Abstract
To reduce the plastic waste problem in agriculture, biodegradable plastic (BP) mulch films have become of key importance thanks to their biodegradability and beneficial effects on crops. However, at present, BPs cannot always replace conventional plastics, because biodegradation is governed by many biotic and abiotic factors under field conditions. This research aimed at isolating and identifying, from soil particles directly attached to the surface of BP samples, the microorganisms responsible of degradation through a combined approach based on biodegradation and molecular tests. For this purpose, a field trial within a Mediterranean apricot orchard was carried out to study the biodegradation of a commercial BP mulch with respect to a no-BP, a conventional apricot management, following the standard agricultural practices, and a subterranean clover cover cropping, either incorporating or leaving its dead mulches on the soil surface. After BP film appeared visibly degraded in field, we isolated from soil particles attached to the polymer surface, a mesophilic bacterium with certain degradative potential assessed by plate and liquid assays, identified by sequencing as Pseudomonas putida. Quantitative real time PCR analysis showed the P. putida was significantly more abundant in PB plots than the other plot treatments. These preliminary results are potentially applicable to accelerate the degradation of BP mulch films and decrease the plastic pollution in agriculture.
1. Introduction
Agricultural plastic mulch films are commonly used for covering cultivated fields to reduce weed pressure, preserve soil structure, maintain soil temperature, improve moisture conservation and increase crop yields [1,2]. Most mulch films are synthetic polymers produced from petroleum-based plastics, such as polyethylene (PE), polypropylene (PP), polyvinyl chloride, polyethylene terephthalate (PET), etc., which are characterized by low costs, ease of manufacture and good versatility [3]. Given the high number of benefits, in the last twenty years, the use of plastic mulches derived from man-made long-chain polymeric molecules has increased dramatically, mainly in the USA and Western Europe. Furthermore, the production of plastic mulches is expected to triple by 2050, thus needing the 20% of the annual global oil consumption for their synthesis [4]. The widespread use of plasticulture in agriculture caused many environmental problems, not only for the high greenhouse gas (GHG) emissions, but also for the depletion of fossil resources, as well as for the removal and disposal of plastic films after use [5].
To address the agricultural plastic waste problem, several environmentally degradable polymeric materials and plastics have been developed since the 1980s and have gained in popularity. Biodegradable plastics (BPs), i.e., biopolymers synthetized by renewable biomass or mimicking microorganisms, exhibit a significant degradation rate, so that, at the end of their life, they can be directly integrated into the soil, thus representing a sustainable alternative to petroleum-based plastics [6]. BPs, in fact, can be degraded by extracellular depolymerases secreted by microbes (bacteria, fungi or algae) into oligomeric or monomeric units [7]. Nowadays, BPs account for about 0.5% of the total annual plastic production, but the European Bioplastics [8] (2019) esteemed that BP production will increase to about 2.43 million tons in 2024.
The most prominent role in BPs production is played by aliphatic and aromatic polyesters: polyhydroxyalkanoate (PHA), polylactic acid (PLA), polybutylene succinate (PBS), polyhydroxybutyrate (PHB), etc. Commercially available BPs are often blended with starch, natural fibers or other polymers (e.g., fillers, plasticizers and dyes) to control and increase their degradable rate after use [9]. BPs can be grouped into microbial synthetic plastics, natural polymer plastics and synthetic biodegradable plastics [10]. The first two typologies of BPs are completely biodegradable, while the latter are destructive biodegradable plastics. Ideally, a PB should have excellent physicochemical and mechanical properties, a programmed degradability and a 100% post-use biodegradability. However, biodegradation, defined as the conversion of plastic monomers or polymers into biomass, CH4, CO2 and H2O through biological processes, cannot be controlled in natural environments [11]. Indeed, biodegradation occurs via various mechanisms, from abiotic processes (photolytic, thermal, mechanical and hydrolytic) to biological degradation (mineralization) and it is governed by different factors, including polymer characteristics (mobility, tacticity, crystallinity, molecular weight and type of functional groups), nature of pre-treatment, type of organism and environmental conditions [10,11]. Generally, biodegradation involves three stages [12]: (i) biodeterioration (aggregation of microorganisms on the surface of BPs and abiotic degradation of mechanical properties), (ii) biofragmentation (fragmentation of polymers into oligomers by depolymerases) and (iii) bioassimilation (break down of oligomers into monomers, biomass, CO2 and H2O).
Unfortunately, only few studies have been published on microbial biodegradation of BPs in soil environments and under soil crop conditions [2,9,13]. Among these few studies, none identified and isolated the microbial communities directly associated with BPs. On the contrary, most studies characterized the microorganisms near BPs [14] and were focused only on fungi [2,13,15]. Therefore, a significant knowledge gap exists in the identification of the specific microbial taxa involved in biodegradation, especially regarding bacteria, as well as in the microorganisms directly colonizing BPs. Given these considerations, the present research aims at (1) evaluating the effect of biodegradable mulching on soil microorganisms and (2) isolating and identifying the microorganisms potentially responsible of degradation under field conditions. We tested the hypothesis that soil microorganisms would degrade BPs and, therefore, our goal was to isolate the microorganisms showing degradative abilities and to quantify them in the soil.
2. Materials and Methods
2.1. Site, Soil, Climate and Soil Temperature
The field trial occurred in central Sicily (37°13′ N, 14°05′ E, 290 m a.s.l., southern Italy) within an apricot (Prunus armeniaca L.) field located in an area devoted to typical Mediterranean crops. The soil at the site was a clayed textured Regosoil [16] with 18.3% sand, 24.3% silt and 57.4% clay, 1.7% organic matter, 9.5% active limestone, an average pH of 8.0 and 1.3‰ total N, 23 mg−1 kg−1 assimilable P2O5 and 622 mg−1 kg−1 exchangeable K2O. The climate was semiarid Mediterranean. Daily rainfall, maximum, average and minimum air temperatures from October 2019 to February 2021, were measured with a meteorological station (Mod. Multirecorder 2.40; ETG, Firenze, Italy) sited at ~15 m from the experimental site. In the same period, the soil temperature was also measured on 7-day intervals at two depths (−10 cm and −20 cm) using a FieldScout EC 450 Meter (Spectrum Technologies, Inc., Aurora, Illinois, U.S.A.; accuracy: ±1%). At −10 cm, compared to the conventional management (CM, 23.6 °C), it was found that the average soil temperature was −2.1 °C in TCC-S, −1.9 °C in TCC-B, +5.9 °C in nBP and +4.1 °C in BP. At −20 cm, compared to CM (22.0 °C), soil temperature was −2.3 °C in TCC-S, −2.0 °C in TCC-B, +4.5 °C in nBP and +3.2 °C in BP.
