The Exogenous Application of Non-Toxic Sulfur Contributes to the Growth-Promoting Effects of Leaf Lettuce (Lactuca sativa L. var. crispa)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. NTS, Calcium, and Magnesium Materials and Experimental Design
2.3. Physiological Analysis
2.4. RNA Extraction and Quantitative Real-Time RT-PCR
2.5. Chlorophyll and Carotenoid Determination
2.6. Measurement of Transient Chlorophyll a Fluorescence
2.7. Hydrogen Peroxide Determination
2.8. Antioxidant Enzyme Activity Assay
2.8.1. Enzyme Extraction
2.8.2. Determination of SOD Activity
2.8.3. Determination of CAT Activity
2.8.4. Determination of GR Activity
2.9. Statistical Analysis
3. Results
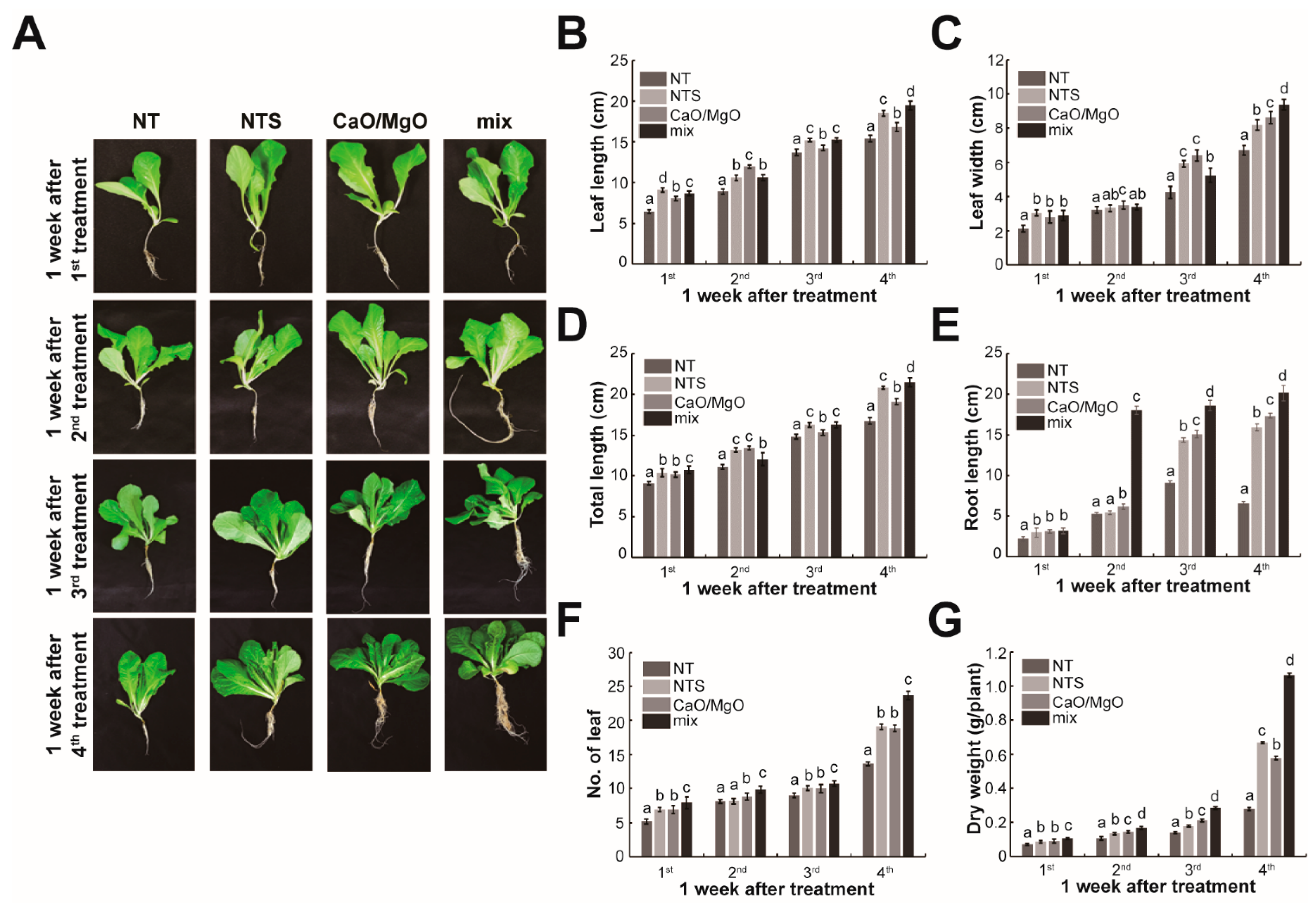
3.1. Treatment with NTS, Ca, and Mg Promotes Growth in Leaf Lettuce
3.2. Treatment with NTS, Ca, and Mg Induced the Biosynthesis-Related mRNA Expression of Auxin and GA in Leaf Lettuce
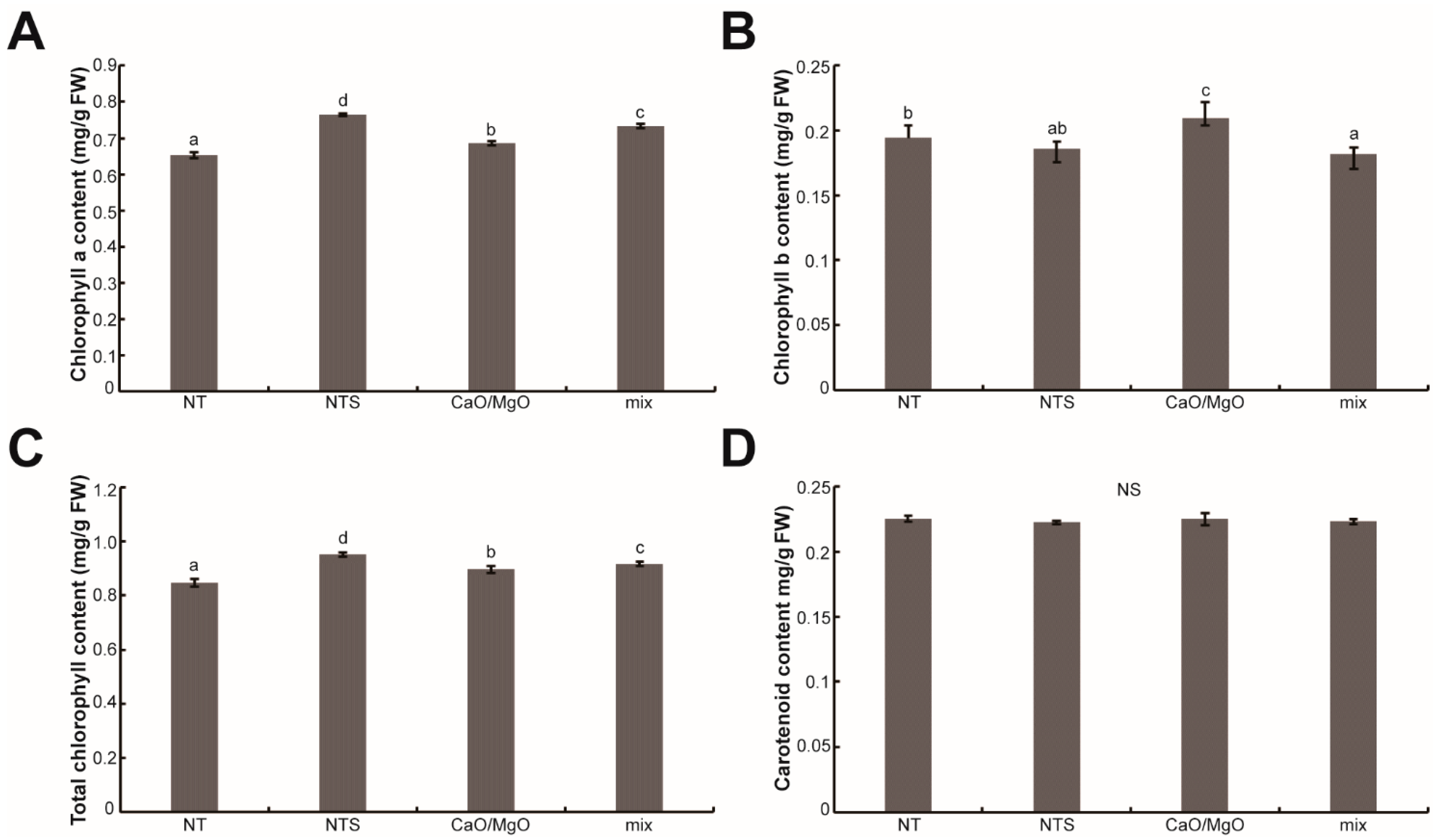
3.3. Treatment with NTS, Ca, and Mg Changed the Photosynthetic Pigment Content in Leaf Lettuce
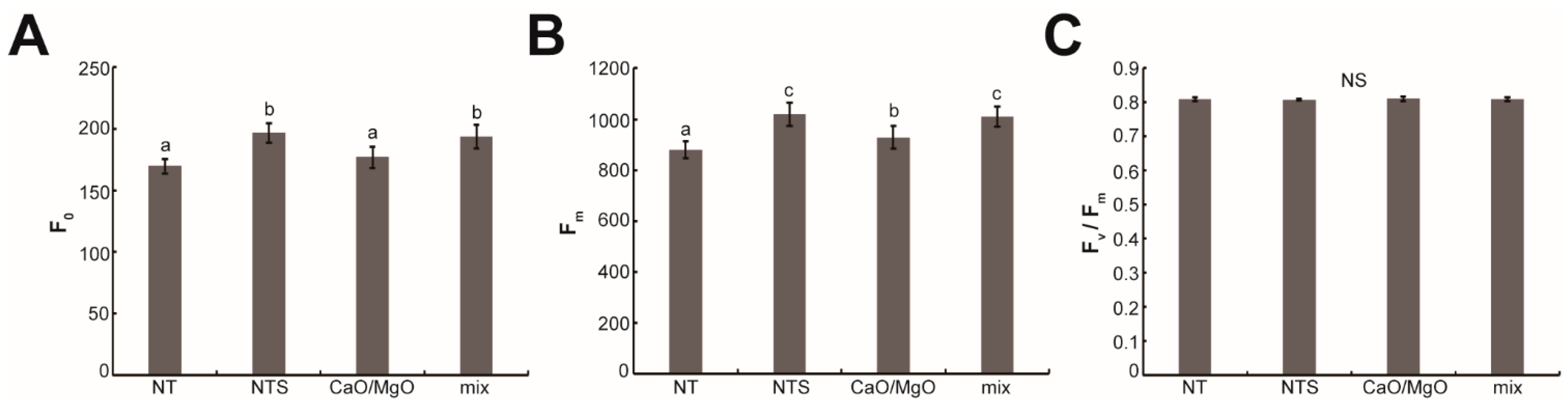
3.4. Treatment with NTS, Ca, and Mg Changed the Chlorophyll Fluorescence Parameters in Leaf Lettuce
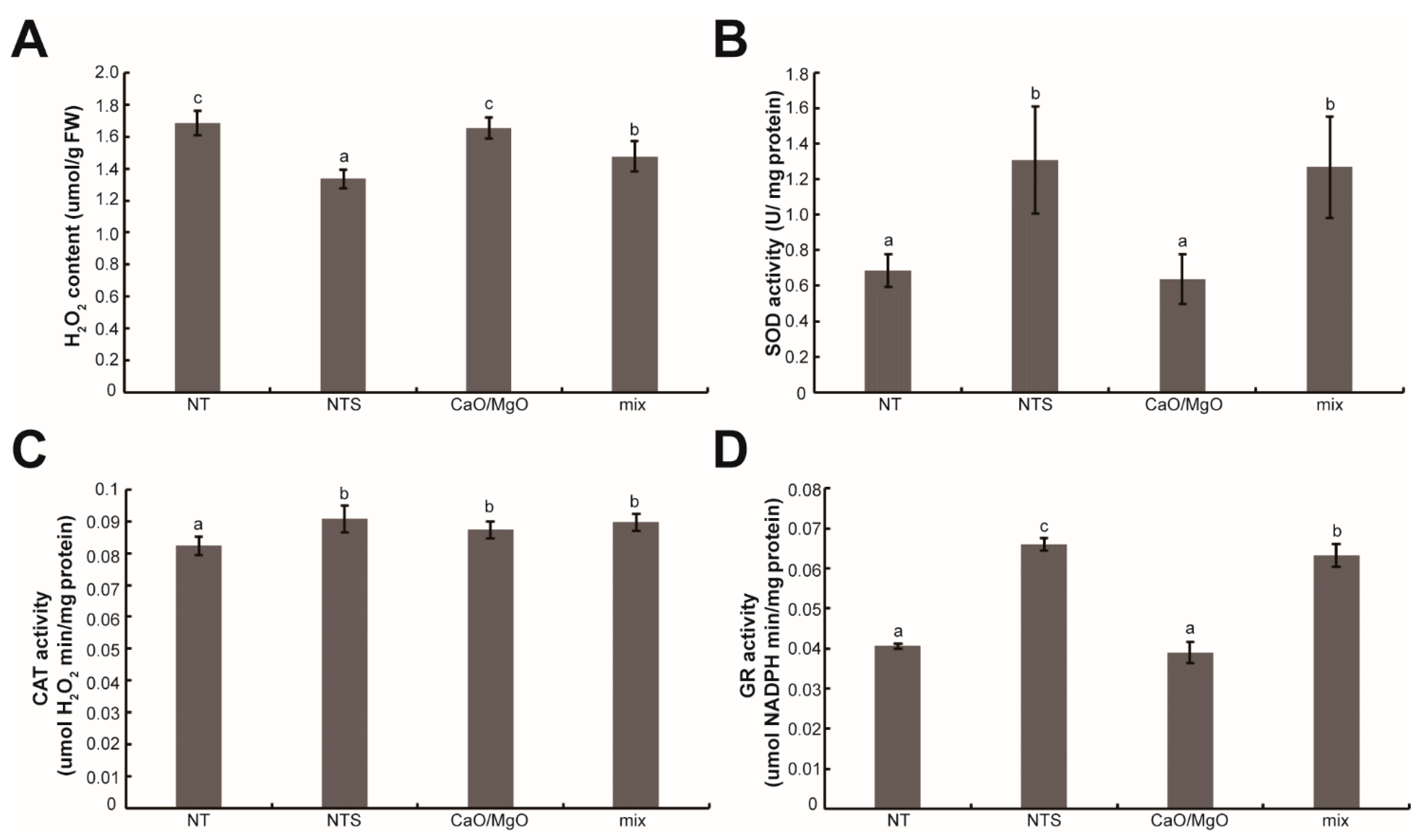
3.5. Treatment with NTS, Ca, and Mg Improved Antioxidant Enzyme Activity in Leaf Lettuce
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alix, A.; Capri, E. Chapter One-Modern agriculture in Europe and the role of pesticides. In Advances in Chemical Pollution, Environmental Management and Protection; Capri, E., Alix, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–22. [Google Scholar]
