Combined Application of Rice Husk Biochar and Lime Increases Phosphorus Availability and Maize Yield in an Acidic Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Soil Collection and Preparation
2.3. Rice Husk Biochar Collection and Characterization
2.4. Experimental Layout and Treatment
2.5. Soil Analysis
2.6. Biochar Analysis
2.7. Plant Material Analysis
2.8. Soil CO2 Flux Emission Measurement
2.9. Percent Relative Data
2.10. Statistical Analysis
3. Results
3.1. Effect of Treatments on Changes in Nutrients of the Post-Harvest Soil
3.2. Effect of Treatments on Changes in Plant Growth and Yield Contributing Characters
3.3. Effect of Treatments on Changes in Plant Nutrient Concentration and Uptake
3.4. Effect of Treatments on Changes in Soil CO2 Emission
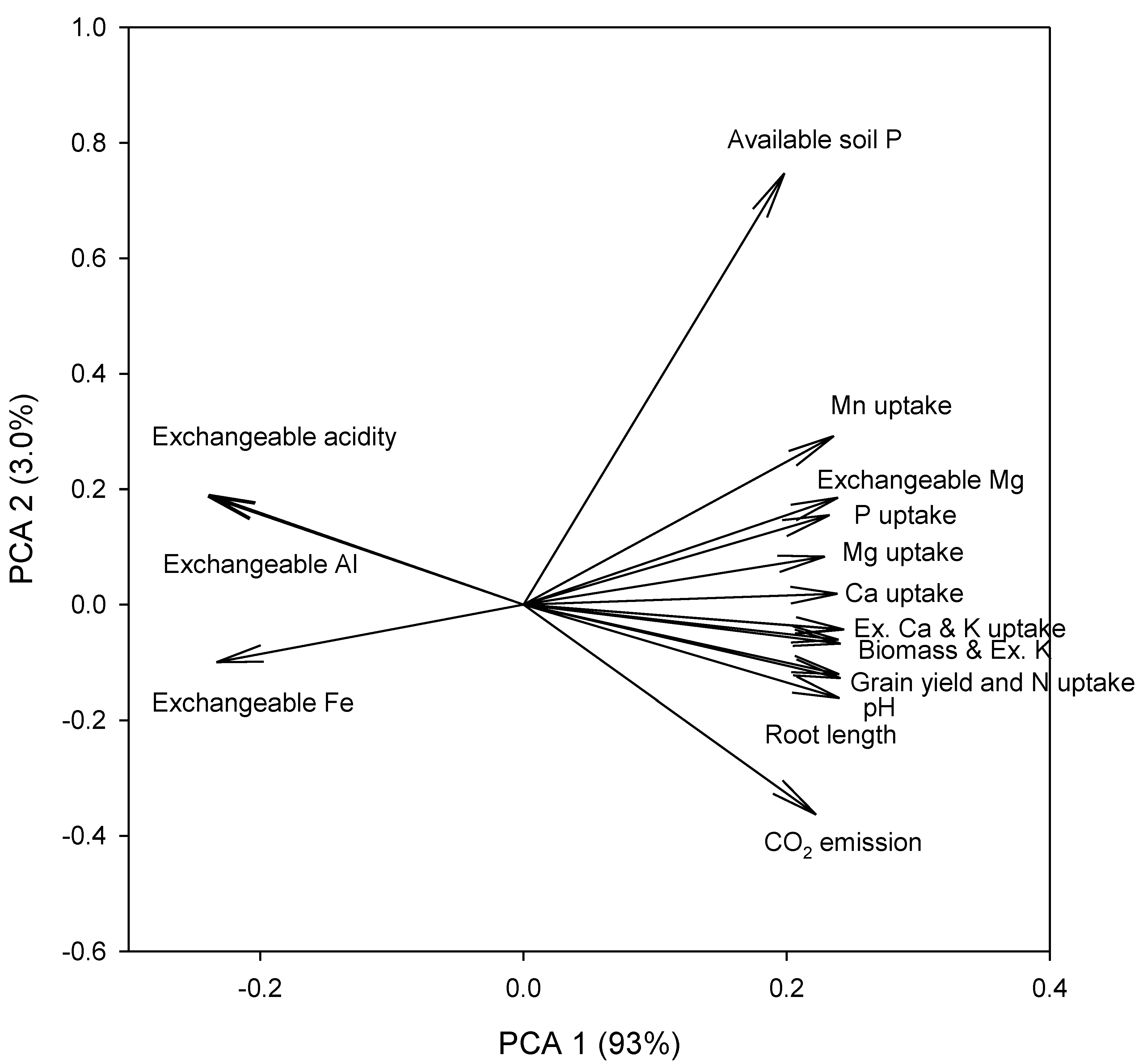
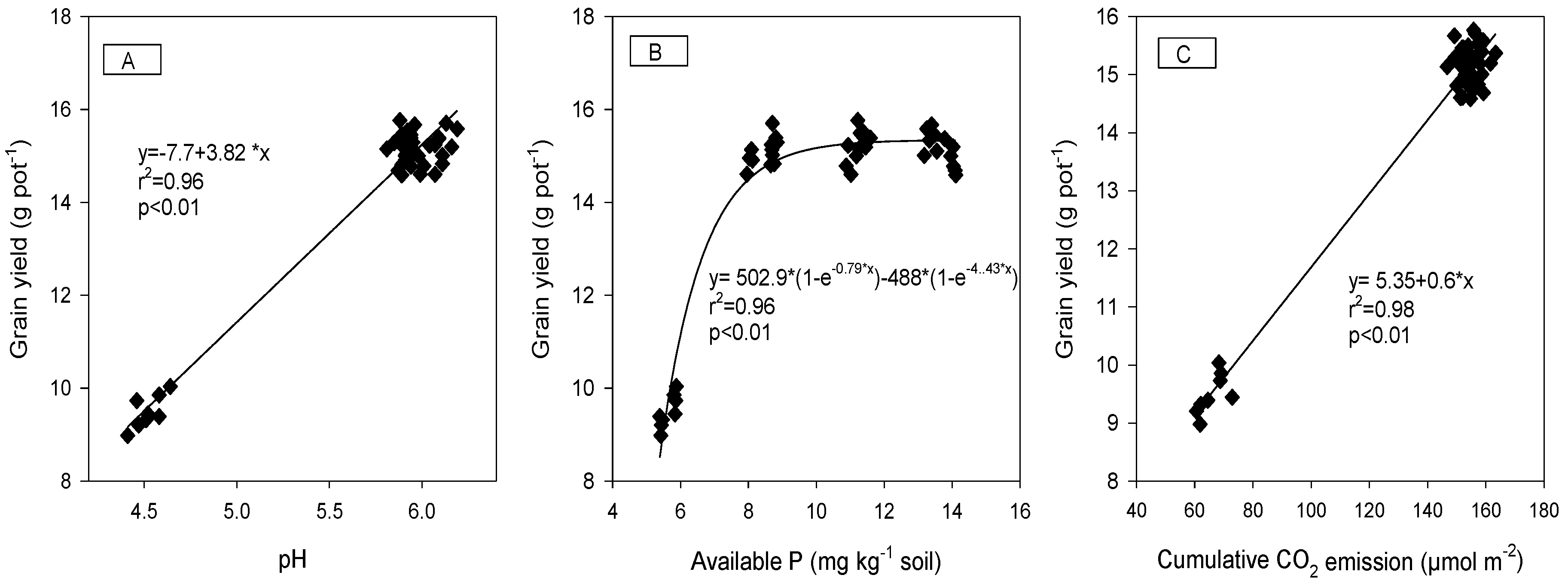
3.5. Relationship between Plant Parameters, Nutrients Uptake, Soil pH, and Nutrients
4. Discussion
4.1. Impact of Treatments on Changes in Nutrients of the Post-Harvest Soil
4.2. Impact of Treatments on Plant Growth and Yield Contributing Characters
4.3. Impact of Treatments on Plant Nutrient Concentration and Uptake
4.4. Impact of Treatments on Changes in Soil CO2 Emission
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaidun, S.W.; Jalloh, M.B.; Awang, A.; Sam, L.M.; Besar, N.A.; Musta, B.; Ahmed, O.H.; Omar, L. Biochar and clinoptilolite zeolite on selected chemical properties of soil cultivated with maize (Zea mays L.). Eurasian J. Soil Sci. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Hasbullah, N.A.; Ahmed, O.H.; Majid, N.M.A. Effects of Amending Phosphatic Fertilizers with Clinoptilolite Zeolite on Phosphorus Availability and Its Fractionation in an Acid Soil. Appl. Sci. 2020, 10, 3162. [Google Scholar] [CrossRef]
- Kalkhoran, S.S.; Pannell, D.J.; Thamo, T.; White, B.; Polyakov, M. Soil acidity, lime application, nitrogen fertility, and greenhouse gas emissions: Optimizing their joint economic management. Agric. Syst. 2019, 176, 102684. [Google Scholar] [CrossRef]
- Joris, H.A.W.; Caires, E.F.; Scharr, D.A.; Bini, A.R.; Haliski, A. Liming in the conversion from degraded pastureland to a no-till cropping system in Southern Brazil. Soil Tillage Res. 2016, 162, 68–77. [Google Scholar] [CrossRef]
- Tiritan, C.S.; Büll, L.T.; Crusciol, C.A.C.; Carmeis Filho, A.C.A.; Fernandes, D.M.; Nascente, A.S. Tillage system and lime application in a tropical region: Soil chemical fertility and corn yield in succession to degraded pastures. Soil Tillage Res. 2016, 155, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Li, G.D.; Conyers, M.K.; Helyar, K.R.; Lisle, C.J.; Poile, G.J.; Cullis, B.R. Long-term surface application of lime ameliorates subsurface soil acidity in the mixed farming zone of south-eastern Australia. Geoderma 2019, 338, 236–246. [Google Scholar] [CrossRef]
- Gazey, C.; Davies, S.; Master, R. Soil Acidity: A Guide for WA Farmers and Consultant, 2nd ed.; Bulletin 4858; Department of Agriculture and Food: Perth, Australia, 2014. [Google Scholar]
- Wang, M.; Xian-Jun, J. Effects of applying lime and calcium montmorillonite on nitrification dynamics in acidic soil. J. Agric. Resour. Environ. 2017, 34, 47. [Google Scholar] [CrossRef]