2.2. Experimental Design and Agronomic Management
The experiment was a randomized complete block design with three replications of five main plots treatments (20 × 10.8 m for each plot) totaling to 15 plots, 216 m2 per treatment and 3240 m2 in total. Treatments included (1) a BP and (2) a no-BP (nBP), (3) a subterranean clover (Trifolium subterraneum L.) cover cropping with soil-surface application of dead mulches (TCC-S) (4) and a subterranean clover cover cropping with soil-incorporation of dead mulches (TCC-B). TCC-S and TCC-B were chosen as they represent a valid agronomic tool in P. armeniaca orchards to improve weed control [17], soil mineral nitrogen [18] and the nutritional status of the trees [19]. These treatments were compared to (5) a conventional management (CM) following the typical agronomic practices for the zone: a winter shallow hoeing at 0.10 m in September, followed by two tine harrowing in February and May to control weeds.
Placement of BP and nBP films occurred in May 2020. BP film was a commercial biodegradable plastic mulch, Agribio® (Agriplast, Vittoria, Italy), according to the normative reference EN 17033, with a thickness of 15 µm and a total visible light emission ≤3%. A no-biodegradable commercial film (nBP) was placed as control, Black 35® (Agriplast, Vittoria, Italy), which was a conventional low-density PE mulch film with a thickness of 35 µm. Apricot cv. Kioto® was planted on January 2018 with a 4.5 × 4.0 m layout, while subterranean clover cv. Mintaro was seeded in October 2019 by adopting a seeding rate of 22.2 kg ha−1. In TCC-S and TCC-B, Rhizobium inoculation was not applied due to previous cultivations of Trifolium spp. These plots received an irrigation up to the field capacity to promote T. subterraneum germination. “Mintaro” is a mid-season Australian cultivar of T. subterraneum var. brachycalycinum with high adaptability in Mediterranean environments, clay and neutral to alkaline soils, good N-fixation and self-reseeding capacity. The total length of the subterranean clover biological cycle was ~210 days, with the emergence occurring in December 2019, the highest vegetative development (~30 cm in height) in March 2020, flowering in April 2020 and total drying of the plants in the first decade of July 2020.
No post-emergence soil tillage was carried out in BP, nBP, TCC-SB and TCC-GM. In all the plots, a self-compensating drip irrigation was used, replacing 100% of the maximum evapotranspiration. About fertilization, N and P2O5 were soil-applied through 50 kg ha−1 Hergoton Plus® (8% total organic N, 26% biologic C and 44.45% total organic matter) and 50 kg ha−1 simple perphosphate, respectively, while Ca was foliar-applied with 150 g hL−1 Biocal® (12% CaO, two times, in April and May 2021). Fungi and insects were controlled with the following commercial organic products, applied as foliar sprays: Cupravit 35 WG® (copper oxychloride 35%, twice in winter at 15 days interval), Thiovit® Jet (80% sulphur, four times at 3 kg ha−1) and LaserTM 240 SC (240 g L−1 spinosad).
2.3. Biodegradation in Plate Assay
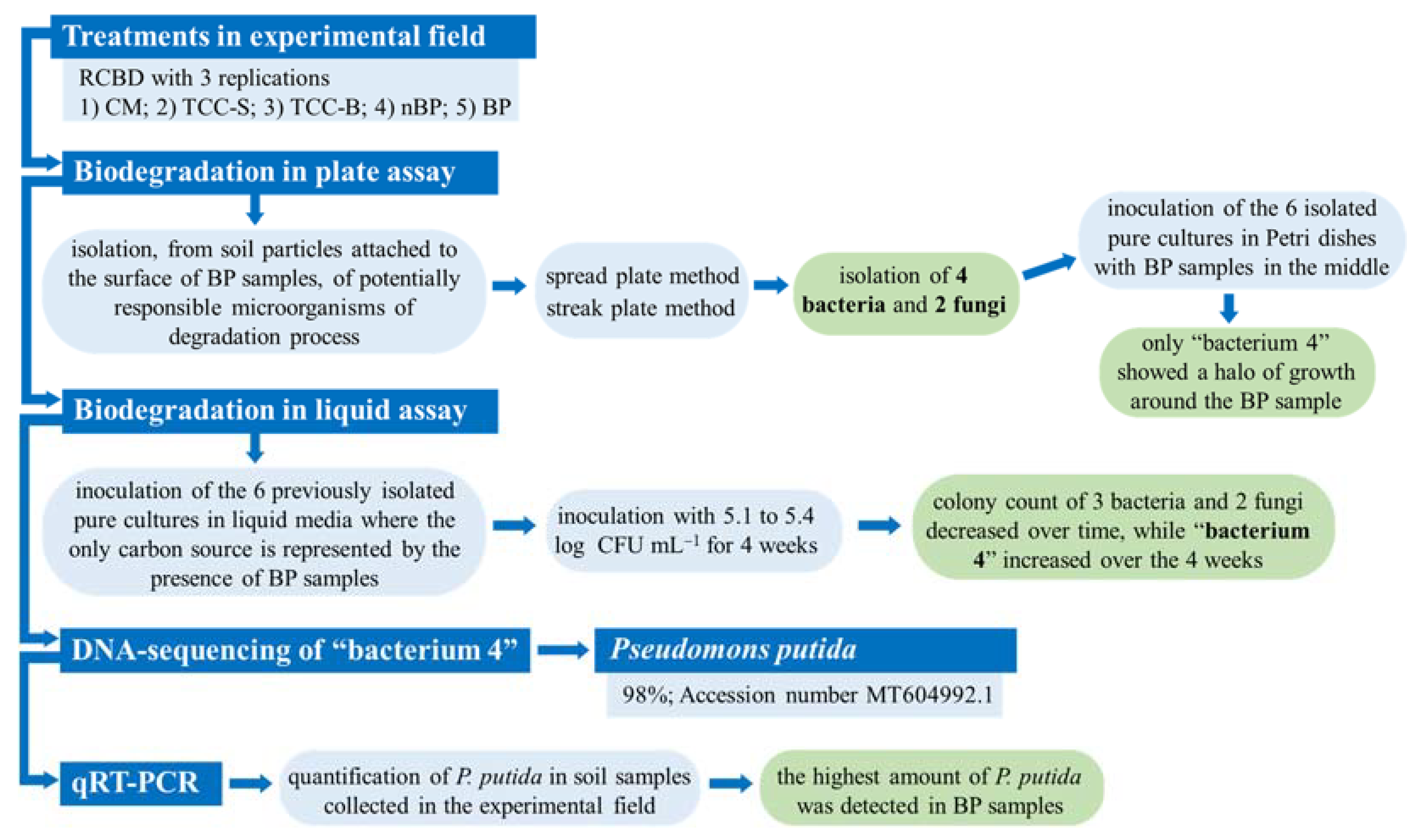
In order to identify the soil microbial communities directly associated with biodegradable mulching, several tests were carried out with the aim of isolating and identifying the microorganisms potentially responsible of the degradation process. Figure 1 shows the general experimental sequence of the activities of our study.
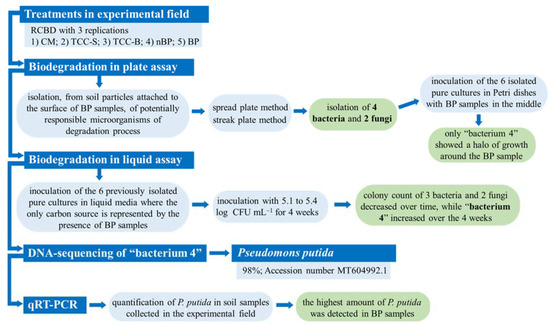
Figure 1.
