Potentiality of Vermicomposting in the South Pacific Island Countries: A Review
Abstract
1. Introduction
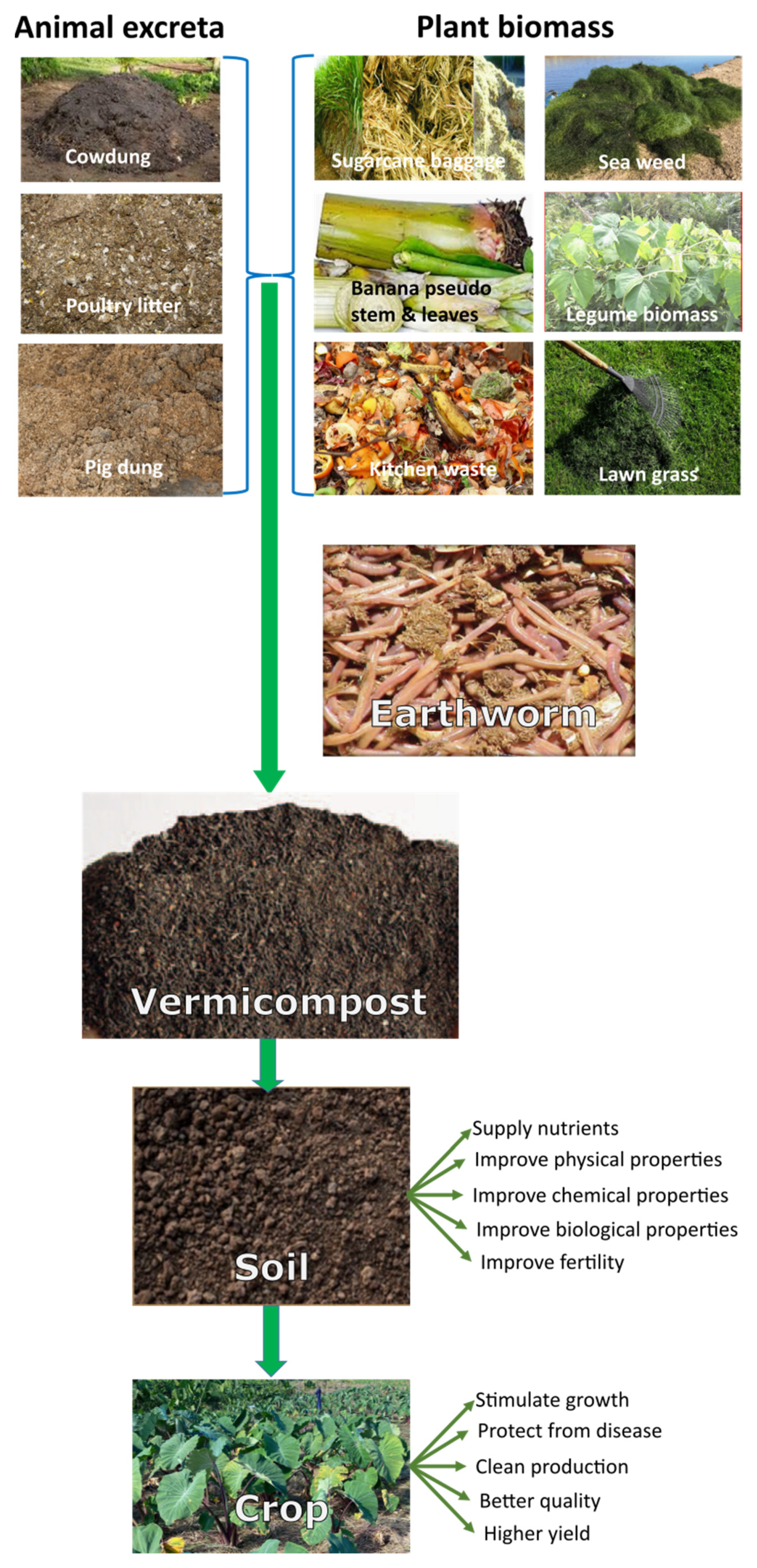
2. The Vermicomposting Process
3. Earthworm Species Used for Vermicomposting
4. Characteristics of Vermicompost
5. Use of Vermicompost
5.1. Plant and Soil Nutrients Supply
5.2. Growth Regulator
5.3. Bio-Pesticide
5.4. Recycling of Solid Waste
6. Potentiality of Vermicompost in South Pacific Island Countries
6.1. Availability and Adaptability of Earthworm Species Suitable for Vermicomposting
6.2. Traditional Organic Farming Systems
6.3. Tropical and Sub-Tropical Climates
6.4. Availability of Biomass for Vermicomposting
6.5. Demand for Biopesticides
6.6. The Low-Tech Nature of Vermicomposting Systems
6.7. Suitability for SPICs Crops
6.8. Required Adaptive Research
- Assessments of the suitability of native earthworm species for vermicomposting;
- Analysis of the suitability of available raw materials (substrate) for vermicomposting;
- Standardization of protocols for vermicompost production in a Pacific context;
- Situation-based crop response studies;
- Awareness development for adoption of vermicompost use.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.; Brown, G.G.; De Deyn, B.G.; van Groenigen, J.K. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef]
- Eurostat. Statistics Explained. Available online: http://ec.europa.eu/eurostat/statistics-explained/ (accessed on 1 March 2018).