- Mia, S.; Singh, B.; Dijkstra, F.A. Chemically oxidized biochar increases ammonium-15 N recovery and phosphorus uptake in a grassland. Biol. Fertil. Soils 2019, 55, 577–588. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Rhizosphere Chemistry in Relation to Plant Nutrition. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London/Waltham, UK; San Diego, CA, USA, 2011; pp. 347–368. [Google Scholar]
- Wu, S.; Zhang, Y.; Tan, Q.; Sun, X.; Wei, W.; Hu, C. Science of the Total Environment Biochar is superior to lime in improving acidic soil properties and fruit quality of Satsuma mandarin. Sci. Total Environ. 2020, 714, 136722. [Google Scholar] [CrossRef]
- Novais, S.V.; Zenero, M.D.O.; Barreto, M.S.C.; Montes, C.R.; Cerri, C.E.P. Phosphorus removal from eutrophic water using modified biochar. Sci. Total Environ. 2018, 63, 825–835. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2020, 64, 222–236. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Olayanju, A.; Ejue, W.S.; Adekanye, T.A.; Adenusi, T.T.; Ayeni, J.F. Effect of Biochar on Soil Properties, Soil Loss, and Cocoyam Yield on a Tropical Sandy Loam Alfisol. Sci. World J. 2020, 2020. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Solaiman, Z.M.; Shafi, M.I.; Beamont, E.; Anawar, H.M. Poultry litter biochar increases mycorrhizal colonisation, soil fertility and cucumber yield in a fertigation system on sandy soil. Agriculture 2020, 10, 480. [Google Scholar] [CrossRef]
- Deluca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Earthscan: New York, NY, USA, 2015; pp. 424–425. [Google Scholar]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota-A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Kahura, M.W.; Hyungi, M.; Min, S.K.; Jeong, G.K. Assessing phosphorus availability in a high pH, biochar amended soil under inorganic and organic fertilization. Ecol. Resilient Infrastruct. 2018, 5, 11–18. [Google Scholar] [CrossRef]
- Maru, A.; Haruna, A.O.; Asap, A.; Majid, N.M.A.; Maikol, N.; Jeffary, A.V. Reducing acidity of tropical acid soil to improve phosphorus availability and Zea mays L. Productivity through efficient use of chicken litter biochar and triple superphosphate. Appl. Sci. 2020, 10, 2127. [Google Scholar] [CrossRef] [Green Version]
- Mia, S.; Van Groenigen, J.W.; Van de Voorde, T.F.J.; Oram, N.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S. Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Hiemstra, T.; Mia, S.; Duhaut, P.B.; Molleman, B. Natural and pyrogenic humic acids at goethite and natural oxide surfaces interacting with phosphate. Environ. Sci. Technol. 2013, 47, 9182–9189. [Google Scholar] [CrossRef] [PubMed]
- Mia, S.; Dijkstra, F.A.; Singh, B. Aging induced changes in biochar’s functionality and adsorption behavior for phosphate and ammonium. Environ. Sci. Technol. 2017, 51, 8359–8367. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.; Senesi, N.; Kunhikrishnan, A.; James, T.; McDowell, R. Dissolved organic carbon: Biogeochemistry, dynamics and agro environmental significance in soils. Adv. Agron. 2010, 110, 1–67. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Wang, J.; Pan, X.; Liu, Y.; Zhang, X.; Xiong, Z. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 2012, 360, 287–298. [Google Scholar] [CrossRef]