- Jallah, J.K.; Mulbah, C.K.; Kiazolu, J.S.; Frank, K.; Morris, M.Z. Efficient fertilizer use for increased crop production: The Liberia experience. Fertil. Res. 1991, 29, 55–64. [Google Scholar] [CrossRef]
- Simonne, E.H.; Gazula, A.; Ozores-Hampton, M.; DeValerio, J.; Hochmuth, R.C. Localized application of fertilizers in vegetable crop production. In Advances in Research on Fertilization Management of Vegetable Crops; Tei, F.N.S., Benincasa, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 149–181. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Environmental Analysis: Chemical Pollutants in Air, Water, Soil, and Solid Wastes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 824. [Google Scholar] [CrossRef]
- Ning, C.-C.; Gao, P.-D.; Wang, B.-Q.; Lin, W.-P.; Jiang, N.-H.; Cai, K.-Z. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef] [Green Version]
- Prabakaran, G.; Vaithiyanathan, D.; Ganesan, M. Fuzzy decision support system for improving the crop productivity and efficient use of fertilizers. Comput. Electron. Agric. 2018, 150, 88–97. [Google Scholar] [CrossRef]
- Nadarajan, S.; Sukumaran, S. Chapter 12—Chemistry and toxicology behind chemical fertilizers. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 195–229. [Google Scholar] [CrossRef]
- Shaji, H.; Chandran, V.; Mathew, L. Chapter 13—Organic fertilizers as a route to controlled release of nutrients. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 231–245. [Google Scholar] [CrossRef]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur Assimilation in Photosynthetic Organisms: Molecular Functions and Regulations of Transporters and Assimilatory Enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A. Metabolic changes sustain the plant life in low-sulfur environments. Curr. Opin. Plant Biol. 2017, 39, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Saito, K. S-Alk(en)ylcysteine sulfoxides in the genus Allium: Proposed biosynthesis, chemical conversion, and bioactivities. J. Exp. Bot. 2019, 70, 4123–4137. [Google Scholar] [CrossRef]
- Nakai, Y.; Maruyama-Nakashita, A. Biosynthesis of Sulfur-Containing Small Biomolecules in Plants. Int. J. Mol. Sci. 2020, 21, 3470. [Google Scholar] [CrossRef]
- Benning, C. Biosynthesis and Function of the Sulfolipid Sulfoquinovosyl Diacylglycerol. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 53–75. [Google Scholar] [CrossRef]
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.T.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and Diversity of Plant Cell Walls: From Algae to Flowering Plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.; Mahmud, J.A.; Nahar, K.; Mohsin, S.M.; Parvin, K.; Fujita, M. Interaction of sulfur with phytohormones and signaling molecules in conferring abiotic stress tolerance to plants. Plant Signal. Behav. 2018, 13, e1477905. [Google Scholar] [CrossRef]
- Capaldi, F.R.; Gratão, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur Metabolism and Stress Defense Responses in Plants. Trop. Plant Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Henriet, C.; Aimé, D.; Térézol, M.; Kilandamoko, A.; Rossin, N.; Combes-Soia, L.; Labas, V.; Serre, R.F.; Prudent, M.; Kreplak, J.; et al. Water stress combined with sulfur deficiency in pea affects yield components but mitigates the effect of deficiency on seed globulin composition. J. Exp. Bot. 2019, 70, 4287–4304. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Juárez-Maldonado, A.; Rincón-Sánchez, F.; Benavides-Mendoza, A. From Elemental Sulfur to Hydrogen Sulfide in Agricultural Soils and Plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Juhasz, A.; Islam, S.; Diepeveen, D.; Zhang, J.; Wang, P.; Ma, W. Impact of mid-season sulphur deficiency on wheat nitrogen metabolism and biosynthesis of grain protein. Sci. Rep. 2018, 8, 2499. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.W.; Fitzgerald, M.A. Physiological and metabolic origin of sulphur for the synthesis of seed storage proteins. J. Plant Physiol. 2001, 158, 447–456. [Google Scholar] [CrossRef]
- Agrawal, H.P.; Mishra, A.K. Sulphur nutrition of soybean. Commun. Soil Sci. Plant Anal. 1994, 25, 1303–1312. [Google Scholar] [CrossRef]
- Zhao, Y.; Bi, D.; Zhao, Q.; Liu, C.; Hu, Z. Physiological and ecological effects of sulfur fertilization on soybean. Ying Yong Sheng Tai Xue Bao 2006, 17, 2376–2380. [Google Scholar]
- Scherer, H.W. Sulphur in crop production—Invited paper. Eur. J. Agron. 2001, 14, 81–111. [Google Scholar] [CrossRef]
- Koh, E.; Surh, J. Influence of Sulfur Fertilization on the Antioxidant Activities of Onion Juices Prepared by Thermal Treatment. Prev. Nutr. Food Sci. 2016, 21, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Caron, J.M.; Bannon, M.; Rosshirt, L.; Luis, J.; Monteagudo, L.; Caron, J.M.; Sternstein, G.M. Methyl sulfone induces loss of metastatic properties and reemergence of normal phenotypes in a metastatic cloudman S-91 (M3) murine melanoma cell line. PLoS ONE 2010, 5, e11788. [Google Scholar] [CrossRef]