Graphical scheme of the experimental activities. RCBD, randomized complete block design; CM, conventional apricot management; TCC-S, Trifolium subterraneum L. cover cropping leaving dead mulch on the soil surface; TCC-B, T. subterraneum cover cropping burying dead mulch in the soil; nBP, no-biodegradable plastic; BP, biodegradable plastic.
BP samples, which, in the field, appeared partially degraded, were taken from the soil and washed with M9 medium [20] to recover the microorganisms adhering to the surface and potentially responsible for degradation. An aliquot of the M9 culture broth was used to inoculate NB (Nutrient Broth; Oxoid, Milan, Italy) and YPD (Yeast Extract-Peptone-Dextrose; Oxoid, Milan, Italy), respectively, to allow bacterial and fungal growth. In NB, cycloheximide was added to inhibit fungal growth (100 μg L−1 added after sterilization); in YPD, chloramphenicol was added to inhibit bacterial growth (100 μg L−1). Bacterial and fungal broths were incubated at 32 °C for 24 h and 25 °C for 48 h, respectively.
Serial dilutions were made with sterile solution and aliquots of all samples were plated for microbial growth using the spread plate method and distributing 0.1 mL dilutions directly over the surface of the plates. PCA (Plate Count Agar; Oxoid, Milan, Italy) and SDA (Sabouraud Dextrose Agar; Oxoid, Milan, Italy) were respectively used as media for growth of aerobic mesophilic bacteria and fungi. The plates were incubated at the optimum growth temperature for 24 and 48 h, respectively. When microorganism growth was visible on the plates, the streak plate method was used in order to isolate different colony types from the mixed culture and, then, the pure cultures were stored at −80 °C with the addition of 20% sterile glycerol.
Based on the different colony morphology, 6 of the isolated colonies (4 bacteria and 2 fungi) were tested individually on BP samples to understand if they were responsible for degradation. For this purpose, 1.8 × 1.8 cm biodegradable plastic samples were sterilized under UV light, put in the middle of Petri dishes and contaminated with the pure cultures. For the contamination, each pure culture was previously grown in 10 mL of liquid medium (NB for bacteria; YPD for fungi); then, 20 mL of the same medium, with an agar concentration of 30 g L−1, was added to obtain a final agar concentration of 20 g L−1 and, finally, poured into Petri dishes. Samples were placed in a thermostat at 25 °C for fungi and 32 °C for bacteria to favor the growth of microorganisms and verify whether polymer biodegradation occurred. A sterile control was performed to verify if the degradation was only due to microorganism activity. The polymer samples were sterilized under UV light, put in the middle of Petri dishes without any inoculum and incubated in the same conditions as above.
2.4. Biodegradation in Liquid Assay
In order to verify if the 4 bacteria and 2 fungi, isolated as mentioned above, were able to grow using the polymer as sole carbon source, biodegradation tests were performed in M9 and YNB (Yeast Nitrogen Based; Oxoid, Italy) liquid media, where the only carbon source was represented by the presence of polymer samples.
A volume of 50 mL of sterile M9 and YNB media for the bacterial and fungal growth, respectively, was poured in Erlenmeyer flasks and inoculated with the pure culture of the microorganism previously isolated, ranging from 5.1 to 5.4 log CFU mL−1, in presence of plastic film 1.8 × 1.8 cm, previously sterilized under UV light. The samples were incubated aerobically at 32 °C and 25 °C, for bacterial and fungal growth, respectively, in the dark, on a rotary oscillator at 120 rpm. Tests were carried out for 4 weeks. At the end of every week, colony counts were made to verify the microbial growth. Four sampling were totally made, one for each week. Analyses were carried out in triplicate for each material. Population densities were expressed as log10 CFU mL−1 of culture broth.
2.5. Real-Time Quantitative PCR Assay of Pseudomonas putida in Soil
In relation to the obtained results regarding the identification of the “bacterium 4” as Pseudomonas putida, which was able to degrade BP films, we decided to quantify, by RT-PCR, the above-mentioned bacterium in soil samples in order to monitor its presence over time among the treatments. Prior to RT-PCR, the soil DNA was extracted following Scavo et al. [21]. The obtained pure DNA was stored at −20 °C until RT-PCR amplification, then it was spectrophotometrically quantified (all with 260:280 ratios above 1.7).
The qRT-PCR is widely used to quantify the PCR product. Data from RT-quantitative PCR experiments were analyzed with the absolute quantification method, which determines the input copy number of the gene of interest by relating the PCR signal to a standard curve [22]. The qRT-PCR was carried out in accordance with Scavo et al. [18]. The obtained cycle threshold (Ct) is the number of cycles required for the fluorescent signal to cross the threshold (i.e., to exceed background level). Ct levels are inversely proportional to the amount of target nucleic acid in the sample.
Real-time PCR of P. putida was performed on soil DNA extracts for each treatment at different sampling times (May 2020, October 2020, December 2020 and February 2021) to monitor its abundance. PCR amplification conditions, using SYBR GREEN technology, were 94 °C for 2 min, 40 cycles of 94 °C for 15 s, 55 °C for 40 s, 72 °C for 40 s. Reactions were 25 µL volumes using Platinum Quantitative PCR Supermix-UDG (Invitrogen). For testing the primers, designed as described below, P. putida DNA (DSM 291) was directly used as a source of DNA template in a 25 µL reaction.
The same strain was used as standard for the calibration curve and subsequent calculation of its amount. Standard curves were derived using known amounts of DNA corresponding to 0.001–100 ng of genomic DNA. Threshold cycle (Ct) values were determined, in triplicate, using 2 µL samples of each soil DNA extract per PCR reaction. Ct values were converted to ng of DNA using the equation derived from the standard curve.
Design of Pseudomonas putida-Specific Amplicon
An alignment of the P. putida 16S rRNA gene was analyzed to identify conserved regions suitable for developing an RT-PCR assay for detection of gene sequences specific to this bacterium. Specific forward Pptf (20-mer (5′-AAGCTAGAGTACGGTAGAGG-3′)) and reverse Pptr (20-mer (5′-ACCAGGGTATCTAATCCTGT-3′)) primers were designed to amplify a 154-bp amplicon from the P. putida 16S rRNA gene to allow its quantification.
2.6. Statistical Analysis
An analysis of variance (ANOVA) was conducted using the CoStat® computer package version 6.003 (CoHort Software, Monterey, CA, USA) to statistically analyze qRT-PCR data. The Bartlett’s and Shapiro–Wilk tests were performed to verify the ANOVA basic assumptions of homoscedasticity and normality, respectively. Prior to ANOVA, qRT-PCR data and populations densities needed a log10(x + 1)-transformation (untransformed data are reported and discussed), in accordance with Lombardo et al. [23]. A generalized linear model (GLM) was performed considering the ‘field treatment’ and ‘soil sampling time’ as fixed factors. Means were separated through the Fisher’s protected Least Significant Difference (LSD) test with α = 0.05.