- Thammasom, N.; Vityakon, P.; Lawongsa, P.; Saenjan, P. Biochar and rice straw have different effects on soil productivity, greenhouse gas emission and carbon sequestration in Northeast Thailand paddy soil. Agric. Nat. Resour. 2016, 50, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Z.; Chen, C.R.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D.K. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Rodrigues, M.; Coelho, M.J.A.; Gasperini, A.M.; Sartor, L.R.; Pavinato, P.S. Changes in soil phosphorus lability promoted by phosphate sources and cover crops. Soi Tillage Res. 2018, 179, 20–28. [Google Scholar] [CrossRef]
- Pedram, K. Genetic Potential of Selected Sweet Corn Inbred Lines and Analysis of Their Combining Ability Assisted by Microsatellite DNA Markers. Ph.D. Thesis, Universiti Putra Malaysia, Seri Kembangan, Selangor, Malaysia, 2012. [Google Scholar]
- Mosharrof, M.; Uddin, M.K.; Jusop, S.; Sulaiman, M.F.; Shamsuzzaman, S.M.; Haque, A.N.A. Changes in Acidic Soil Chemical Properties and Carbon Dioxide Emission Due to Biochar and Lime Treatments. Agriculture 2021, 11, 219. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Laboratory Methods Manual; Soil Survey Laboratory Investigations Report; U.S. Department of Agriculture Lincoln, Nebraska: Washington, DC, USA, 2014. [Google Scholar]
- Tan, K.H. Soil and plant test. In Soil Sampling, Preparation, and Analysis, 2nd ed.; Tan, K.H., Ed.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2005; pp. 98–134. [Google Scholar]
- Benton, J.J. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9780429132117. [Google Scholar]
- Schollenberger, C.J.; Simon, R.H. Determination of exchange capacity and exchangeable bases in soil-ammonium acetate method. Soil Sci. 1945, 59, 13–24. [Google Scholar] [CrossRef]
- Elisa, A.A.; Ninomiya, S.; Shamshuddin, J.; Roslan, I. Alleviating aluminum toxicity in an acid sulfate soil from Peninsular Malaysia by calcium silicate application. Solid Earth 2016, 7, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Douglas, L.A.; Bremner, J.M. Extraction and colorimetric determination of urea in soils. Soil Sci. Soc. Am. 1970, 34, 859–862. [Google Scholar] [CrossRef]
- Ahmedna, M.; Marshall, W.E.; Rao, R.M. Production of granular activated carbon from select agricultural by-products and evaluation of their physical, chemical, and adsorption properties. Bioresour. Technol. 1998, 71, 113–123. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperature. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Lai, L. Utilization of Rice Straw Biochar and Urea to Mitigate Greenhouse Gases Emission in Sustainable Rice Production. Ph.D. Thesis, Universiti Putra Malaysia, Seri Kembangan, Selangor, Malaysia, 2019. [Google Scholar]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Optimal leaf positions for SPAD meter measurement in rice. Front. Plant Sci. 2016, 7, 719. [Google Scholar] [CrossRef] [Green Version]
- Lija, M.; Ahmed, O.H.; Susilawati, K. Maize (Zea mays L.) nutrient use efficiency as affected by formulated fertilizer with Clinoptilolite Zeolite. Emir. J. Food Agric. 2014, 26, 284–292. [Google Scholar] [CrossRef]
- Cottenie, A. Soil testing and plant testing as a basis of fertilizer recommendation. FAO Soil Bull. 1980, 38, 70–73. [Google Scholar]
- Rabileh, M.A.; Shamshuddin, J.; Panhwar, Q.A.; Rosenani, A.B.; Anuar, A.R. Effects of biochar and/or dolomitic limestone application on the properties of Ultisol cropped to maize under glasshouse conditions. Can. J. Soil Sci. 2015, 95, 37–47. [Google Scholar] [CrossRef]
- Snyder, C.S.; Bruulsema, T.W. Nutrient Use Efficiency and Effectiveness in North America: Indices of Agronomic and Environmental Benefit; International Plant Nutrition Institute: Norcross, GA, USA, 2007; p. 4. [Google Scholar]