- Song, X.; Liu, M.; Wu, D.; Griffiths, B.S.; Jiao, J.; Li, H.; Hu, F. Interaction matters: Synergy between vermicompost and PGPR agents improves soil quality, crop quality and crop yield in the field. Appl. Soil Ecol. 2015, 89, 25–34. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil. Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Devi, K.N.; Singh, T.B.; Athokpam, H.S.; Singh, N.B.; Samurailatpam, D. Influence of inorganic, biological and organic manures on nodulation and yield of soybean (Glycine max Merril, L.) and soil properties. Aust. J. Crop. Sci. 2013, 7, 1407–1415. [Google Scholar]
- Przemieniecki, S.W.; Skwiercz, A.; Damszel, M.; Telesiński, A.; Zapałowska, A.; Sierota, Z.; Gorczyca, A. Ecology, biology and enzymatic activity of the rhizosphere planted with Larix decidua seedlings after addition of vermicompost. Appl. Soil Ecol. 2021, 168, 104101. [Google Scholar] [CrossRef]
- Jack, A.L.; Thies, J.E. Compost and vermicompost as amendments promoting soil health. In Biological Approaches to Sustainable Soil Systems; Uphoff, N., Ed.; CRC Press: New York, NY, USA, 2006; pp. 453–466. [Google Scholar]
- Jindo, K.; Chocano, C.; De Aguilar, J.M.; Gonzalez, D.; Hernandez, T.; Garcia, C. Impact of compost application during 5 years on crop production, soil microbial activity, carbon fraction, and humification process. Commun. Soil Sci. Plant Anal. 2016, 47, 1907–1919. [Google Scholar] [CrossRef]
- Dignac, M.-F.; Derrien, D.; Barré, P.; Barot, S.; Cécillon, L.; Chenu, C.; Chevallier, T.; Freschet, G.T.; Garnier, P.; Guenet, B.; et al. Increasing soil carbon storage: Mechanisms, effects of agricultural practices and proxies. A review. Agron. Sust. Dev. 2017, 37, 14. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jahiruddin, M.; Kader, M.A.; Islam, M.R.; Solaiman, Z.M. Sugarcane bagasse biochar increases soil carbon sequestration and yields of maize and groundnut in charland ecosystem. Arch. Agron. Soil Sci. 2021. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 2017. [Google Scholar]
- Kulkarni, S.J. Vermicomposting—A boon for waste minimization and soil quality. Int. J. Res. Rev. 2017, 4, 76–81. [Google Scholar]
- Mia, S.; Ektear, U.; Kader, M.A.; Ahsan, M.A.; Mannan, M.A.; Hossain, M.M.; Solaiman, Z.M. Pyrolysis and co-composting of municipal organic waste in Bangladesh: A quantitative estimate of recyclable nutrients, and economics benefits. Waste Manag. 2018, 75, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Rekha, G.S.; Kaleena, P.K.; Elumalai, D.; Srikumaran, M.P.; Maheswari, V.N. Effects of vermicompost and plant growth enhancers on the exo-morphological features of Capsicum annum (Linn.) Hepper. Int. J. Recycl. Org. Waste Agric. 2018, 7, 83–88. [Google Scholar] [CrossRef]
- Mondal, T.; Datta, J.K.; Mondal, N. KInfluence of indigenous inputs on the properties of old alluvial soil in a mustard cropping system. Arch. Agron. Soil Sci. 2015, 61, 1319–1332. [Google Scholar] [CrossRef]
- Taheri, R.E.; Ansari, M.H.; Razavi, N.A. Influence of cow manure and its vermicomposting on the improvement of grain yield and quality of rice (Oryza sativa L.) in field conditions. Appl. Ecol. Environ. Res. 2017, 16, 97–110. [Google Scholar] [CrossRef]
- Ali, M.A.; Inubushi, K.; Kim, P.J.; Amin, S. Management of paddy soil towards low greenhouse gas emissions and sustainable rice production in the changing climatic conditions. In Soil Contamination and Alternatives for Sustainable Development; IntechOpen: London, UK, 2019. [Google Scholar]
- Dhanuja, C.; Abbasi, T.; Abbasi, S.A. Fertilization of paddy cultivation with vermicompost: A critical mini review. Org. Arg. 2020, 10, 309–325. [Google Scholar] [CrossRef]
- Chan, P.L.S.; Griffiths, D.A. The vermicomposting of pre-treated pig manure. Biol. Wastes 1988, 24, 57–69. [Google Scholar] [CrossRef]
- Subler, S.; Edwards, C.; Metzger, J. Comparing vermicomposts and composts. Biocycle 1998, 39, 63–66. [Google Scholar]
- Atiyeh, R.M.; Arancon, N.Q.; Edwards, C.A.; Metzger, J.D. Influence of earthworm-processed pig manure on the growth and yield of greenhouse tomatoes. Bioresour. Technol. 2000, 75, 175–180. [Google Scholar] [CrossRef]
- Wang, X.-X.; Zhao, F.; Zhang, G.; Zhang, Y.; Yang, L. Vermicompost improves tomato yield and quality and the biochemical properties of soils with different tomato planting history in a greenhouse study. Front. Plant Sci. 2017, 8, 1978. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dutta, S.K.; Kikon, Z.J.; Kuotsu, R.; Sarkar, D.; Satapathy, B.S.; Deka, B.C. Recycling of agricultural wastes to vermicomposts: Characterization and application for clean and quality production of green bell pepper (Capsicum annuum L.). J. Clean. Prod. 2021, 315, 128115. [Google Scholar] [CrossRef]
- FAO. “Samoa”. FAO Regional Office for Asia and the Pacific. Available online: http://www.fao.org/asiapacific/perspectives/agricultural-statistics/global-strategy/results-in-the-region/samoa/en/ (accessed on 15 December 2018).