3. Results
3.1. Weather Trend
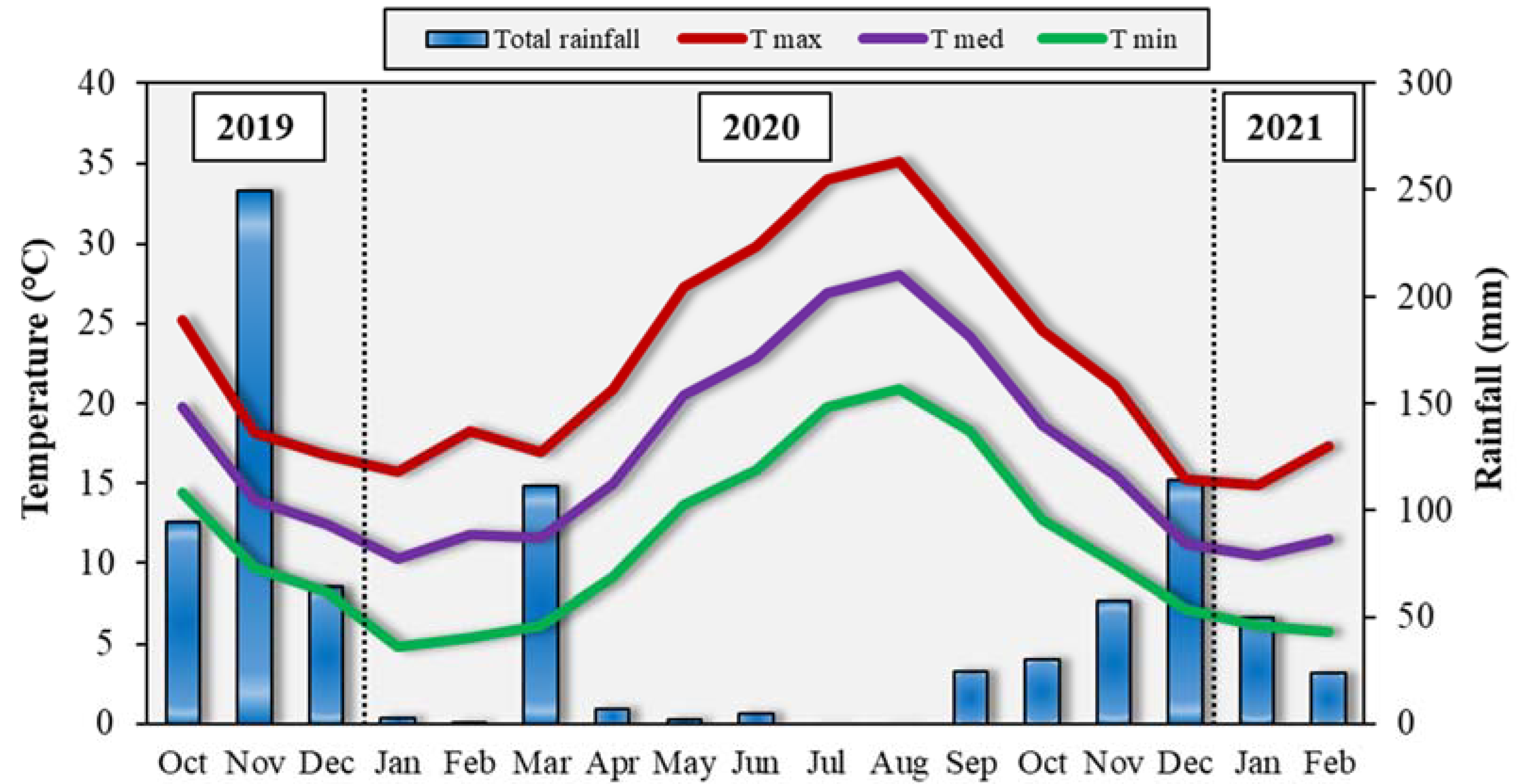
During the field experiment, weather trend was consistent with the climate of the zone (Figure 2). Most of rainfall experienced in autumn–winter, with the sum of November 2019 (249.6 mm), March 2020 (111.4 mm) and December 2020 (113.8 mm) accounting for 57% of the total precipitation fell in the period October 2019–February 2021 (837.8 mm). On the contrary, a very drought summer occurred, with only 7.2 mm of rainfall between May and August. In addition, the mean air temperatures followed the typical climatic trend. The highest maximum air temperature was recorded in August (~35 °C) and the lowest minimum one in January 2020 (4.7 °C).
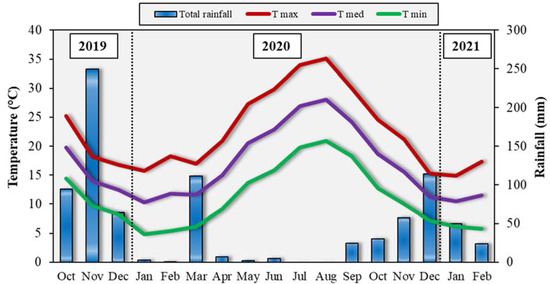
Figure 2.
Total rainfall and average monthly maxima and minima air temperatures in the experimental field from October 2019 to February 2021.