- Iqbal, J.; Hu, R.G.; Feng, M.; Lin, S.; Malghani, S.; Ali, I.M. Microbial biomass, and dissolved organic carbon and nitrogen strongly affect soil respiration in different land uses: A case study at Three Gorges Reservoir Area, South China. Agric. Ecosyst. Environ. 2010, 137, 294–307. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, L.; Cheng, H.; Yue, S.; Li, S. Effects of biochar application on CO2 emissions from a cultivated soil under semiarid climate conditions in Northwest China. Sustainability 2017, 9, 1482. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Waheed, A. Screening of local/exotic accessions of lentil (Lens culinaris Medic.) for salt tolerance at two growth stages. Plant Soil 1990, 128, 167–176. [Google Scholar] [CrossRef]
- Abdulrahman, D.K.; Othman, R.; Saud, H.M. Effects of empty fruit bunch biochar and nitrogen-fixing bacteria on soil properties and growth of sweet corn. Malays. J. Soil Sci. 2016, 8, 177–194. [Google Scholar]
- Tang, C.; Sparling, G.P.; McLay, C.D.A.; Raphael, C. Effect of short-term legume residue decomposition on soil acidity. Aust. J. Soil Res. 1999, 37, 561–573. [Google Scholar] [CrossRef]
- Ch’ng, H.Y.; Ahmed, O.H.; Majid, N.M.N.A.; Jalloh, M.B. Improving soil phosphorus availability and yield of Zea mays L. using biochar and compost derived from agro-industrial wastes. Ital. J. Agron. 2019, 14, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Mensah, A.K.; Frimpong, K.A. Biochar and/or compost applications improve soil properties, growth, and yield of maize grown in acidic rainforest and coastal savannah soils in Ghana. Int. J. Agron. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Panhwar, Q.A.; Naher, U.A.; Shamshuddin, J.; Ismail, M.R. Effects of biochar and ground magnesium limestone application, with or without bio-Fertilizer addition, on biochemical properties of an acid sulfate soil and rice yield. Agronomy 2020, 10, 1100. [Google Scholar] [CrossRef]
- Masud, M.M.; Abdulaha-Al Baquyb, M.; Akhtera, S.; Sena, R.; Barmana, A.; Khatuna, M.R. Liming effects of poultry litter derived biochar on soil acidity amelioration and maize growth. Ecotoxicol. Environ. Saf. 2020, 202, 110865. [Google Scholar] [CrossRef]
- Baquy, M.A.A.; Jiang, J.; Xu, R.K. Biochars derived from crop straws increased the availability of applied phosphorus fertilizer for maize in Ultisol and Oxisol. Environ. Sci. Pollut. Control Ser. 2020, 27, 5511–5522. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.A.; Jiang, J.; Li, J.Y.; Shi, R.Y.; Mehmood, K.; Baquy, M.A.A.; Xu, R.K. Amelioration of soil acidity, Olsen-P, and phosphatase activity by manure- and peatderived biochars in different acidic soils. Arab. J. Geosci. 2018, 11, 272. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.S.; Bol, R. Biochar, climate change and soil: A review to guide future research. CSIRO Land Water Sci. Rep. 2009, 5, 1–57. [Google Scholar]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macêdo, J.L.V.; Blum, W.E.H.; Zech, W. Long-term effect of manure, charcoal and mineral fertilization on crop production and fertility on highly weathered central amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of biochar amendment on fertility of a southeastern Coastal Plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, K.; Baquy, M.A.A.; Xu, R.K. Influence of nitrogen fertilizer forms and crop straw biochars on soil exchange properties and maize growth on an acidic Ultisol. Int. J. Agron. 