber, A.; de Oliveira Anese, R. Dynamic controlled atmosphere and ultralow oxygen storage on ‘Gala’ mutants quality maintenance. Food Chem. 2015, 188, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Erkan, M.; Pekmezc, M.; Gübbük, H.; Karafiah, I. Effects of controlled atmosphere storage on scald development and postharvest physiology of ‘Granny Smith’ Apples. Turk. J. Agric. 2004, 28, 43–48. [Google Scholar]
- Tran, D.T.; Verlinden, B.E.; Hertog, M.; Nicolaï, B.M. Monitoring of extremely low oxygen control atmosphere storage of ‘Greenstar’ apples using chlorophyll fluorescence. Sci. Hortic. 2015, 184, 18–22. [Google Scholar] [CrossRef]
- Prange, R.K.; Wright, A.H.; DeLong, J.M.; Zanella, A. A review on the successful adoption of dynamic controlled-atmosphere (DCA) storage as a replacement for diphenylamine (DPA), the chemical used for control of superficial scald in apples and pears. Acta Hortic. 2015, 1071, 389–396. [Google Scholar] [CrossRef]
- Weber, A.; Brackmann, A.; Both, V.; Pavanello, E.P.; de Oliveira Anese, R.; Thewes, F.R.; Anese, R.D.O.; Rodrigo, F. Respiratory quotient: Innovative method for monitoring ‘Royal Gala’ apple storage in a dynamic controlled atmosphere. Sci. Agric. 2015, 72, 28–33. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. Effects of location within the tree canopy on carbohydrates, organic acids, amino acids and phenolic compounds in the fruit peel and flesh from three apple (Malus × domestica) cultivars. Hortic. Res. 2014, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bessemans, N.; Verboven, P.; Verlinden, B.E.; Nicolaï, B.M. A novel type of dynamic controlled atmosphere storage based on the respiratory quotient (RQ-DCA). Postharvest Biol. Technol. 2016, 115, 91–102. [Google Scholar] [CrossRef]
- Wright, A.H.; DeLong, J.M.; Gunawardena, A.H.; Prange, R.K. The interrelationship between the lower oxygen limit, chlorophyll fluorescence and the xanthophyll cycle in plants. Photosynth. Res. 2011, 107, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Veltman, R.H.; Verschoor, J.A.; van Dugteren, J.H.R. Dynamic control system (DCS) for apples (Malus domestica Borkh. cv ‘Elstar’): Optimal quality through storage based on product response. Postharvest Biol. Technol. 2003, 27, 79–86. [Google Scholar] [CrossRef]
- Watkins, C.B.; Bramlage, W.J.; Cregoe, B.A. Superficial scald of ‘Granny Smith’ apples is expressed as a typical chilling injury. J. Am. Soc. Hortic. Sci. 1995, 120, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Opara, U.L. Texture measurement approaches in fresh and processed foods—A review. Food Res. Int. 2013, 51, 823–835. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.O. Effects of bruising and storage duration on physiological response and quality attributes of pomegranate fruit. Sci. Hortic. 2020, 267, 1–7. [Google Scholar] [CrossRef]
- Al-Said, F.A.; Opara, L.U.; Al-Yahyai, R.A. Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman. J. Food Eng. 2009, 90, 129–134. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Daus, A.; Porat, R. Changes in sensory quality and aroma volatile composition during prolonged storage of ‘Wonderful’ pomegranate fruit. Int. J. Food Sci. Technol. 2013, 48, 1569–1578. [Google Scholar] [CrossRef]
- Poirier, B.C.; Mattheis, J.P.; Rudell, D.R. Extending ‘Granny Smith’ apple superficial scald control following long-term ultra-low oxygen controlled atmosphere storage. Postharvest Biol. Technol. 2020, 161, 111062. [Google Scholar] [CrossRef]
- Lavilla, T.; Puy, J.; López, M.L.; Recasens, I.; Vendrell, M. Relationships between volatile production, fruit quality, and sensory evaluation in Granny Smith apples stored in different controlled-atmosphere treatments by means of multivariate analysis. J. Agric. Food Chem. 1999, 47, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Hunt, M.; Barnett, L.E. Cultivar and growing region determine the antioxidant polyphenolic concentration and composition of apples grown in New Zealand. J. Agric. Food Chem. 2005, 53, 3065–3070. [Google Scholar] [CrossRef]
- Zanella, A. Control of apple superficial scald and ripening—A comparison between 1-methylcyclopropene and diphenylamine postharvest treatments, initial low oxygen stress and ultra-low oxygen storage. Postharvest Biol. Technol. 2003, 27, 69–78. [Google Scholar] [CrossRef]