- Wilson, C. Organic Farmers Cultivate rural success in Samoa. Available online: http://www.ipsnews.net/2014/09/organic-farmers-cultivate-rural-success-in-samoa/ (accessed on 18 April 2018).
- Suruban, C. Influence of Selected Composted Organic Amendments and their Rates of Application on Nitrogen Mineralization and Soil Productivity using Chinese Cabbage (Brassica rapa. L. var. chinensis) as an Indicator Crop. Master’s Thesis, University of South Pacific, Suva, Fiji, 2019. [Google Scholar]
- Theunissen, J.; Ndakidemi, P.A.; Laubscher, C.P. Potential of vermicompost produced from plant waste on the growth and nutrient status in vegetable production. Int. J. Phys. Sci. 2010, 5, 1964–1973. [Google Scholar]
- Lazcano, C.; Revilla, P.; Malvar, R.A.; Domínguez, J. Yield and fruit quality of four sweet corn hybrids (Zea mays) under conventional and integrated fertilization with vermicompost. J. Sci. Food. Agric. 2011, 91, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Yeong, W.T.; Lim, P.; Shak, K.P.Y. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Lofty, J.R. Biology of Earthworms; Chapman and Hall: London, UK, 1972; pp. 1–288. [Google Scholar]
- Ndegwa, P.M.; Thompson, S.A. Integrating composting and vermicomposting in the treatment and bioconversion of biosolids. Bioresour. Technol. 2001, 76, 107–112. [Google Scholar] [CrossRef]
- Jusselme, M.D.; Pruvost, C.; Motard, E.; Giusti-Miller, S.; Frechault, S.; Alphonse, V.; Balland-Bolou-Bi, C.; Dajoz, I.; Mora, P. Increasing the ability of a green roof to provide ecosystem services by adding organic matter and earthworms. Agric. Ecosyst. 2019, 143, 61–69. [Google Scholar] [CrossRef]
- Chanda, G.K.; Bhunia, G.; Chakraborty, S.K. The effect of vermicompost and other fertilizers on cultivation of tomato plants. J. Hortic. For. 2011, 3, 42–45. [Google Scholar]
- Wu, T.Y.; Lim, L.S.; Lim, P.N.; Shak, K.P.Y. Biotransformation of biodegradable solid wastes into organic fertilizers using composting or/and vermicomposting. Chem. Eng. Trans. 2014, 39, 1579–1584. [Google Scholar]
- Lee, K.E. Earthworms Their Ecology and Relationships with Soils and Land Use; Academic Press: Sydney, Australia, 1985. [Google Scholar]
- FiBL; IFOAM. The World of Organic Agriculture, Statistics and Emerging Trends 2014. In FiBL-IFOAM Report; Helga, W., Lernoud, J., Eds.; Research Institute of Organic Agriculture (FiBL); Frick and International Federation of Organic Agriculture Movement (IFOAM): Bonn, Germany, 2014; Available online: https://www.fibl.org/fileadmin/documents/shop/1636-organic-world-2014.pdf (accessed on 19 December 2019).
- Sharma, S.; Pradhan, K.; Satya, S.; Vasudevan, P. Potentiality of earthworms for waste management and in other uses—A review. J. Am. Sci. 2005, 1, 4–16. [Google Scholar]
- Padmavathiamma, P.K.; Li, L.Y.; Kumari, U.R. An experimental study of vermi-biowaste composting for agricultural soil improvement. Bioresour. Technol. 2008, 99, 1672–1681. [Google Scholar] [CrossRef]
- Adhikary, S. Vermicompost, the story of organic gold: A review. Agric. Sci. 2012, 3, 905–917. [Google Scholar] [CrossRef]
- Nagavallemma, K.P.; Wani, S.P.; Stephane, L.; Padmaja, V.V.; Vineela, C.; Rao, B.M.; Sahrawat, K.L. Vermicomposting: Recycling wastes into valuable organic fertilizer. In Global Theme on Agroecosystems Report No. 8; Patancheru 502,324; International Crops Research Institute for the Semi-Arid Tropics: Andhra Pradesh, India, 2004; p. 20. Available online: https://www.researchgate.net/publication/26513646_Vermicomposting_recycling_wastes_into_valuable_organic_fertilizer. (accessed on 15 June 2021).