2018, 64, 834–849. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhan, Y.; Zhu, L. Reduced carbon sequestration potential of biochar in acidic soil. Sci. Total Environ. 2016, 572, 129–137. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gagnon, B.; Bertrand, N. In situ mineralization of dairy cattle manure as determined using soil-surface carbon dioxide fluxes. Soil Sci. Soc. Am. J. 2006, 70, 744–752. [Google Scholar] [CrossRef]
- Mosharrof, M.; Uddin, M.K.; Sulaiman, M.F.; Mia, S.; Shamsuzzaman, S.M.; Haque, A.N.A. Combined Application of Biochar and Lime Increases Maize Yield and Accelerates Carbon Loss from an Acidic Soil. Agronomy 2021, 11, 1313. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Parkin, T.B. Effect of biochar on soil greenhouse gas emissions at the laboratory and field scales. Soil Syst. 2019, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Chenfei, L.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Qin, H.; Fuhrmann, J.J. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, B.; Bolan, N.; Novak, J.; Ok, Y.S.; Van Zwieten, L.; Singh, B.P.; Kirkham, M.B.; Choppala, G.; Spokas, K.; et al. Designing advanced biochar products for maximizing greenhouse gas mitigation potential. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1367–1401. [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, Y.F.; Chang, S.X.; Zhang, J.J.; Jiang, P.K.; Zhou, G.M.; Shen, Z.M. Contrasting effects of bamboo leaf and its biochar on soil CO2 efflux and labile organic carbon in an intensively managed Chinese chestnut plantation. Biol. Fertil. Soils 2014, 50, 1109–1119. [Google Scholar] [CrossRef]



| Properties | Soil |
|---|---|
| Textural Class | Sandy clay loam |
| % Sand | 69.27 |
| % Silt | 2.28 |
| % Clay | 28.44 |
| pH | 4.61 |
| CEC (cmolc kg−1) | 5.77 |
| Total C (%) | 1.41 |
| Total N (%) | 0.07 |
| Total S (%) | 0.05 |
| Exchangeable K (cmolc kg−1) | 0.22 |
| Exchangeable Ca (cmolc kg−1) | 1.46 |
| Exchangeable Mg (cmolc kg−1) | 0.42 |
| Exchangeable Al (cmolc kg−1) | 2.49 |
| Available P (mg kg−1) | 5.21 |
| Extractable Fe (mg kg−1) | 99.44 |
| Extractable Mn (mg kg−1) | 4.64 |
| NH4+-N (mg kg−1) | 16.41 |
| NO3--N (mg kg−1) | 11.37 |
| Properties | RHB |
|---|---|
| Moisture Content (%) | 6 |
| Ash Content (%) | 32.40 |
| pH | 8.15 |
| CEC (cmolc kg−1) | 48.12 |
| Total C (%) | 24.86 |
| Total N (%) | 1.13 |
| Total S (%) | 0.15 |
| Exchangeable K (cmolc kg−1) | 17.45 |
| Exchangeable Ca (cmolc kg−1) | 19.46 |
| Exchangeable Mg (cmolc kg−1) | 13.96 |
| Available P (mg kg−1) | 3098.40 |
| Extractable Fe (mg kg−1) | 43.06 |
| Extractable Mn (mg kg−1) | 23.51 |
| Treatment Code | Description of the Treatments |
|---|---|
| T1 | Control (no treatments and fertilizer) |
| T2 | Recommended rate of NPK (t ha−1) |
| T3 | 100% dolomitic limestone + 10 t ha−1 rice husk biochar + 100% TSP |
| T4 | 100% dolomitic limestone + 10 t ha−1 rice husk biochar + 75% TSP |
| T5 | 100% dolomitic limestone + 10 t ha−1 rice husk biochar + 50% TSP |
| T6 | 75% dolomitic limestone + 10 t ha−1 rice husk biochar + 100% TSP |
| T7 | 75% dolomitic limestone + 10 t ha−1 rice husk biochar + 75% TSP |
| T8 | 75% dolomitic limestone + 10 t ha−1 rice husk biochar + 50% TSP |