3.2. Assessment of Biodegradation in Plate Assay
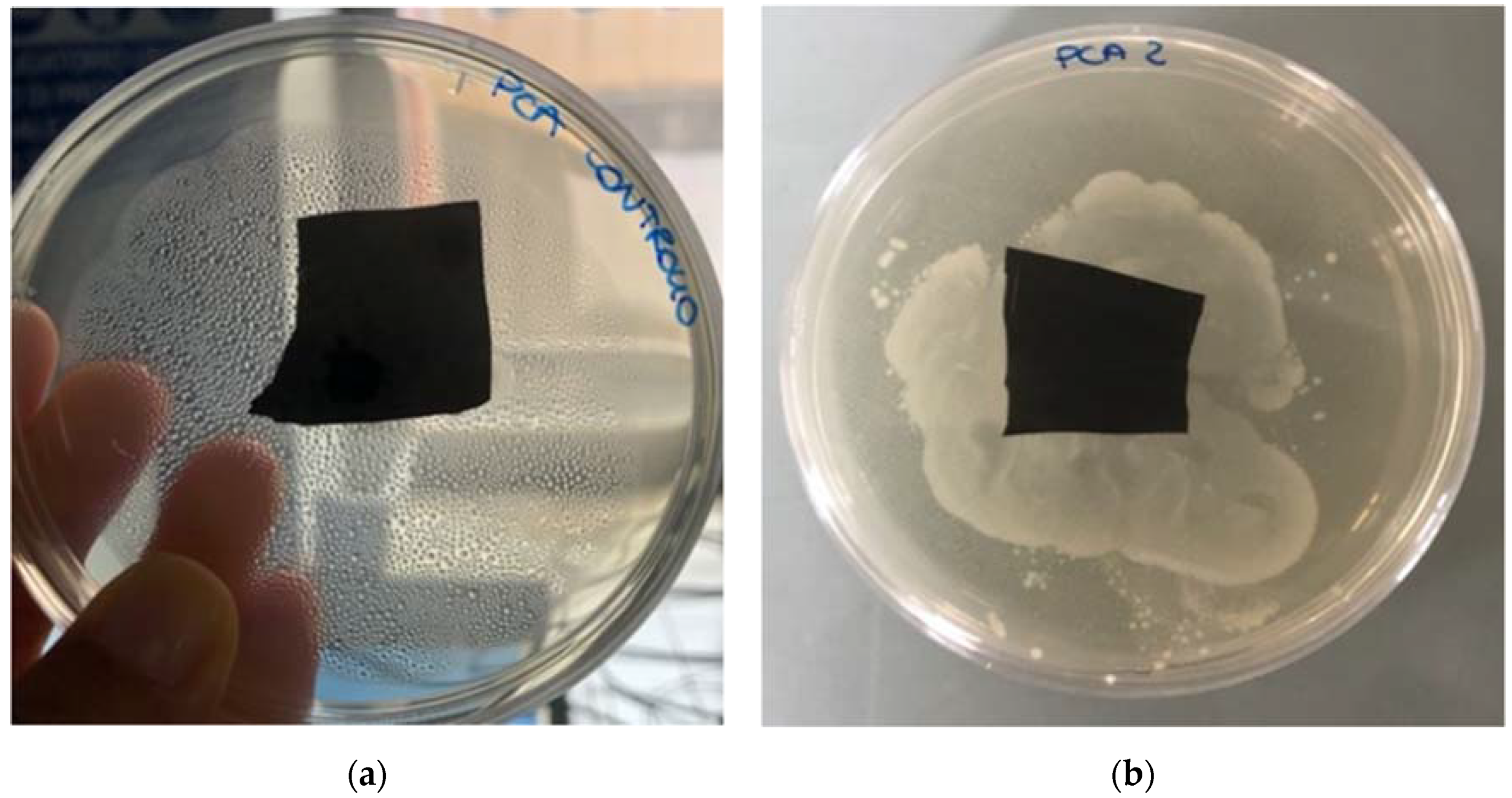
In order to isolate microorganisms potentially responsible for BP degradation in soil, polymer degraded samples were taken from the field and used to recover the microorganisms present in soil particles attached to the surface of the sample. All BP samples used for this test exhibited a considerable surface deformation. Besides biodegradation studies, we screened and isolated microorganisms potentially responsible for BP degradation. Totally, four bacteria and two fungi were isolated with the streak plate method in relation to the different colony morphology visible in Petri dishes. Each pure culture was used as inoculum in plate assay in the presence of BP film to verify if its biodegradation occurred. Among the six tested pure cultures, only one bacterium was recognized with certain potential to degrade the film. After 14 days of bacterial contamination, the degradation level of BP samples was evaluated due to certain visual physical changes detected in the inoculated samples and a consistent microbial growth on the surface and all around the films, as compared to the blank (Figure 3). In fact, in the blank (sterile control) no microbial growth was detected on the surface and all around the sample. The same results were also obtained for the other three isolated bacteria and two fungi and none halo of growth was observed around the plastic film in the Petri dishes.
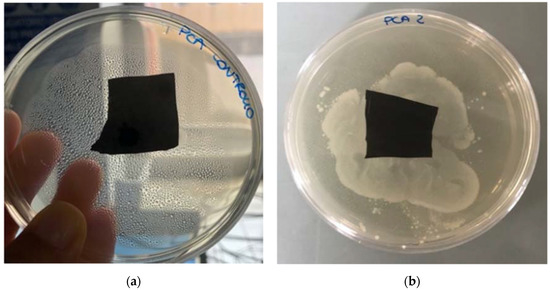
Figure 3.
(a) BP film not inoculated as control and (b) BP film 14 days after the inoculation with Pseudomonas putida.
3.3. Assessment of Biodegradation in Liquid Assay
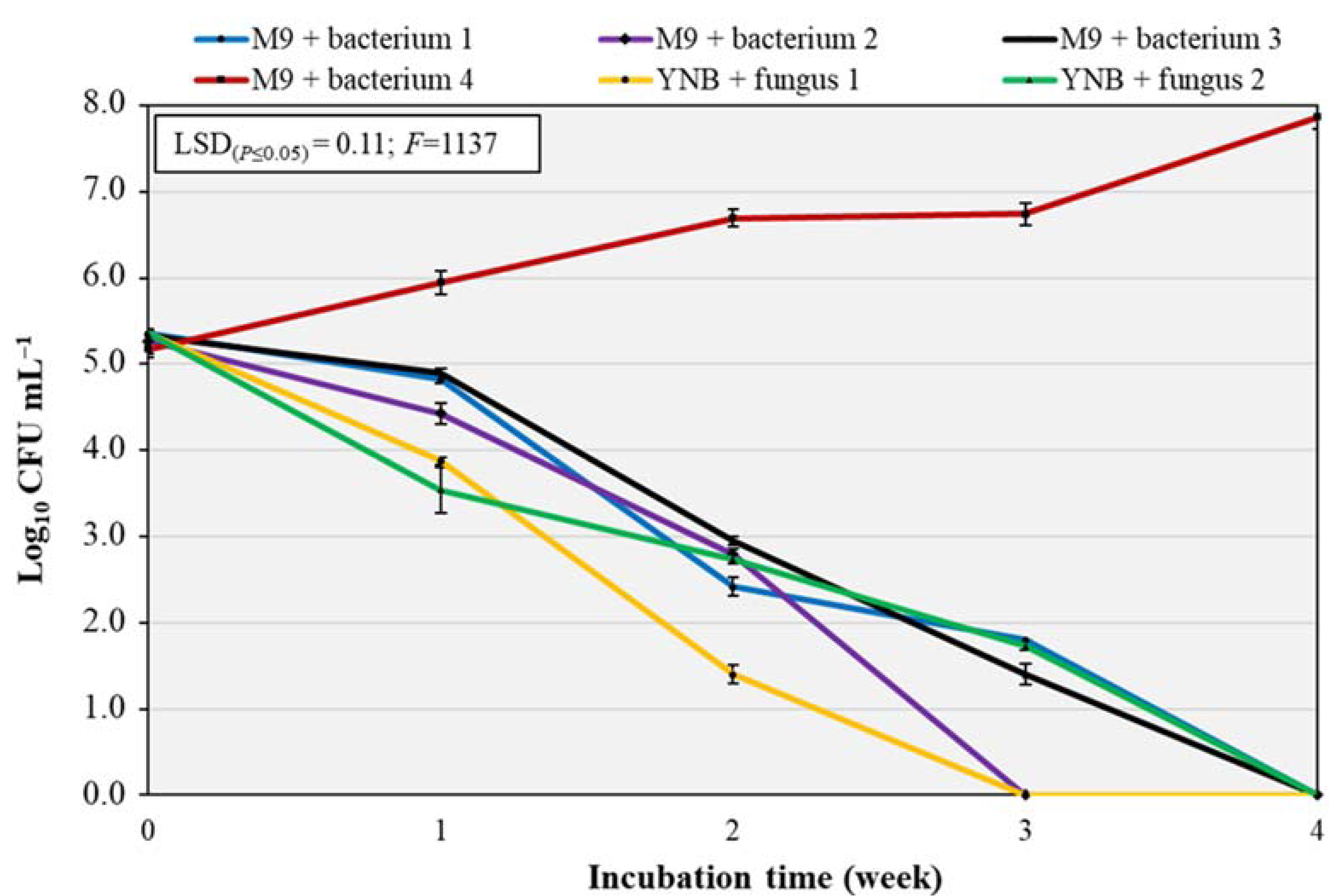
This test was performed to confirm the results of the biodegradation in plate assay and to verify if the six isolated microorganisms were able to grow in a liquid medium using the polymer film as the only carbon source. The test was carried out for 4 weeks. The two-way interaction ‘treatment × incubation time’ was significant at p ≤ 0.001 (Figure 4), with the latter factor providing the largest source of variance (54%, F = 10202). At the beginning of the experiment, the initial contamination by each pure culture was in a range from 5.1 to 5.4 log CFU mL−1. The trend for three bacteria and two fungi was similar, since bacterial and fungal growth decreased after the first week, until we detected microbial counts after 3 weeks for “bacterium 2” and “fungus 1” and after 4 weeks for “bacteria 1 and 3” and “fungus 2”. “Bacterium 4” showed an opposite trend, since its growth increased during the 4 weeks (from 5.2 log CFU mL−1 at T0 to 7.9 log CFU mL−1 after 4 weeks), demonstrating that this microorganism was able to use the polymer as energy and carbon source for its metabolism. “Bacterium 4” is the same microorganism that showed a visible growth on the surface and around the polymer in plate assay. This bacterium was DNA-sequenced and identified by using the NCBI library as P. putida (98%, Accession number MT604992.1).