- Hemalatha, B. Vermicomposting of fruit waste and industrial sludge. Int. J. Adv. Eng. Technol. 2012, 3, 60–63. [Google Scholar]
- Joshi, R.; Singh, J.; Vig, A.P. Vermicompost as an effective organic fertilizer and biocontrol agent: Effect on growth, yield and quality of plants. Rev. Environ. 2015, 14, 137–159. [Google Scholar] [CrossRef]
- Ansari, A.A.; Sukhraj, K. Effect of vermiwash and vermicompost on soil parameters and productivity of okra (Abelmoschus esculentus) in Guyana. Afr. J. Agric. Res. 2010, 5, 1794–1798. [Google Scholar]
- Solano, K.D.G.; Mendoza, M.D.L.N.R.; Téllez, L.I.T.; Escudero, J.S.; Cué, J.L.G. Chemical properties of vermicompost “teas”. Rev. Mex. Cienc. Agric. 2013, 5, 901–911. [Google Scholar]
- Chaudhary, D.R.; Bhandari, S.C.; Shukla, L.M. Role of vermicompost in sustainable agriculture—A review. Agric. Rev. 2004, 25, 29–39. [Google Scholar]
- Tognetti, C.; Laos, F.; Mazzarino, M.J.; Hernandez, M.T. Composting vs. vermicomposting: A comparison of end product quality. Compost Sci. Util. 2005, 13, 6–13. [Google Scholar] [CrossRef]
- Gutiérrez-Miceli, F.A.; Santiago-Borraz, J.; Molina, J.A.M.; Nafate, C.C.; Abud-Archila, M.; Llaven, M.A.O.; Ricon-Rosales, R.; Dendooven, L. Vermicompost as a soil supplement to improve growth, yield and fruit quality of tomato (Lycopersicum esculentum). Bioresour. Technol. 2007, 98, 2781–2786. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.; Ghosh, G.K.; Ghosal, P.K.; Banik, P. Changes in organic-C, N, P and K and enzyme activities in vermicompost of biodegradable organic wastes under liming and microbial inoculants. Bioresour. Technol. 2007, 98, 2485–2494. [Google Scholar] [CrossRef]
- Tripathi, G.; Bhardwaj, P. Decomposition of kitchen waste amended with cow manure using an epigeic species (Eisenia fetida) and anecic species (Lampito mauriti). Bioresour. Technol. 2004, 92, 215–218. [Google Scholar] [CrossRef]
- Garg, P.; Gupta, A.; Satya, S. Vermicomposting of different types of waste using Eisenia foetida: A comparative study. Bioresour. Technol. 2005, 97, 391–395. [Google Scholar] [CrossRef]
- Hernández, A.; Castillo, H.; Ojeda, D.; Arras, A.; López, J.; Sánchez, E. Effect of vermicompost and compost on lettuce production. Chil. J. Agric. Res. 2010, 70, 583–589. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.R.; Kumar, S.; Gupta, R.K.; Patil, R.T. Vermicompost substitution influences growth, physiological disorders, fruit yield and quality of strawberry (Fragaria x ananassa Duch). Bioresour. Technol. 2008, 99, 8507–8511. [Google Scholar] [CrossRef]
- Murmu, K.; Gosh, C.B.; Swain, K.D. Yield and quality of tomato growth under organic and conventional nutrient management. Arch. Agron. 2013, 59, 1311–1321. [Google Scholar]
- Dominguez, J. Edwards, C.A. Vermicomposting organic wastes: A review. In Soil Zoology for Sustainable Development in the 21st Century; Hanna, S.H.S., Mikhail, W.Z.A., Eds.; IUCN: Cairo, Egypt, 2004; pp. 369–395. [Google Scholar]
- Yasir, M.; Aslam, Z.; Kim, S.W.; Lee, S.-W.; Jeon, C.O.; Chung, Y.R. Bacterial community composition and chitinase gene diversity of vermicompost with antifungal activity. Bioresour. Technol. 2009, 100, 4396–4403. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P. Influences of vermicomposts on field strawberries: Part 2. Effects on soil microbial and chemical properties. Bioresour. Technol. 2006, 97, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Wasnik, K. Effect of vermicompost and chemical fertilizer on growth, herb, oil yield, nutrient uptake, soil fertility, and oil quality of rosemary. Commun. Soil Sci. Plant Anal. 2013, 44, 2691–2700. [Google Scholar] [CrossRef]
- Vasanthi, D.; Kumaraswamy, K. Efficacy of vermicompost to improve soil fertility and rice yield. J. Indian Soc. Soil Sci. 1999, 47, 268–272. [Google Scholar]
- Edwards, C.A.; Arancon, N.Q.; Vasko-Bennett, M.; Askar, A.; Keeney, G.; Little, B. Suppression of green peach aphid (Myzus persicae) (Sulz.), citrus mealybug (Planococcus citri) (Risso), and two spotted spider mite (Tetranychus urticae) (Koch.) attacks on tomatoes and cucumbers by aqueous extracts from vermicomposts. Crop Prot. 2010, 29, 80–93. [Google Scholar] [CrossRef]
- Barthod, J.; Dignac, M.-F.; Le Mer, G.; Bottinelli, N.; Watteau, F.; Kogel-Knabner, I.; Rumpel, C. How do earthworms affect organic matter decomposition in the presence of clay-sized minerals? Soil Biol. Biochem. 2020, 143, 107730. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Chaudhuri, P.S.; Datta, M. Response of paddy (Var. Trc-87-251) crop on amendment of the field with different levels of vermicomposts. Asian J. Microbiol. Biotech. Environ. Sci. 2001, 3, 191–196. [Google Scholar]
- Sáez, J.A.; Pérez-Murcia, M.D.; Vico, A.; Martínez-Gallardo, M.R.; Andreu-Rodríguez, F.J.; López, M.J.; Bustamante, M.A.; Sanchez-Hernandez, J.C.; Moreno, J.; Moral, R. Olive mill wastewater-evaporation ponds long term stored: Integrated assessment of in situ bioremediation strategies based on composting and vermicomposting. J. Hazard. Mater. 2021, 402, 123481. [Google Scholar] [CrossRef] [PubMed]