- Ju, Z.; Curry, E.A. Stripped corn oil emulsion alters ripening, reduces superficial scald, and reduces core flush in ‘Granny Smith’ apples and decay in ‘d’ Anjou’ pears. Postharvest Biol. Technol. 2000, 20, 185–193. [Google Scholar] [CrossRef]
- Johnson, D.S.; Colgan, R.J. Low ethylene controlled atmosphere induces adverse effects on the quality of ‘Cox’s Orange Pippin’ apples treated with aminoethoxyvinylglycine during fruit development. Postharvest Biol. Technol. 2003, 27, 59–68. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P.; Blankenship, S. Development of apple superficial scald, soft scald, core flush, and greasiness is reduced by MCP. J. Agric. Food Chem. 1999, 47, 3063–3068. [Google Scholar] [CrossRef]
- De Ell, J.R.; Khanizadeh, S.; Saad, F.; Ferree, D.C. Factors affecting apple fruit firmness—A review. J. Am. Pomol. Soc. 2001, 55, 8–27. [Google Scholar]
- Rebeaud, S.G.; Gasser, F. Fruit quality as affected by 1-MCP treatment and DCA storage—A comparison of the two methods. Eur. J. Hortic. Sci. 2015, 80, 18–24. [Google Scholar] [CrossRef]
- Zanella, A.; Rossi, O. Post-harvest retention of apple fruit firmness by 1-methylcyclopropene (1-MCP) treatment or dynamic CA storage with chlorophyll fluorescence (DCA-CF). Eur. J. Hortic. Sci. 2015, 80, 11–17. [Google Scholar] [CrossRef]
- Graell, J.; Larrigaudiere, C.; Vendrell, M. Effect of low-oxygen atmospheres on quality and superficial scald of ‘Top red’ apples. Food Sci. Technol. Int. 1997, 3, 203–211. [Google Scholar] [CrossRef]
- Magazin, N.; Keserović, Z.; Milić, B.; Dorić, M. Fruits quality of granny smith apples treated with 1- methylcyclopropene or diphenylamine and stored under ulo conditions. Acta Hortic. 2013, 981, 619–624. [Google Scholar] [CrossRef]
- Ding, C.K.; Chachin, K.; Hamauzu, Y.; Ueda, Y.; Imahori, Y. Effects of storage temperatures on physiology and quality of loquat fruit. Postharvest Biol. Technol. 1998, 14, 309–315. [Google Scholar] [CrossRef]
- Melgarejo, P.; Salazar, D.M.; Artes, F. Organic acids and sugars composition of harvested pomegranate fruits. Eur. Food Res. Technol. 2000, 211, 185–190. [Google Scholar] [CrossRef]
- Both, V.; Thewes, F.R.; Brackmann, A.; de Freitas Ferreira, D.; Pavanello, E.P.; Wagner, R. Effect of low oxygen conditioning and ultralow oxygen storage on the volatile profile, ethylene production and respiration rate of ‘Royal Gala’ apples. Sci. Hortic. 2016, 209, 156–164. [Google Scholar] [CrossRef]
- Lafer, G. Effect of different CA storage conditions on storability and fruit quality of organically grown ‘Uta’ pears. Acta Hortic. 2011, 909, 757–760. [Google Scholar] [CrossRef]
- Matich, A.J.; Banks, N.H.; Rowan, D.D. Modification of α-farnesene levels in cool-stored ‘Granny Smith’ apples by ventilation. Postharvest Biol. Technol. 1998, 14, 159–170. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Paliyath, G.; Murr, D.P. Biosynthesis of alpha-farnesene and its relation to superficial scald development in ‘Delicious’ apples. J. Am. Soc. Hort. Sci. 1998, 123, 882–886. [Google Scholar] [CrossRef] [Green Version]
- Sabban-Amin, R.; Feygenberg, O.; Belausov, E.; Pesis, E. Low oxygen and 1-MCP pretreatments delay superficial scald development by reducing reactive oxygen species (ROS) accumulation in stored ‘Granny Smith’ apples. Postharvest Biol. Technol. 2011, 62, 295–304. [Google Scholar] [CrossRef]
- Anet, E.F.L.J. Superficial scald, a functional disorder of stored apples. XI. Apple antioxidants. J. Sci. Food Agric. 1974, 25, 299–304. [Google Scholar] [CrossRef]
- Huelin, F.E.; Coggiola, I.M. Superficial scald, a functional disorder of stored apples. IV. Effect of variety, maturity, oiled wraps and diphenylamine on the concentration of alpha-farnesene in the fruit. J. Sci. Food Agric. 1968, 19, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, B.D.; Villalobos-Acuña, M.; Mitcham, E.J.; Mattheis, J.P. Superficial scald susceptibility and alpha-farnesene metabolism in ‘Bartlett’ pears grown in California and Washington. Postharvest Biol. Technol. 2009, 53, 43–50. [Google Scholar] [CrossRef]
- Mditshwa, A. The Potential of Dynamic Controlled Atmospheres and Possible Mechanisms in Mitigating Superficial Scald in Apples cv. ‘Granny Smith’. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, December 2015. Available online: https://scholar.sun.ac.za/handle/10019.1/97690 (accessed on 20 January 2021).