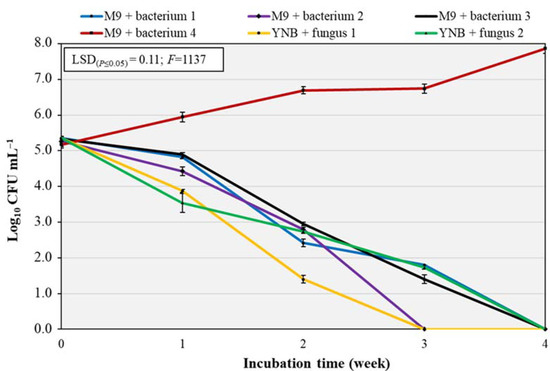
Figure 4.
Population densities (log10 CFU mL−1) of the six isolated pure cultures in liquid media with BP samples as sole carbon source over four weeks. The Least Significant Difference (LSD) interaction was calculated with the Fisher’s protected LSD test at α = 0.05. Vertical bars indicate the standard deviation of the mean. F, F of Fisher.
3.4. Quantification of P. putida in Soil
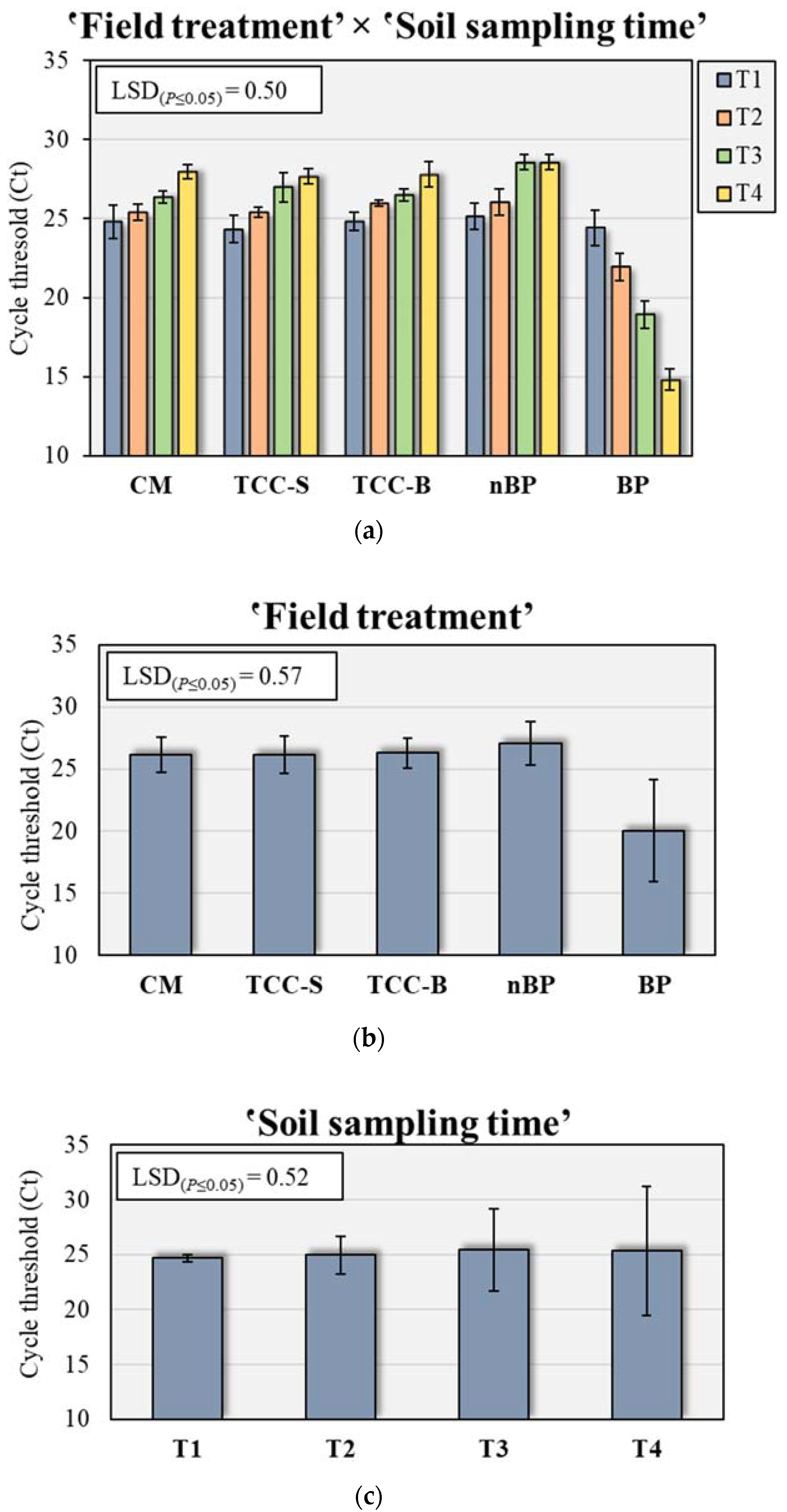
Based on the above-mentioned results, we decided to monitor the behavior of and quantify the bacterium P. putida in soil DNA extracts of the apricot orchard in the five treatments under study by qRT-PCR. Specific primers were designed to amplify an amplicon of 154 bp from the P. putida 16S rRNA gene to allow its quantification. ANOVA demonstrated that the presence of P. putida in the soil was significantly affected by treatment (F = 199.5, p ≤ 0.001), sampling time (F = 3.8, p ≤ 0.05) and their interaction (F = 38.0, p ≤ 0.001), with the treatment accounting for 82.7% of the total variance. Considering the inverse relationship between Ct levels and the amount of target nucleic acids in the sample, BP plots were highly colonized by P. putida in all the sampling times, with Ct values ranging from 14.7 in T4 to 24.3 in T1 (Figure 5a). Averaged over sampling times, the amount of P. putida nucleic acids was the highest in BP (20.0) and the lowest in nBP (27.0), while no significant differences were observed between CM and subterranean clover plots (Figure 5b). Regarding the effect of sampling time, P. putida was significantly higher in T3 (25.5) and T4 (25.4), while T1 showed the lowest amount (24.7) (Figure 5c).