- Demir, Z. Effects of vermicompost on soil physicochemical properties and lettuce (Lactuca sativa Var. Crispa) yield in greenhouse under different soil water regimes. Commun. Soil Sci. Plant Anal. 2019, 50, 2151–2168. [Google Scholar] [CrossRef]
- Pandit, N.P.; Ahmad, N.; Maheshwari, S.K. Vermicomposting biotechnology an eco-loving approach for recycling of solid organic wastes into valuable biofertilizers. J. Biofertil. Biopestic. 2012, 3, 1–8. [Google Scholar] [CrossRef]
- Atiyeh, R.; Subler, S.; Edwards, C.; Bachman, G.; Metzger, J.; Shuster, W. Effects of vermicomposts and composts on plant growth in horticultural container media and soil. Pedobiologia 2000, 44, 579–590. [Google Scholar] [CrossRef]
- Sangwan, P.; Garg, V.K.; Kaushik, C.P. Growth and yield response of marigold to potting media containing vermicompost produced from different wastes. Environmentalist 2010, 30, 123–130. [Google Scholar] [CrossRef]
- Oliva Llaven, M.A.; Jimenez, J.L.G.; Coro, B.I.C.; Rincon-Rosales, R.; Molina, J.M.; Dendooven, L.; Gutierrez-Miceli, F.A. Fruit characteristics of bell pepper cultivated in sheep manure vermicompost substituted soil. J. Plant. Nutr. 2008, 31, 1585–1598. [Google Scholar] [CrossRef]
- Moghadam, A.R.L.; Ardebill, Z.O.; Saidi, F. Vermicompost induced changes in growth and development of Lilium Asiatic hybrid var. Navona. Afr. J. Agric. Res. 2012, 7, 2609–2621. [Google Scholar]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P.; Metzger, J.D.; Lee, S.; Welch, C. Effects of vermicomposts on growth and marketable fruits of field-grown tomatoes, peppers and strawberries. Pedobiologia 2003, 47, 731–735. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P.; Welch, C.; Metzger, J.D. Influences of vermicomposts on field strawberries: 1. Effects on growth and yields. Bioresour. Technol. 2004, 93, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Levinsh, G. Vermicompost treatment differentially affects seed germination, seedling growth and physiological status of vegetable crop species. Plant Growth Regul. 2011, 65, 169–178. [Google Scholar] [CrossRef]
- Rivera, M.C.; Wright, E.R. Research on vermicompost as plant growth promoter and disease suppressive substrate in Latin America. Dyn. Soil Dyn. Plant 2009, 3, 32–40. [Google Scholar]
- Aguilar-Benitez, G.; Pena-Valdivia, C.B.; Garcia-Nava, J.R.; Ramirez-Vallejo, P.; Benedicto-Valdes, S.G.; Molina-Galan, J.D. Yield of common bean (Phaseolus vulgaris L.) in relation to substrate vermicompost concentration and water deficit. Agrociencia 2012, 46, 37–50. [Google Scholar]
- Amooaghaie, R.; Golmohammadi, S. Effect of vermicompost on growth, essential oil, and health of Thymus Vulgaris. Compost Sci. Util. 2017, 25, 166–177. [Google Scholar] [CrossRef]
- Verma, R.; Maurya, B.R.; Meena, V.S. Integrated effect of bio-organics with chemical fertilizer on growth and yield and quality of cabbage (Brassica oleracea var capitata). Indian J. Agri. Sci. 2014, 84, 914–919. [Google Scholar]
- Moraditochaee, M.; Bozorgi, H.R.; Halajisani, N. Effects of vermicompost application and nitrogen fertilizer rates on fruit yield and several attributes of eggplant (Solanum melongena L.) in Iran. World Appl. Sci. J. 2011, 15, 174–178. [Google Scholar]
- Alam, M.N.; Jahan, M.S.; Ali, M.K.; Ashraf, M.A.; Islam, M.K. Effect of vermicompost and Chemical fertilizers on growth, yield, and yield components of potato in barind soil of Bangladesh. J. Appl. Sci. Res. 2007, 3, 1879–1888. [Google Scholar]
- Azarmi, R.; Ziveh, P.S.; Satari, M.R. Effect of vermicompost on growth, yield and nutrition status of tomato (Lycopersicul esculentum). Pak. J. Biol. Sci. 2008, 11, 1797–1802. [Google Scholar] [CrossRef]
- Vijaya, K.S.; Seethalakshmi, S. Contribution of Parthenium vermicompost in altering growth, yield and quality of Alelmoschus esculentus (I) Moench. Biotechnol. Adv. 2011, 11, 44–47. [Google Scholar]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P.; Metzger, J.D.; Lucht, C. Effects of vermicomposts produced from cattle manure, food waste and paper waste on the growth and yield of peppers in the field. Pedobiologia 2005, 49, 297–306. [Google Scholar] [CrossRef]
- Gajalakshmi, S.; Abbasi, S.A. Earthworms and vermicomposting. Indian J. Biothechnol. 2004, 3, 486–494. [Google Scholar]
- Papathanasiou, F.; Papadopoulos, I.; Tsakiris, I.; Tamoutsidis, E. Vermicompost as a soil supplement to improve growth, yield and quality of lettuce (Lactuca sativa L.). J. Food Agric. Environ. 2012, 10, 677–682. [Google Scholar]
- Uma, B.; Malathi, M. Vermicompost as a soil supplement to improve growth and yield of Amaranthus species. Res. J. Agric. Biol. Sci. 2009, 5, 1054–1060. [Google Scholar]
- Sarma, B.K.; Singh, D.P.; Pandey, S.K.; Singh, H.B. Vermicompost as modulator of plant growth and disease suppression. Dyn. Soil Dyn. Plant 2010, 4, 58–66. [Google Scholar]
- Arancon, N.Q.; Edwards, C.A.; Lee, S.S.; Yardim, E. Management of plant parasitic nematodes by applications of vermicompost. In Brighton Crop Protection Conference Pests and Diseases; BCPC Registered Office: Brighton, UK, 2002; Volume 8B-2, pp. 705–716. [Google Scholar]
- Munroe, G. Manual of on-Farm Vermicomposting and Vermiculture; Organic Agriculture Centre of Canada: Truro, NS, Canada, 2007; Available online: https://www.scirp.org/journal (accessed on 15 December 2018).