| T9 | 75% dolomitic limestone + 15 t ha−1 rice husk biochar + 100% TSP |
| T10 | 75% dolomitic limestone + 15 t ha−1 rice husk biochar + 75% TSP |
| T11 | 75% dolomitic limestone + 15 t ha−1 rice husk biochar + 50% TSP |
| Treatment | pH | Available P (mg kg−1) | Exchangeable K (cmolc kg−1) | Exchangeable Ca (cmolc kg−1) | Exchangeable Mg (cmolc kg−1) | Exchangeable Al (cmolc kg−1) | Extractable Fe (mg kg−1) | Extractable Mn (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
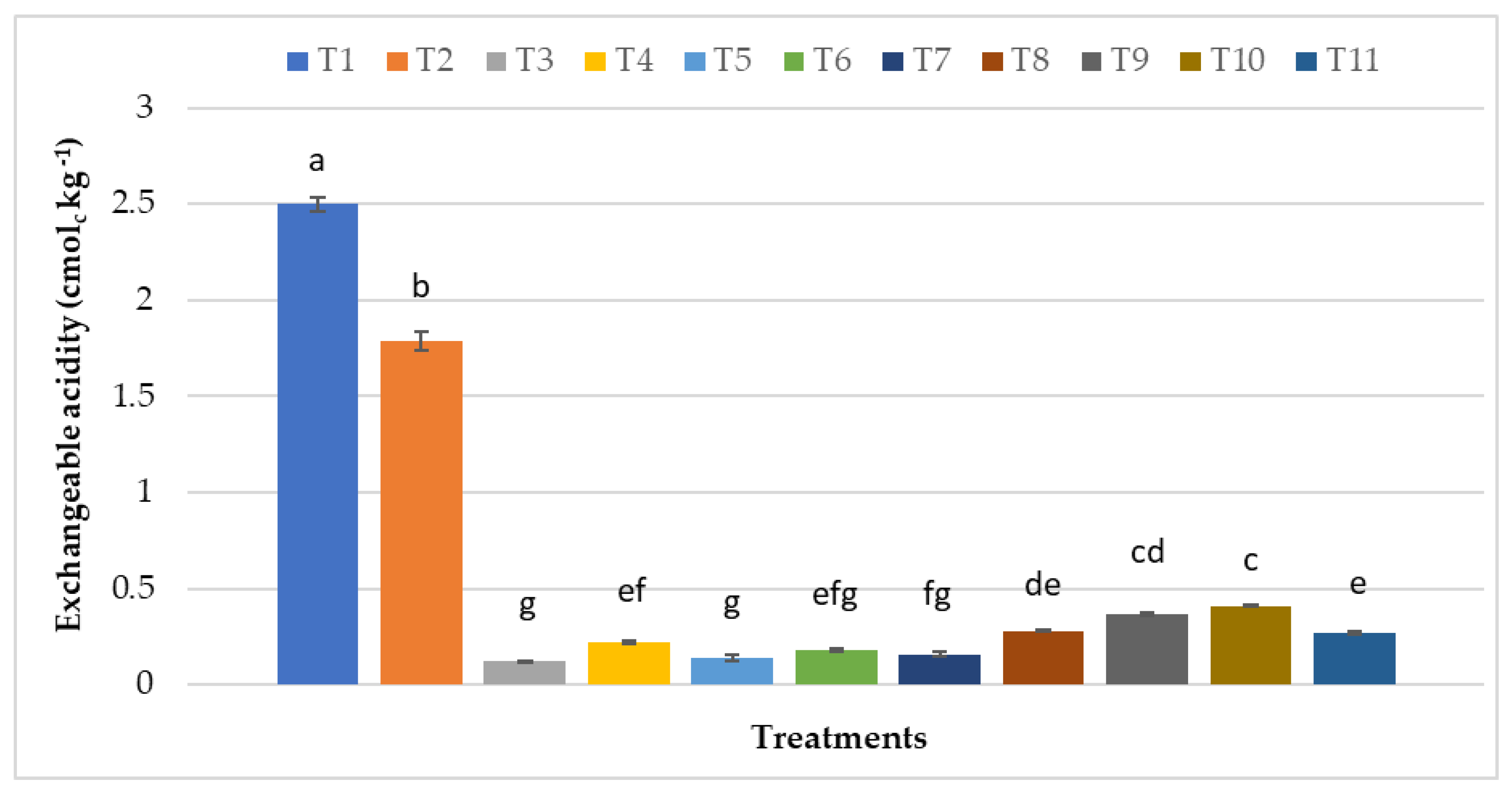
| T1 | 4.49 e ± 0.036 | 5.43 h ± 0.017 | 0.34 e ± 0.022 | 1.43 g ± 0.013 | 0.44 g ± 0.012 | 2.53 a ± 0.013 | 80.05 a ± 1.56 | 4.67 f ± 0.041 |
| T2 | 4.55 e ± 0.039 | 5.85 g ± 0.015 | 0.47 d ± 0.025 | 1.60 f ± 0.013 | 0.55 g ± 0.025 | 2.48 a ± 0.021 | 84.09 a ± 1.31 | 4.71 f ± 0.016 |
| T3 | 6.14 a ± 0.023 | 13.56 b ± 0.206 | 1.37 bc ± 0.015 | 4.05 b ± 0.013 | 1.47 a ± 0.015 | 0.08 f ± 0.011 | 51.66 bc ± 1.14 | 6.58 a ± 0.016 |
| T4 | 5.91 d ± 0.013 | 11.41 c ± 0.078 | 1.34 c ± 0.019 | 3.97 bc ± 0.015 | 1.31b cd ± 0.019 | 0.18 d ± 0.015 | 47.25 cd ± 1.01 | 6.11 c ± 0.025 |
| T5 | 6.10 ab ± 0.013 | 8.74 e ± 0.029 | 1.35 bc ± 0.018 | 3.96 cd ± 0.021 | 1.19 f ± 0.016 | 0.09 ef ± 0.018 | 55.03 b ± 1.06 | 5.84 e ± 0.021 |
| T6 | 5.93 cd ± 0.015 | 13.44 b ± 0.048 | 1.43 ab ± 0.018 | 3.84 e ± 0.023 | 1.33 bc ± 0.013 | 0.24 d ± 0.019 | 45.86 d ± 0.70 | 6.27 b ± 0.022 |
| T7 | 6.02 cb ± 0.019 | 11.01 d ± 0.065 | 1.38 bc ± 0.015 | 4.05 b ± 0.017 | 1.26 de ± 0.019 | 0.12 ef ± 0.017 | 43.36 d ± 1.50 | 6.08 c ± 0.015 |
| T8 | 5.95 cd ± 0.017 | 8.05 f ± 0.035 | 1.39 bc ± 0.013 | 3.81 e ± 0.022 | 1.22 ef ± 0.024 | 0.17 de ± 0.018 | 54.46 b ± 1.48 | 5.95 de ± 0.019 |
| T9 | 5.90 d ± 0.015 | 14.05 a ± 0.033 | 1.49 a ± 0.017 | 4.15 a ± 0.011 | 1.46 a ± 0.013 | 0.39 bc ± 0.016 | 46.49 cd ± 1.28 | 6.48 a ± 0.019 |
| T10 | 5.87 d ± 0.026 | 11.33 cd ± 0.043 | 1.34 c ± 0.018 | 4.14 a ± 0.013 | 1.34 b ± 0.019 | 0.44 b ± 0.016 | 47.21 cd ± 1.28 | 6.27 b ± 0.022 |
| T11 | 5.92 cd ± 0.013 | 8.73 e ± 0.042 | 1.39 cb ± 0.016 | 3.88 de ± 0.026 | 1.28 cd ± 0.019 | 0.34 c ± 0.024 | 53.46 b ± 0.78 | 6.05 cd ± 0.018 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatment | Plant Height (cm) | Dry Biomass (g) | Root Length (cm) | SPAD Value |
|---|---|---|---|---|
| T1 | 179.20 c ± 1.86 | 22.32 c ± 1.82 | 46.94 b ± 1.21 | 40.70 c ± 0.684 |
| T2 | 195.07 b ± 1.53 | 39.37 b ± 2.21 | 53.65 b ± 1.00 | 43.70 bc ± 0.62 |
| T3 | 225.66 a ± 2.86 | 84.01 a ± 1.90 | 73.01 a ± 1.67 | 50.45 a ± 0.924 |
| T4 | 224.52 a ± 4.06 | 79.23 a ± 1.93 | 76.16 a ± 1.25 | 49.03 a ± 0.578 |
| T5 | 225.30 a ± 2.89 | 80.03 a ± 1.84 | 77.18 a ± 1.61 | 52.03 a ± 0.657 |
| T6 | 222.26 a ± 2.53 | 84.31 a ± 1.82 | 77.35 a ± 1.94 | 50.75 a ± 1.372 |
| T7 | 223.92 a ± 2.20 | 81.87 a ± 2.05 | 79.61 a ± 4.09 | 50.68 a ± 1.148 |
| T8 | 224.86 a ± 1.98 | 75.37 a ± 2.09 | 75.23 a ± 2.35 | 48.95 ab ± 1.383 |
| T9 | 230.45 a ± 2.22 | 78.91 a ± 1.77 | 71.95 a ± 1.84 | 49.78 a ± 1.193 |
| T10 | 226.07 a ± 1.81 | 81.62 a ± 1.95 | 73.24 a ± 2.26 | 49.63 a ± 1.497 |
| T11 | 222.91 a ± 1.81 | 77.45 a ± 2.15 | 76.90 a ± 2.06 | 50.88 a ± 1.13 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatment | Cob Length (cm) | Fresh Cob Weight (g) | No. of Grain per Cob | Yield (t ha−1) |
|---|---|---|---|---|
| T1 | 18.28 a ± 0.559 | 172.98 c ± 1.69 | 323 d ± 5.82 | 9.22 c ± 0.090 |
| T2 | 19.50 b ± 0.507 | 183.15 c ± 2.33 | 480 c ± 4.19 | 9.77 c ± 0.126 |