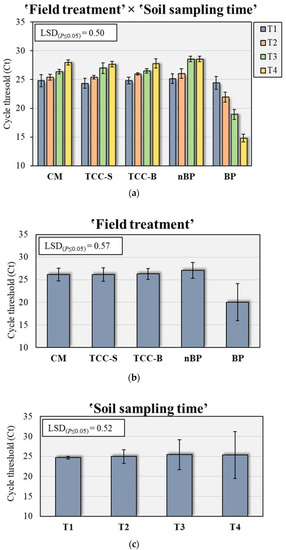
Figure 5.
(a) Amount of Pseudomons putida detected by qRT-PCR over five different field treatments (b) and four soil sampling times (c). The Least Significant Difference (LSD) interaction was calculated with the Fisher’s protected LSD test at α = 0.05. Each bar indicates means ± standard deviation. Ct levels are inversely proportional to the amount of target nucleic acids in the soil sample. CM, conventional apricot management; TCC-S, Trifolium subterraneum L. cover cropping leaving dead mulch on the soil surface; TCC-B, T. subterraneum cover cropping burying dead mulch in the soil; nBP, no-biodegradable plastic; BP, biodegradable plastic; T1, May 2020; T2, October 2020; T3, December 2020; T4, February 2021.
4. Discussion
To evaluate the effect of biodegradable mulching on soil microflora and with the aim of isolating and identifying the microorganisms responsible for degradation, a combined approach, based on biodegradation and molecular tests, was herein described to obtain an overview.
Placement of BP and nBP films in the experimental field occurred in May 2020. The plastic materials were monitored over time. In September 2020, they appeared visibly degraded, with strong evidence of cracks and thinning. It was therefore decided to collect samples from plastics to isolate the microorganisms attached to the surface of the materials. The adopted procedure represents a first-pass technique for isolating potential BP degraders from soil and was successfully used to isolate, from BP, a mesophilic bacterium able to grow using the polymer as the sole carbon source. This microorganism was identified, by sequencing, as P. putida. Pseudomonas putida are ubiquitous bacteria frequently present in water, in soils and, especially, in the plant rhizosphere [24,25]. These aerobic, Gram-negative Pseudomonas show diverse spectra of metabolic versatility and niche-specific adaptations [26,27]. Some of them may also be involved in the biodegradation of natural or man-made toxic chemical compounds [28,29].
Kasirajan and Ngouajio et al. [1] reviewed a list of BP-degrading microorganisms. However, only few studies report so far the use of microorganisms, in particular fungal strains belonging to Aspergillus and Penicillium genera and spore forming bacteria belonging to Bacillus and Clostridium genera, to enhance the degradation of used BP mulch films in soil environment [30]. Indeed, Koitabashi and coauthors [2] reported a study about the potential of using BP-degrading microorganisms, such as phylloplane fungi isolated from Poaceae members, for accelerating the degradation of used BP mulch films in agricultural fields. The authors isolated the strain B47-9 from healthy leaves of barley, that could be safely utilized for acceleration of degradation of BP mulch film after use. Furthermore, Fukushima et al. [31,32] found that the bacterium B. licheniformis was among those responsible for the biodegradation of poly(lactic acid) and poly(ε-caprolactone) and their nanocomposites in a mature compost. Recently, Bandopadhyay et al. [9] combined the amplicon sequencing and the qPCR to study the soil microbial communities associated with BPs and nBPs in two locations. They found a higher presence of Methylobacterium, Arthrobacter and Sphingomonas in BP soils than nBP ones, also reporting these microbial consortia were able to degrade the plastics.
In our research, we found that the isolate P. putida showed a high ability to use BP film both in plate and liquid assays. In the plate assay, the isolated bacterium colonized BP sample with a strong evidence of a growth halo surrounding the polymer. In the liquid assay, its ability to use BP as carbon and energy sources was confirmed with an increase in the population density from the initial contamination of 5.1 to 7.9 log CFU mL−1 after 4 weeks. In order to understand if the in vitro obtained results reflected the behavior of P. putida in the experimental field and to assess the effect of biodegradable mulching on this microorganism, its quantification by RT-PCR was performed in soil samples over the time periods in between treatments. The qRT-PCR technology has been recently used to rapidly quantify microorganisms associated with biodegradable plastic mulch films in soil [9]. In our work, a higher amount of P. putida in BP plots was detected, compared to the other treatments. This was probably due to carbon availability, on one hand, and to the increase of soil temperature, on the other. Indeed, it is well-known that soil temperature is one of the most important factors affecting microorganism growth. Here, BP caused a +4.1 °C in the 0–10 cm soil depth and a +3.2 °C in the 0–20 cm layer, in respect to CM. Brodhagen and coauthors [7] have fully reported that temperature is one of the factors driving microbial metabolism and affecting the biodegradation rate. Biologically, the enhancement of enzymatically catalyzed reactions by warmer temperatures results in an increase in the overall metabolic rate of microbial communities, which implies increased production of cells and enzymes. Overall, our results indicate the ability of the mesophilic P. putida to proliferate and actively degrade the commercial BP film in agricultural soils.
5. Conclusions
In this study, we isolated and identified the mesophilic bacterium P. putida from soil particles attached to the surface of BP mulching film, which was found to be a microorganism responsible for the biodegradation. The same bacterium was also found to be significantly more abundant in BP plots than the other treatments. Only little information on the role of mesophilic bacteria in the biodegradation of plastic mulch films in agricultural soils has been reported; for this reason, our preliminary results are scientifically relevant. The isolation of plastic-degrading microorganisms could lead to their use for amendments to soil where plastics need to be degraded or to accelerate the degradation process, thus reducing the plastic disposal problem in agriculture. However, it is important to perform further specific studies in order to identify and characterize the enzymes responsible for the degradation of BP film and the biochemical processes involved.