- Ali, M.; Griffiths, A.J.; Williams, K.P.; Jones, D.L. Evaluating the growth characteristics of lettuce in vermicompost and green waste compost. Eur. J. Soil Biol. 2007, 43, S316–S319. [Google Scholar] [CrossRef]
- Owagboriaye, F.; Dedeke, G.; Bamidele, J.; Aladesida, A.; Isibor, P.; Feyisola, R.; Adeleke, M. Biochemical response and vermiremediation assessment of three earthworm species (Alma millsoni, Edrilus eugeniae and Libyodrilus violaceus) in soil contaminated with glyphosate-based herbicide. Ecol. Indic. 2020, 108, 105678. [Google Scholar] [CrossRef]
- Rao, K.R. Induced host plant resistance in the management of sucking insect pests of groundnut. Ann. Plant. Protect. Sci. 2002, 10, 45–50. [Google Scholar]
- Basco, M.J.; Bisen, K.; Keswani, C.; Singh, H.B. Biological management of Fusarium wilt of tomato using bio fortified vermicompost. Mycosphere 2017, 8, 467–483. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Galvis, P.A.; Edwards, C.A. Suppression of insect pest populations and damage to plants by vermicomposts. Bioresour. Technol. 2005, 96, 1137–1142. [Google Scholar] [CrossRef]
- Patriquin, D.G.; Barnes, D.; Abboud, A. Diseases, pests and soil fertility. In Soil Management Sustainable Agriculture; Cook, H.F., Lee, C., Eds.; Wye College Press: Wye, UK, 1995; pp. 161–174. [Google Scholar]
- Prestidge, R.A.; McNeill, S. Auchenorrhyncha-host plant interactions leafhoppers and grasses. Ecol. Entomol. 1983, 8, 331–339. [Google Scholar] [CrossRef]
- Asciutto, K.; Rivera, M.C.; Wright, E.R.; Morisigue, D.; López, M.V. Effect of vermicompost on the growth and health of Impatiens wallerana. Phyton 2006, 75, 115–123. [Google Scholar]
- Atiyeh, R.M.; Edwards, C.A.; Subler, S.; Metzger, J.D. Pig manure vermicompost as a component of a horticultural bedding plant medium: Effects on physicochemical properties and plant growth. Bioresour. Technol. 2001, 78, 11–20. [Google Scholar] [CrossRef]
- Basheer, M.; Agrawal, O.P. Management of paper waste by vermicomposting using epigeic earthworm, Eudrilus eugeniae in Gwalior, India. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 42–47. [Google Scholar]
- Villar, I.; Alves, D.; Mato, S. Product quality and microbial dynamics during vermicomposting and maturation of compost from pig manure. Waste Manag. 2017, 69, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Singh, J.; Vig, A.P. Potential utilization of bagasse as feed material for earthworm Eisenia fetida and production of vermicompost. Springerplus 2015, 4, 11. [Google Scholar] [CrossRef]
- Ansari, A.A.; Jaikishun, S. Vermicomposting of sugarcane bagasse and rice straw and its impact on the cultivation of Phaseolus vulgaris L. in Guyana, South America. J. Agric. Technol. 2011, 7, 225–234. [Google Scholar]
- Ansari, A.A.; Ismail, S.A. Role of earthworms in vermitechnology. J. Agric. Technol. 2012, 8, 403–415. [Google Scholar]
- Easton, E.G. Earthworms (Oligochaeta) from islands of the southwestern Pacific, and a note on two species from Papua New Guinea. N. Zealand J. Zool. 1984, 11, 111–128. [Google Scholar] [CrossRef]
- Blakemore, R.J. Review of Pacific Ocean Earthworms Updated from Easton (1984) and Lee (1981). Available online: http://www.annelida.net/earthworm/Pacific/Pacific%20Species.pdf (accessed on 18 December 2020).
- James, S.W. An illustrated key to the earthworms of the Samoan Archipelago. Micronesica 2004, 37, 1–13. [Google Scholar]
- Lal, R. Grace Road Food Company Introduces Earthworm Farming Here. 2015. Available online: https://grgroup.com/grace-road-food-introduces-earthworm-farming-here (accessed on 17 December 2018).
- FAO. Organic Farming and Fair Trade in Pacific Island Countries. Natural Resources Management and Environment Department. Report prepared by Willie Fay Bell. 53p. 2009. Available online: http://www.fao.org/3/ak356e/ak356e.pdf (accessed on 16 June 2021).
- Greiner, H.G.; Stonehouse, A.M.T.; Tiegs, S.D. Cold tolerance among composting earthworm species to evaluate invasion potential. Am. Midl. Nat. 2011, 166, 349–357. [Google Scholar] [CrossRef]
- Samoa Meteorology Division. Climate of Samoa. Available online: http://www.samet.gov.ws/index.php/climate-of-samoa (accessed on 18 December 2018).