- Kang, D.Y.; Sp, N.; Jo, E.S.; Kim, H.D.; Kim, I.H.; Bae, S.W.; Jang, K.J.; Yang, Y.M. Nontoxic sulfur enhances growth hormone signaling through the JAK2/STAT5b/IGF1 pathway in C2C12 cells. Int. J. Mol. Med. 2020, 45, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Hong, D.Y.; Park, J.H.; Joung, Y.H.; Darvin, P.; Kim, S.Y.; Na, Y.M.; Hwang, T.S.; Ye, S.K.; Moon, E.S.; et al. Methylsulfonylmethane suppresses breast cancer growth by down-regulating STAT3 and STAT5b pathways. PLoS ONE 2012, 7, e33361. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.H.; Darvin, P.; Kang, D.Y.; Sp, N.; Byun, H.J.; Lee, C.H.; Lee, H.K.; Yang, Y.M. Methylsulfonylmethane Inhibits RANKL-Induced Osteoclastogenesis in BMMs by Suppressing NF-kappaB and STAT3 Activities. PLoS ONE 2016, 11, e0159891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreekantan Preetha, N.; Kang, D.Y.; Darvin, P.; Kim, D.N.; Joung, Y.H.; Kim, S.Y.; Cho, K.H.; Do, C.H.; Park, K.D.; Lee, J.-H.; et al. Induction of in vitro ketosis condition and suppression using methylsulfonylmethane by altering ANGPTL3 expression through STAT5b signaling mechanism. Anim. Cells Syst. 2015, 19, 30–38. [Google Scholar] [CrossRef]
- Miller, L.E. Methylsulfonylmethane decreases inflammatory response to tumor necrosis factor-α in cardiac cells. Am. J. Cardiovasc. Dis. 2018, 8, 31–38. [Google Scholar] [PubMed]