| T3 | 23.18 a ± 0.332 | 286.68 ab ± 2.31 | 624 ab ± 5.18 | 15.29 ab ± 0.122 |
| T4 | 22.50 a ± 0.492 | 290.03 a ± 2.27 | 622 ab ± 4.19 | 15.47 a ± 0.119 |
| T5 | 22.48 a ± 0.496 | 286.73 ab ± 3.39 | 628 ab ± 4.21 | 15.29 ab ± 0.181 |
| T6 | 23.75 a ± 0.247 | 290.60 a ± 2.21 | 635 a ± 3.86 | 15.50 a ± 0.119 |
| T7 | 23.15 a ± 0.184 | 279.45 ab ± 2.53 | 630 ab ± 5.21 | 14.90 ab ± 0.136 |
| T8 | 22.45 a ± 0.403 | 279.43 ab ± 2.05 | 611 b ± 5.05 | 14.90 ab ± 0.110 |
| T9 | 23.10 a ± 0.381 | 276.80 b ± 1.64 | 622 ab ± 5.74 | 14.77 b ± 0.080 |
| T10 | 22.90 a ± 0.443 | 287.75 ab ± 1.48 | 616 ab ± 3.38 | 15.35 ab ± 0.08 |
| T11 | 23.25 a ± 0.366 | 282.45 ab ± 1.90 | 618 ab ± 4.91 | 15.07 ab ± 0.101 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatment | N Concentration (%) | P Concentration (%) | K Concentration (%) | Ca Concentration (%) | Mg Concentration (%) |
|---|---|---|---|---|---|
| T1 | 0.80 g ± 0.013 | 0.11 e ± 0.009 | 0.55 f ± 0.017 | 0.09 f ± 0.006 | 0.10 g ± 0.006 |
| T2 | 1.30 f ± 0.013 | 0.18 d ± 0.009 | 1.04 e ± 0.011 | 0.11 f ± 0.009 | 0.13 g ± 0.008 |
| T3 | 2.25 bc ± 0.016 | 0.26 bc ± 0.008 | 2.37 a ± 0.006 | 0.54 cd ± 0.009 | 0.33 b ± 0.006 |
| T4 | 2.16 cd ± 0.022 | 0.28 ab ± 0.006 | 2.26 d ± 0.006 | 0.50 de ± 0.005 | 0.26d ef ± 0.006 |
| T5 | 2.30 b ± 0.022 | 0.24 c ± 0.008 | 2.28 cd ± 0.009 | 0.58 bc ± 0.009 | 0.28 de ± 0.003 |
| T6 | 2.41 a ± 0.021 | 0.28 ab ± 0.006 | 2.25 d ± 0.006 | 0.63 a ± 0.010 | 0.23 f ± 0.011 |
| T7 | 2.25 bc ± 0.021 | 0.23 c ± 0.008 | 2.31 bc ± 0.008 | 0.60 ab ± 0.009 | 0.38 a ± 0.004 |
| T8 | 2.14 d ± 0.013 | 0.24 c ± 0.006 | 2.26 d ± 0.004 | 0.54 cd ± 0.009 | 0.25 ef ± 0.006 |
| T9 | 2.02 e ± 0.028 | 0.29 ab ± 0.006 | 2.33 ab ± 0.004 | 0.60 ab ± 0.013 | 0.29 cd ± 0.012 |
| T10 | 2.18 cd ± 0.020 | 0.31 a ± 0.008 | 2.37 a ± 0.013 | 0.54 cd ± 0.006 | 0.32 bc ± 0.006 |
| T11 | 2.30 b ± 0.019 | 0.28 ab ± 0.005 | 2.33 ab ± 0.005 | 0.48 e ± 0.013 | 0.24 f ± 0.009 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatment | N (mg Plant−1) | P (mg Plant−1) | K (mg Plant−1) | Ca (mg Plant−1) | Mg (mg Plant−1) |
|---|---|---|---|---|---|
| T1 | 178.56 g ± 2.88 | 24.00 e ± 1.91 | 123.32 i ± 3.91 | 20.83 g ± 1.08 | 21.21 f ± 1.44 |
| T2 | 511.81 f ± 5.08 | 71.42 d ± 3.43 | 408.46 h ± 4.36 | 44.09 g ± 3.37 | 50.20 f ± 2.95 |
| T3 | 1886.02 b ± 13.06 | 216.33 b ± 6.30 | 1986.84 a ± 5.42 | 451.55 c ± 7.18 | 273.03 a ± 5.42 |
| T4 | 1711.37 d ± 17.42 | 217.32 b ± 5.19 | 1786.64 f ± 5.11 | 394.17 ef ± 3.79 | 229.77 bc ± 10.23 |
| T5 | 1840.69 bc ± 17.59 | 190.08 c ± 6.00 | 1826.69 de ± 6.83 | 462.17 bc ± 6.83 | 221.43 cd ± 3.14 |
| T6 | 2027.66 a ± 17.72 | 231.86 ab ± 5.44 | 1892.76 c ± 5.44 | 526.94 a ± 8.78 | 191.81 de ± 9.35 |
| T7 | 1844.12 bc ± 17.49 | 186.26 c ± 6.14 | 1889.15 c ± 6.14 | 491.22 b ± 7.47 | 272.22 a ± 6.99 |
| T8 | 1615.59 e ± 9.40 | 177.12 c ± 4.87 | 1703.36 g ± 3.08 | 408.88 de ± 6.44 | 186.69 e ± 4.85 |
| T9 | 1590.04 e ± 22.43 | 229.33 ab ± 4.29 | 1838.63 d ± 3.22 | 473.46 bc ± 10.19 | 224.18 c ± 9.90 |
| T10 | 1781.36 cd ± 16.45 | 250.98 a ± 6.12 | 1934.39 b ± 10.54 | 440.75 cd ± 4.71 | 257.10 ab ± 5.27 |
| T11 | 1777.48 cd ± 15.00 | 219.28 b ± 3.65 | 1802.65 ef ± 3.71 | 371.77 f ± 10.00 | 176.20 e ± 6.61 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Correlation | pH | Av. P | Exch. K | Exch. Ca | Exch. Mg | Exch. Al | Exct. Fe | Exct. Mn | Exch. Acidity | Plant Biomass | Root Length | Grain Yield | N Uptake | P Uptake | K Uptake | Ca Uptake | Mg Uptake | CO2 Emission |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.00 | |||||||||||||||||