- Australian Bureau of Meteorology and CSIRO. Climate Change in the Pacific: Scientific Assessment and New Research. Volume 1 (Regional Overview) & 2 (Country Reports). 2011. Available online: https://www.pacificclimatechangescience.org/publications/reports/report-climate-change-in-the-pacific-scientific-assessment-and-new-research/ (accessed on 1 June 2021).
- Stanley, D. Climate. Text from Moon Handbooks South Pacific. Available online: http://cookislands.southpacific.org/cookislands/climate.html (accessed on 18 December 2018).
- Jandaik, S.; Kumar, V.; Thakur, P. Vermiwash: Plant Growth Enhancer and antifungal Agent. Int. J. Extensive Res. 2015, 2, 38–41. [Google Scholar]
- Taylor, M.; Iosofa, T. Taro Leaf Blight Manual; Secretariat of the Pacific Community: Suva, Fiji, 2013. [Google Scholar]
- Davis, R.I.; Henderson, J.; Jones, L.M.; McTaggart, A.R.; O’Dwyer, C.; Tsatsia, F.; Fanai, C.; Rossel, J.B. First record of a wilt disease of banana plants associated with phytoplasmas in Solomon Islands. Australas. Plant Dis. Notes 2015, 10, 1–6. [Google Scholar] [CrossRef][Green Version]
- Biernbum, J.A. Vermicomposting and Vermiculture Systems for Cold Climates; Department of Horticulture, Michigan State University: East Lansing, MI, USA, 2015; pp. 1–19. [Google Scholar]
- Maheswarappa, H.P. In-situ waste management in integrated nutrient management system under coconut (Cocos nucifera)- Based high density multi-species cropping system in tropical soils of India. Indian J. Agric. Sci. 2008, 78, 924–928. [Google Scholar]
- Nedunchezhiyan, M.; Byju, G.; Dash, S.N.; Ranasingh, N. Selected soil enzyme activities, soil microbial biomass carbon, and root yield as influenced by organic production systems in sweet potato. Commun. Soil Sci. Plant Anal. 2013, 44, 1322–1339. [Google Scholar] [CrossRef]
- Li, P.; Wu, M.; Kang, G.; Zhu, B.; Li, H.; Hu, F.; Jiao, J. Soil quality response to organic amendments on dryland red soil in subtropical China. Geoderma 2020, 373, 114416. [Google Scholar] [CrossRef]
- Chirinda, N.; Trujillo, C.; Loaiza, S.; Salazar, S.; Luna, J.; Encinas, L.A.T.; Becerra López Lavalle, L.A.; Tran, T. Nitrous oxide emissions from cassava fields amended with organic and inorganic fertilizers. Soil Use Manag. 2021, 37, 257–263. [Google Scholar] [CrossRef]
- Omar, N.F.; Hassan, S.A.; Yusoff, U.K.; Abdullah, N.A.P.; Wahab, P.E.M.; Sinniah, U.R. Phenolics, flavonoids, antioxidant activity and cyanogenic glycosides of organic and mineral-base fertilized cassava tubers. Molecules 2012, 17, 2378–2387. [Google Scholar] [CrossRef]
- Venkatakrishnan, D.; Manimaran, S. Organic manures and industrial by-products influence the yield attributes and yield of sugarcane. Indian J. Agric. Res. 2020, 54, 541–544. [Google Scholar]
- Benitez, M.M.; Zapanta, R.J.S.; Briones, E.D. Postharvest quality and antioxidant content of organic eggplant (Solanum melongena L.) in modified atmosphere packaging. Acta Hortic. 2015, 1088, 261–266. [Google Scholar] [CrossRef]
- Tesfay, T.; Gebremariam, M.; Gebretsadik, K.; Hagazi, M.; Girmay, S. Tomato yield and economic performance under vermicompost and mineral fertilizer applications. Open Agric. 2018, 12, 262–269. [Google Scholar] [CrossRef]
- Biswas, I.; Mitra, D.; Senapati, A.; Mitra, D.; Chattaraj, S.; Ali, M.; Basak, G.; Panneerselvam, P.; Mohapatra, P.K.D. Valorization of vermicompost with bacterial fermented chicken feather hydrolysate for the yield improvement of tomato plant: A novel organic combination. Int. J. Recycl. Org. Waste Agric. 2021, 10, 29–42. [Google Scholar]
- Uma Maheswari, T.; RajKumar, M. Influence of organic inputs in augmenting the growth and yield attributes of okra (Abelmoschus esculentus (L.) Moench.). Plant Arch. 2020, 20, 2968–2970. [Google Scholar]
- Dalorima, T.; Khandaker, M.M.; Zakaria, A.J.; Hasbullah, M. Impact of organic fertilizations in improving bris soil conditions and growth of watermelon (Citrullus lanatus). Bulg. J. Agric. Sci. 2018, 24, 112–118. [Google Scholar]