- Kang, D.Y.; Sp, N.; Jo, E.S.; Rugamba, A.; Kim, H.D.; Kim, I.H.; Park, J.C.; Bae, S.W.; Jang, K.J.; Yang, Y.M. Non-toxic sulfur inhibits LPS-induced inflammation by regulating TLR-4 and JAK2/STAT3 through IL-6 signaling. Mol. Med. Rep. 2021, 24, 485. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.S.; Sp, N.; Kang, D.Y.; Rugamba, A.; Kim, I.H.; Bae, S.W.; Liu, Q.; Jang, K.-J.; Yang, Y.M. Sulfur Compounds Inhibit High Glucose-Induced Inflammation by Regulating NF-κB Signaling in Human Monocytes. Molecules 2020, 25, 2342. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D.Y.; Kim, H.D.; Rugamba, A.; Jo, E.S.; Park, J.-C.; Bae, S.W.; Lee, J.-M.; Jang, K.-J. Natural Sulfurs Inhibit LPS-Induced Inflammatory Responses through NF-κB Signaling in CCD-986Sk Skin Fibroblasts. Life 2021, 11, 427. [Google Scholar] [CrossRef]
- Gilbert, F.A. The Place of Sulfur in Plant Nutrition. Bot. Rev. 1951, 17, 671–691. [Google Scholar] [CrossRef]
- Palmer, R.V.; Zhao, F.J.; McGrath, S.P.; Hawkesford, M.J. Sulphur supply and the optimisation of the yield of wheat. In Plant Nutrition: Food Security and Sustainability of Agro-Ecosystems through Basic and Applied Research; Horst, W.J., Schenk, M.K., Bürkert, A., Claassen, N., Flessa, H., Frommer, W.B., Goldbach, H., Olfs, H.W., Römheld, V., Sattelmacher, B., et al., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 836–837. [Google Scholar] [CrossRef]
- Messick, D. Correcting Sulphur Deficiency for Higher Productivity. FAI Seminar, New Delhi, India, December 5–7, The Sulphur Institute (TSI), Washington, United States. 2007. Available online: https://www.yumpu.com/en/document/view/30788633/correcting-sulphur-deficiency-for-higher-productivity (accessed on 12 August 2021).