- Anet, E.F.L.J.; Coggiola, I.M. Superficial scald, a functional disorder of stored apples. X. Control of alpha-farnesene autoxidation. J. Sci. Food Agric. 1974, 25, 293–298. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Impact of suppression of ethylene action or biosynthesis on flavor metabolites in apple (Malus domestica Borkh) Fruits. J. Agric. Food Chem. 2004, 52, 5694–5701. [Google Scholar] [CrossRef]
- Shaham, Z.; Lers, A.; Lurie, S. Effect of heat or 1-methylcyclopropene on antioxidative enzyme activities and antioxidants in apples in relation to superficial scald development. J. Amer. Soc. Hort. Sci. 2003, 128, 761–766. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, A.; Ben, J. Antioxidant properties of two apple cultivars during long-term storage. Food Chem. 2003, 80, 303–307. [Google Scholar] [CrossRef]
- Tarozzi, A.; Marchesi, A.; Cantelli-forti, G.; Hrelia, P. Cold-storage affects antioxidant properties of apples in Caco-2 cells. J. Nutr. 2004, 134, 1105–1109. [Google Scholar] [CrossRef] [Green Version]
- Ahn, T.; Paliyath, G.; Murr, D.P. Antioxidant enzyme activities in apple varieties and resistance to superficial scald development. Food Res. Int. 2007, 40, 1012–1019. [Google Scholar] [CrossRef]

| Season | Storage Duration (Months) | Shelf-Life (Days) | Superficial Scald (%) | |||
|---|---|---|---|---|---|---|
| RLOS + ULO | RLOS + CA | DCA-CF | RA | |||
| 2015 | 2 | 0 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| 7 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | ||
| 4 | 0 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 90.86 ± 8.45 b | |
| 7 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 100.00 ± 0.00 a | ||
| 6 | 0 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 100.00 ± 0.00 a | |
| 7 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 100.00 ± 0.00 a | ||
| 8 | 0 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 100.00 ± 0.00 a | |
| 7 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 100.00 ± 0.00 a | ||
| 10 | 0 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 100.00 ± 0.00 a | |
| 7 | 0.00 ± 0.00 d | 7.06 ± 7.96 d | 0.00 ± 0.00 d | 100.00 ± 0.00 a | ||
| 2016 | 2 | 0 | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| 7 | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 84.92 ± 6.80 a | ||
| 4 | 0 | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 29.24 ± 10.63 b | |
| 7 | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 100.00 ± 0.00 a | ||
| 6 | 0 | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 97.48 ± 2.22 a | |
| 7 | 32.38 ± 24.12 b | 0.00 ± 0.00 c | 3.36 ± 1.07 c | 100.00 ± 0.00 a | ||
| 8 | 0 | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ±0.00 c | 100.00 ± 0.00 a | |
| 7 | 0.00 ± 0.00 c | 5.17 ± 5.45 c | 1.31 ± 2.26 c | 100.00 ± 0.00 a | ||
| 10 | 0 | 2.78 ± 2.64 c | 4.88 ±2.32 c | 0.00 ± 0.00 c | 100.00 ± 0.00 a | |
| 7 | 32.04 ± 13.60 b | 32.15 ± 7.79 b | 2.41 ± 1.63 def | 100.00 ± 0.00 a | ||
| Pr > F | ||||||
| Season | 2015 | 2016 | ||||
| Treatment (A) | <0.0001 | <0.0001 | ||||
| Storage duration (B) | <0.0001 | <0.0001 | ||||
| Shelf life (C) | 0.0186 | <0.0001 | ||||
| A × B | <0.0001 | <0.0001 | ||||
| A × C | 0.0729 | <0.0001 | ||||
| B × C | 0.0729 | <0.0001 | ||||
| A × B × C | 0.0003 | <0.0001 | ||||
| Season | Storage Duration (Months) | Shelf-Life (Days) | Coreflush (%) | |||
|---|---|---|---|---|---|---|
| RLOS + ULO | RLOS + CA | DCA-CF | RA | |||
| 2015 | 2 | 0 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g |