| Av. P | 0.73 | 1.00 * | ||||||||||||||||
| Exch. K | 0.97 * | 0.77 * | 1.00 * | |||||||||||||||
| Exch. Ca | 0.98 * | 0.77 * | 0.99 * | 1.00 * | ||||||||||||||
| Exch. Mg | 0.95 * | 0.86 * | 0.97 * | 0.97 * | 1.00 * | |||||||||||||
| Exch Al | −0.99 * | −0.72 * | −0.98 * | −0.98 * | −0.94 * | 1.00 * | ||||||||||||
| Exct. Fe | −0.92 * | −0.82 * | −0.94 * | −0.95 * | −0.92 * | 0.93 * | 1.00 * | |||||||||||
| Exct. Mn | 0.93 * | 0.90 * | 0.95 * | 0.96 * | 0.99 * | −0.92 * | −0.93 * | 1.00 * | ||||||||||
| Exch. acidity | −0.98 * | −0.72 * | −0.97 * | −0.97 * | −0.94 * | 0.98 * | 0.90 * | −0.91 * | 1.00 * | |||||||||
| Plant biomass | 0.96 * | 0.78 * | 0.97 * | 0.97 * | 0.95 * | −0.96 * | −0.92 * | 0.93 * | −0.98 * | 1.00 * | ||||||||
| Root length | 0.91 * | 0.63 * | 0.91 * | 0.90 * | 0.84 * | −0.91 * | −0.88 * | 0.82 * | −0.93 * | 0.90 * | 1.00 * | |||||||
| Grain yield | 0.98 * | 0.74 * | 0.97 * | 0.98 * | 0.95 * | −0.98 * | −0.93 * | 0.93 * | −0.98 * | 0.97 * | 0.91 * | 1.00 * | ||||||
| N uptake | 0.97 * | 0.75 * | 0.96 * | 0.96 * | 0.94 * | −0.97 * | −0.92 * | 0.92 * | −0.98 * | 0.97 * | 0.92 * | 0.98 * | 1.00 * | |||||
| P uptake | 0.90 * | 0.81 * | 0.94 * | 0.95 * | 0.96 * | −0.90 * | −0.91 * | 0.94 * | −0.92 * | 0.93 * | 0.82 | 0.94 * | 0.93 * | 1.00 * | ||||
| K uptake | 0.98 * | 0.79 * | 0.98 * | 0.99 * | 0.97 * | −0.98 * | −0.94 * | 0.96 * | −0.98 * | 0.98 * | 0.91 * | 0.99 * | 0.99 * | 0.96 * | 1.00 * | |||
| Ca uptake | 0.96 * | 0.81 * | 0.97 * | 0.96 * | 0.94 * | −0.96 * | −0.95 * | 0.93 * | −0.95 * | 0.96 * | 0.88 * | 0.96 * | 0.97 * | 0.91 * | 0.97 * | 1.00 * | ||
| Mg uptake | 0.92 * | 0.78 * | 0.89 * | 0.94 * | 0.92 * | −0.91 * | −0.90 * | 0.91 * | −0.91 * | 0.91 * | 0.83 * | 0.91 * | 0.91 * | 0.87 * | 0.94 * | 0.91 * | 1.00 * | |
| CO2 emission | 0.99 * | 0.76 * | 0.99 * | 0.99 * | 0.97 * | −0.99 * | −0.94 * | 0.95 * | −0.98 * | 0.97 * | 0.90 * | 0.99 * | 0.97 * | 0.93 * | 0.99 * | 0.96 * | 0.92 * | 1.00 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosharrof, M.; Uddin, M.K.; Sulaiman, M.F.; Mia, S.; Shamsuzzaman, S.M.; Haque, A.N.A. Combined Application of Rice Husk Biochar and Lime Increases Phosphorus Availability and Maize Yield in an Acidic Soil. Agriculture 2021, 11, 793. https://doi.org/10.3390/agriculture11080793
Mosharrof M, Uddin MK, Sulaiman MF, Mia S, Shamsuzzaman SM, Haque ANA. Combined Application of Rice Husk Biochar and Lime Increases Phosphorus Availability and Maize Yield in an Acidic Soil. Agriculture. 2021; 11(8):793. https://doi.org/10.3390/agriculture11080793
Chicago/Turabian StyleMosharrof, Mehnaz, Md. Kamal Uddin, Muhammad Firdaus Sulaiman, Shamim Mia, Shordar M. Shamsuzzaman, and Ahmad Numery Ashfaqul Haque. 2021. "Combined Application of Rice Husk Biochar and Lime Increases Phosphorus Availability and Maize Yield in an Acidic Soil" Agriculture 11, no. 8: 793. https://doi.org/10.3390/agriculture11080793
APA StyleMosharrof, M., Uddin, M. K., Sulaiman, M. F., Mia, S., Shamsuzzaman, S. M., & Haque, A. N. A. (2021). Combined Application of Rice Husk Biochar and Lime Increases Phosphorus Availability and Maize Yield in an Acidic Soil. Agriculture, 11(8), 793. https://doi.org/10.3390/agriculture11080793