Author Contributions
Conceptualization, C.A. and G.M.; methodology, S.F., A.R., G.M., A.S. and C.A.; investigation and data curation, S.F., A.S. and C.A.; writing—original draft preparation, A.S. and C.A.; writing—review and editing, A.S., C.A. and G.M.; supervision, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Catania, Project “Piano per la Ricerca di Ateneo 2020—Linea di Intervento 2”, grant number 5A722192151.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the FONTANAZZA farm (Caltanissetta, Italy) for hosting the field experiment.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Koitabashi, M.; Noguchi, M.T.; Sameshima-Yamashita, Y.; Hiradate, S.; Suzuki, K.; Yoshida, S.; Watanabe, T.; Shinozaki, Y.; Tsushima, S.; Kitamoto, H.K. Degradation of biodegradable plastic mulch films in soil environment by phylloplane fungi isolated from gramineous plants. AMB Express 2012, 2, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreeßen, C.; Steinbüchel, A. Recent developments in non-biodegradable biopolymers: Precursors, production processes, and future perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 143–157. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. The New Plastics Economy: Rethinking the Future of Plastics. Industry Agenda. 2016. Available online: http://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf (accessed on 15 June 2021).
- Cherubini, F.; Bird, N.D.; Cowie, A.; Jungmeier, G.; Schlamadinger, B.; Woess-Gallasch, S. Energy- and greenhouse gas-based LCA of biofuel and bioenergy systems: Key issues, ranges and recommendations. Resour. Conserv. Recycl. 2009, 53, 434–447. [Google Scholar] [CrossRef]
- Kapanen, A.; Schettini, E.; Vox, G.; Itavaara, M. Performance and environmental impact of biodegradable films in agriculture: A field study on protected cultivation. J. Polym. Environ. 2008, 16, 109–122. [Google Scholar] [CrossRef]
- Brodhagen, M.; Peyron, M.; Miles, C.; Inglis, D.A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 2015, 99, 1039–1056. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Data 2019. Global Production Capacities of Bioplastics 2019–2024. Available online: https://docs.european-bioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2019.pdf (accessed on 14 June 2021).
- Bandopadhyay, S.; Liquet y Gonzalez, J.E.; Henderson, K.B.; Anunciado, M.B.; Hayes, D.G.; DeBruyn, J.M. Soil microbial communities associated with biodegradable plastic mulch films. Front. Microbiol. 2020, 11, 587074. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Kyrikou, I.; Briassoulis, D. Biodegradation of agricultural plastic films: A critical review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, K.; Ishii, N.; Inoue, Y.; Yazawa, K.; Tagaya, T.; Yotsumoto, T.; Kazahaya, J.; Nagai, D. Characterization of a mesophilic aliphatic-aromatic copolyester-degrading fungus. Polym. Degrad. Stab. 2009, 94, 1190–1196. [Google Scholar] [CrossRef]
- Muroi, F.; Tachibana, Y.; Kobayashi, Y.; Sakurai, T.; Kasuya, K. Influences of poly(butylene adipate-co-terephthalate) on soil microbiota and plant growth. Polym. Degrad. Stab. 2016, 129, 338–346. [Google Scholar] [CrossRef]
- Bailes, G.; Lind, M.; Ely, A.; Powell, M.; Moore-Kucera, J.; Miles, C.; Inglis, D.; Brodhagen, M. Isolation of native soil microorganisms with potential for breaking down biodegradable plastic mulch films used in agriculture. J. Vis. Exp. 2013, 75, 50373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; U.S. Gov. Print. Office: Washington, DC, USA, 1999.
- Restuccia, A.; Scavo, A.; Lombardo, S.; Pandino, G.; Fontanazza, S.; Anastasi, U.; Abbate, C.; Mauromicale, G. Long-term effect of cover crops on species abundance and diversity of weed flora. Plants 2020, 9, 1506. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Lombardo, S.; Fontanazza, S.; Abbate, C.; Pandino, G.; Anastasi, U.; Onofri, A.; Mauromicale, G. Improving soil health, weed management and nitrogen dynamics by Trifolium subterraneum cover cropping. Agron. Sustain. Dev. 2020, 40, 18. [Google Scholar] [CrossRef]
- Lombardo, S.; Restuccia, A.; Abbate, C.; Anastasi, U.; Fontanazza, S.; Scavo, A.; Guarnaccia, P.; La Malfa, S.; Pandino, G.; Mauromicale, G. Trifolium subterraneum cover cropping for improving the nutritional status of a Mediterranean apricot orchard. J. Sci. Food Agric. 2021, 101, 3767–3777. [Google Scholar] [CrossRef]
- Gennari, M.; Negrè, M. Degradation of fluazifop-butyl by soil microorganism. Pestic. Soil Water 1991, 47, 67–73. [Google Scholar]
- Scavo, A.; Restuccia, A.; Abbate, C.; Mauromicale, G. Seeming field allelopathic activity of Cynara cardunculus L. reduces the soil weed seed bank. Agron. Sustain. Dev. 2019, 39, 41. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lombardo, S.; Abbate, C.; Pandino, G.; Parisi, B.; Scavo, A.; Mauromicale, G. Productive and physiological response of organic potato grown under highly calcareous soils to fertilization and mycorrhization management. Agronomy 2020, 10, 1200. [Google Scholar] [CrossRef]
- Timmis, K.N. Pseudomonas putida: A cosmopolitan opportunist par excellence. Environ. Microbiol. 2002, 4, 779–781. [Google Scholar] [CrossRef]
- Dos Santos, V.A.; Heim, S.; Moore, E.R.; Stratz, M.; Timmis, K.N. Insights into the genomic basis of niche specificity of Pseudomonas putida KT2440. Environ. Microbiol. 2004, 6, 1264–1286. [Google Scholar] [CrossRef] [PubMed]
- Rojo, F. Carbon catabolite repression in Pseudomonas: Optimizing metabolic versatility and interactions with the environment. FEMS Microbiol. Rev. 2010, 34, 658–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Monchy, S.; Taghavi, S.; Zhu, W.; Ramos, J.L.; van der Lelie, D. Comparative genomics and functional analysis of niche- specific adaptation in Pseudomonas putida. FEMS Microbiol. Rev. 2011, 35, 299–323. [Google Scholar] [CrossRef] [Green Version]
- Holloway, B. Pseudomonas in the late twentieth century. In Pseudomonas Molecular Biology and Biotechnology; Galli, E., Silver, S., Witholt, B., Eds.; American Society for Microbiology: Washington, DC, USA, 1992; pp. 1–8. [Google Scholar]
- Ramos, J.L.; Krell, T.; Danield, C.; Segura, A.; Duque, E. Responses of Pseudomonas to small toxic molecules by a mosaic of domains. Curr. Opin. Microbiol. 2009, 12, 215–220. [Google Scholar] [CrossRef]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable plastic mulches: Impact on the agricultural biotic environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Rizzarelli, P.; Camino, G. Biodegradation trend of poly(ε-caprolactone) and nanocomposites. Mater. Sci. Eng. C Mater. Biol. Appl. 2010, 30, 566–574. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).