- Verma, V.K.; Patel, R.K.; Deshmukh, N.A.; Jha, A.K.; Ngachan, S.V.; Singha, A.K.; Deka, B.C. Response of ginger and turmeric to organic versus traditional production practices at different elevations under humid subtropics of north-eastern India. Ind. Crops Prod. 2019, 136, 21–27. [Google Scholar] [CrossRef]
- Kirad, K.S.; Barche, S.; Singh, D.B. Integrated nutrient management in papaya (Carica papaya L.) cv. Surya. Acta Hortic. 2010, 851, 377–380. [Google Scholar] [CrossRef]
| Organic Waste | pH (-) | TOC (%) | TN (%) | TP (%) | TK (%) | TS (%) | Zn (mg/kg) | Cu (mg/kg) | Fe (%) | Mn (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| KW | - | 10.30 | 0.85 | 0.15 | - | - | - | - | - | - |
| ST | 6.55 | 24.62 | 1.14 | 0.46 | 1.61 | - | - | - | - | - |
| CD | 7.04 | 32.16 | 3.60 | 0.23 | 0.89 | - | - | - | - | - |
| FW | 7.30 | 34.0 | 1.30 | 2.70 | 9.20 | - | - | - | - | - |
| PL + SP | 8.89 | 16.70 | 2.06 | 0.67 | 5.09 | 0.48 | 440 | 51 | 0.50 | 300 |
| PL + AZ | 8.23 | 16.50 | 1.82 | 0.87 | 3.68 | 0.41 | 520 | 49 | 0.40 | 390 |
| PL + BA | 9.01 | 15.40 | 1.54 | 0.74 | 3.99 | 0.46 | 415 | 45 | 0.45 | 280 |
| PL + PS | 8.91 | 16.20 | 1.54 | 1.06 | 7.23 | 0.58 | 520 | 61 | 0.50 | 590 |
| CD + SP | 6.92 | 20.90 | 1.75 | 0.25 | 1.84 | 0.47 | 290 | 10 | 0.60 | 100 |
| CD + AZ | 6.35 | 19.30 | 2.17 | 0.30 | 1.70 | 0.49 | 285 | 20 | 0.59 | 210 |
| CD + BA | 7.00 | 16.50 | 1.89 | 0.24 | 1.65 | 0.46 | 215 | 9 | 0.78 | 110 |
| CD + PS | 6.75 | 29.30 | 2.24 | 0.34 | 1.51 | 0.41 | 225 | 12 | 0.60 | 310 |
| PD + SP | 7.34 | 19.00 | 2.20 | 0.35 | 2.05 | 0.33 | 210 | 19 | 0.70 | 120 |
| PD + AZ | 6.69 | 28.70 | 2.20 | 0.32 | 1.76 | 0.40 | 220 | 22 | 0.81 | 115 |
| PD + BA | 7.15 | 20.10 | 2.17 | 0.22 | 2.10 | 0.28 | 150 | 12 | 1.10 | 110 |
| PD + PS | 6.80 | 13.90 | 2.48 | 0.56 | 2.15 | 0.36 | 230 | 19 | 1.00 | 300 |
| Element | Vermicompost | Farmyard Manure | Bacterial Compost |
|---|---|---|---|
| N (%) | 1.3–1.84 | 1.10 | 1.40 |
| P (%) | 0.92–1.3 | 0.42 | 0.016 |
| K (%) | 0.21–1.2 | 2.00 | 0.55 |
| Earthworm Species | Reference |
|---|---|
| I. Indigenous | |
| Perionyx exavatus | [102] |
| Pontoscolex corethrurus | [102] |
| Polypheretima elongate | [104] |
| Eudrilus eugeniae | [103] |
| II. Introduced | |
| Eisenia fetida | [105] |
| Crops | Vermicompost Application Rate | Reference |
|---|---|---|
| I. Field crops | ||
| Taro (Colocasia esculenta) | 5–10 t/ha | [115] |
| Sweet potato (Ipomoea batatas) | 10–15 t/ha | [116,117] |
| Cassava (Manihot esculenta) | 5–10 t/ha | [118,119] |
| Sugarcane (Saccharum officinarum) | 5 t/ha | [120] |
| II. Horticulture crops | ||
| Eggplant (Solanum melongena L.) | 3–6 t/ha | [121] |
| Tomato (Solanum lycopersicum) | 5 t/ha | [122,123] |
| Okra (Abelmoschus esculentus) | 5 t/ha | [124] |
| Capsicum (Capsicum annuum) | 10 t/ha | [24] |
| Water melon (Citrullus lanatus) | 10 t/ha | [125] |
| Ginger (Zingiber officinale) | 2–5 t/ha | [126] |
| Turmeric (Curcuma longa) | 2–5 t/ha | [126] |
| Papaya (Carica papaya) | 20 kg/plant | [127] |
| Coconut (Cocos nucifera L.) | 2–20 kg/plant | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierre-Louis, R.C.; Kader, M.A.; Desai, N.M.; John, E.H. Potentiality of Vermicomposting in the South Pacific Island Countries: A Review. Agriculture 2021, 11, 876. https://doi.org/10.3390/agriculture11090876
Pierre-Louis RC, Kader MA, Desai NM, John EH. Potentiality of Vermicomposting in the South Pacific Island Countries: A Review. Agriculture. 2021; 11(9):876. https://doi.org/10.3390/agriculture11090876
Chicago/Turabian StylePierre-Louis, Randy Carlie, Md. Abdul Kader, Nandakumar M Desai, and Eleanor H John. 2021. "Potentiality of Vermicomposting in the South Pacific Island Countries: A Review" Agriculture 11, no. 9: 876. https://doi.org/10.3390/agriculture11090876
APA StylePierre-Louis, R. C., Kader, M. A., Desai, N. M., & John, E. H. (2021). Potentiality of Vermicomposting in the South Pacific Island Countries: A Review. Agriculture, 11(9), 876. https://doi.org/10.3390/agriculture11090876