| 7 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | ||
| 4 | 0 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | |
| 7 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | ||
| 6 | 0 | 0.00 ± 0.00 g | 66.66 ± 5.77 f | 80.00 ± 0.00 d | 0.00 ± 0.00 g | |
| 7 | 76.67 ± 5.77 de | 86.67 ± 11.55 c | 100.00 ± 0.00 a | 0.00 ± 0.00 g | ||
| 8 | 0 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | |
| 7 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | ||
| 10 | 0 | 80.00 ± 0.00 d | 93.33 ± 5.77 b | 100.00 ± 0.00 a | 73.33 ± 11.54 e | |
| 7 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | ||
| 2016 | 2 | 0 | 26.67 ± 20.81 def | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 0.00 ± 0.00 g |
| 7 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | ||
| 4 | 0 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | |
| 7 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | ||
| 6 | 0 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 43.33 ± 5.77 bcd | 0.00 ± 0.00 g | |
| 7 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | ||
| 8 | 0 | 20.00 ± 0.00 ef | 30.00 ± 10.00 cde | 100.00 ± 0.00 a | 0.00 ± 0.00 g | |
| 7 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g | ||
| 10 | 0 | 60.00 ± 10.00 b | 53.33 ± 11.55 b | 100.00 ± 0.00 a | 46.67 ± 5.77 bc | |
| 7 | 0.00 ± 0.00 g | 10.00 ±17.32 fg | 0.00 ± 0.00 g | 0.00 ± 0.00 g | ||
| Pr > F | ||||||
| Season | 2015 | 2016 | ||||
| Treatment (A) | <0.0001 | <0.0001 | ||||
| Storage duration (B) | <0.0001 | <0.0001 | ||||
| Shelf life (C) | <0.0001 | <0.0001 | ||||
| A × B | <0.0001 | <0.0001 | ||||
| A × C | <0.0001 | <0.0001 | ||||
| B × C | <0.0001 | <0.0001 | ||||
| A × B × C | <0.0001 | <0.0001 | ||||
| Pearson’s Correlation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Superficial Scald | MHO | α-Farnesene | Total Phenolic Content | Radical Scavenging Activity | ||||||
| Parameter | r | p | r | p | r | p | r | p | r | p |
| 6 methyl-5-hepten-2-one | 0.6610 | <0.0001 | - | - | 0.2701 | <0.0001 | −0.2061 | <0.0001 | −0.2547 | <0.0001 |
| α-farnesene | 0.3187 | <0.0001 | 0.2701 | <0.0001 | - | - | - | NS * | - | NS |
| Total phenolic content | −0.3527 | <0.0001 | −0.2061 | <0.0001 | - | NS | - | - | 0.4191 | <0.0001 |
| Radical scavenging activity | −0.3048 | <0.0001 | −0.2545 | <0.0001 | - | NS | 0.4191 | <0.0001 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawhena, T.G.; Fawole, O.A.; Opara, U.L. Application of Dynamic Controlled Atmosphere Technologies to Reduce Incidence of Physiological Disorders and Maintain Quality of ‘Granny Smith’ Apples. Agriculture 2021, 11, 491. https://doi.org/10.3390/agriculture11060491
Kawhena TG, Fawole OA, Opara UL. Application of Dynamic Controlled Atmosphere Technologies to Reduce Incidence of Physiological Disorders and Maintain Quality of ‘Granny Smith’ Apples. Agriculture. 2021; 11(6):491. https://doi.org/10.3390/agriculture11060491
Chicago/Turabian StyleKawhena, Tatenda Gift, Olaniyi Amos Fawole, and Umezuruike Linus Opara. 2021. "Application of Dynamic Controlled Atmosphere Technologies to Reduce Incidence of Physiological Disorders and Maintain Quality of ‘Granny Smith’ Apples" Agriculture 11, no. 6: 491. https://doi.org/10.3390/agriculture11060491
APA StyleKawhena, T. G., Fawole, O. A., & Opara, U. L. (2021). Application of Dynamic Controlled Atmosphere Technologies to Reduce Incidence of Physiological Disorders and Maintain Quality of ‘Granny Smith’ Apples. Agriculture, 11(6), 491. https://doi.org/10.3390/agriculture11060491