- Dougher, T.A.; Bugbee, B. Differences in the Response of Wheat, Soybean and Lettuce to Reduced Blue Radiation. Photochem. Photobiol. 2001, 73, 199–207. [Google Scholar] [CrossRef]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Górnik, K.; Lahuta, L.B. Application of phytohormones during seed hydropriming and heat shock treatment on sunflower (Helianthus annuus L.) chilling resistance and changes in soluble carbohydrates. Acta Physiol. Plant. 2017, 39, 118. [Google Scholar] [CrossRef] [Green Version]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis, 2nd ed.; Bergmeyer, H.U., Ed.; Academic Press: Cambridge, MA, USA, 1974; pp. 673–684. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Harberd, N.P. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 2003, 421, 740–743. [Google Scholar] [CrossRef]
- Hirai, M.Y.; Fujiwara, T.; Awazuhara, M.; Kimura, T.; Noji, M.; Saito, K. Global expression profiling of sulfur-starved Arabidopsis by DNA macroarray reveals the role of O-acetyl-l-serine as a general regulator of gene expression in response to sulfur nutrition. Plant J. 2003, 33, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Hirai, M.Y.; Saito, K. Post-genomics approaches for the elucidation of plant adaptive mechanisms to sulphur deficiency. J. Exp. Bot. 2004, 55, 1871–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforova, V.J.; Kopka, J.; Tolstikov, V.; Fiehn, O.; Hopkins, L.; Hawkesford, M.J.; Hesse, H.; Hoefgen, R. Systems rebalancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of Arabidopsis plants. Plant Physiol. 2005, 138, 304–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terry, N. Effects of sulfur on the photosynthesis of intact leaves and isolated chloroplasts of sugar beets. Plant Physiol. 1976, 57, 477–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resurreccion, A.P.; Makino, A.; Bennett, J.; Mae, T. Effects of sulfur nutrition on the growth and photosynthesis of rice. Soil Sci. Plant Nutr. 2001, 47, 611–620. [Google Scholar] [CrossRef]
- Skudra, I.; Ruza, A. Effect of Nitrogen and Sulphur Fertilization on Chlorophyll Content in Winter Wheat. Rural Sustain. Res. 2017, 37, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R. Acclimation by the thylakoid membranes to growth irradiance and the partitioning of nitrogen between soluble and thylakoid proteins. Funct. Plant Biol. 1988, 15, 93–106. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Öquist, G.; Chow, W.S.; Anderson, J.M. Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 1992, 186, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Mauromicale, G.; Ierna, A.; Marchese, M. Chlorophyll fluorescence and chlorophyll content in field-grown potato as affected by nitrogen supply, genotype, and plant age. Photosynthetica 2006, 44, 76. [Google Scholar] [CrossRef]
- Aarabi, F.; Naake, T.; Fernie, A.R.; Hoefgen, R. Coordinating sulfur pools under sulfate deprivation. Trends Plant Sci. 2020, 25, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.X.; Phua, S.Y.; Van Breusegem, F. Secondary sulfur metabolism in cellular signalling and oxidative stress responses. J. Exp. Bot. 2019, 70, 4237–4250. [Google Scholar] [CrossRef] [Green Version]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant. Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Chandra, N.; Pandey, N. Influence of Sulfur Induced Stress on Oxidative Status and Antioxidative Machinery in Leaves of Allium cepa L. Int. Sch. Res. Not. 2014, 2014, 568081. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-C.; Sp, N.; Kim, H.D.; Kang, D.Y.; Kim, I.H.; Bae, S.W.; Yang, Y.; Jang, K.-J. The Exogenous Application of Non-Toxic Sulfur Contributes to the Growth-Promoting Effects of Leaf Lettuce (Lactuca sativa L. var. crispa). Agriculture 2021, 11, 769. https://doi.org/10.3390/agriculture11080769
Park J-C, Sp N, Kim HD, Kang DY, Kim IH, Bae SW, Yang Y, Jang K-J. The Exogenous Application of Non-Toxic Sulfur Contributes to the Growth-Promoting Effects of Leaf Lettuce (Lactuca sativa L. var. crispa). Agriculture. 2021; 11(8):769. https://doi.org/10.3390/agriculture11080769
Chicago/Turabian StylePark, Jong-Chan, Nipin Sp, Hyoung Do Kim, Dong Young Kang, Il Ho Kim, Se Won Bae, Young Yang, and Kyoung-Jin Jang. 2021. "The Exogenous Application of Non-Toxic Sulfur Contributes to the Growth-Promoting Effects of Leaf Lettuce (Lactuca sativa L. var. crispa)" Agriculture 11, no. 8: 769. https://doi.org/10.3390/agriculture11080769





