A New Grafting Method for Watermelon to Inhibit Rootstock Regrowth and Enhance Scion Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Design
2.2. Plant Material and Cultivation
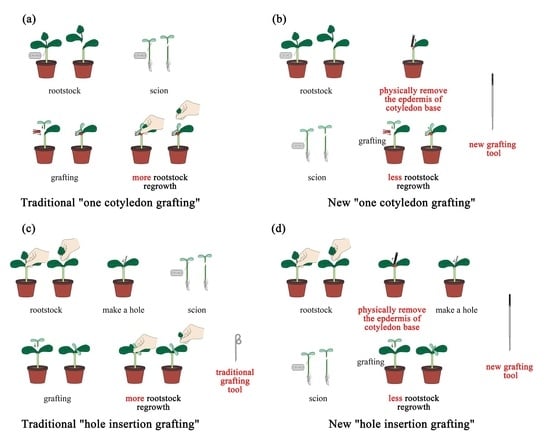
2.3. Grafting
2.4. New Grafting Tool Development and Usage
2.5. Grafted Survival Measurement
2.6. Measurement of Rootstock Regrowth Rate
2.7. Anatomical Study of Rootstock Cotyledon Base
2.8. Measurement of Watermelon Scion Dry Weight, Leaf Area of Watermelon Scion, and Rootstock Regrowth
2.9. Working Efficiency Measurement
2.10. Data Analyses
3. Results
3.1. New Grafting Method had no Significant Effect on the Graft Survival Rate
3.2. New Grafting Method Largely Decreased the Rootstock Regrowth Rate
3.3. New Grafting Method Enhances Scion Growth
3.4. New Grafting Method Is Labor Saving
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mudge, K. A History of Grafting. Hortic. Rev. 2009, 35, 437–494. [Google Scholar]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.L.; Hoyos Echevarria, P.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Huang, Y.; Kong, Q.S.; Chen, F.; Bie, Z.L. The history, current status and future prospects of vegetable grafting in China. Acta Hortic. 2015, 1086, 31–39. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.S.; Dangitsis, C. Impact of scion and rootstock seedling quality selection on the vigor of watermelon–interspecific squash grafted seedlings. Agriculture 2020, 10, 326. [Google Scholar] [CrossRef]
- Devi, P.; Lukas, S.; Miles, C. Advances in watermelon grafting to increase efficiency and automation. Horticulturae 2020, 6, 88. [Google Scholar] [CrossRef]
- Yetısir, H.; Sari, N.; Yucel, S. Rootstock resistance to Fusarium wilt and effect on watermelon fruit yield and quality. Phytoparasitica 2003, 31, 163–169. [Google Scholar] [CrossRef]
- Thies, J.A.; Ariss, J.J.; Kousik, C.S.; Hassell, R.L.; Levi, A. Resistance to southern root-knot nematode (Meloidogyne incognita) in wild watermelon (Citrullus lanatus var. citroides). J. Nematol. 2016, 48, 14–19. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Wang, L.M.; Jiao, Y.Y.; Chen, C.; Zhao, L.; Mei, M.J.; Yu, Y.L.; Bie, Z.L.; Huang, Y. Pumpkin rootstock improves nitrogen use efficiency of watermelon scion by enhancing nutrient uptake, cytokinin content, and expression of nitrate reductase genes. Plant Growth Regul. 2017, 82, 233–246. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lu, X.M.; Yan, B.; Li, B.; Sun, J.; Guo, S.R.; Tezuka, T. Bottle gourd rootstock-grafting affects nitrogen metabolism in NaCl-stressed watermelon leaves and enhances short-term salt tolerance. J. Plant Physiol. 2013, 170, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.F.; Wang, X.B.; Cheng, F.; Cao, H.S.; Liang, H.; Lu, J.Y.; Kong, Q.S.; Bie, Z.L. iTRAQ-based quantitative proteomics analysis of cold stress-induced mechanisms in grafted watermelon seedlings. J. Proteom. 2019, 192, 311–320. [Google Scholar] [CrossRef]
- Yavuz, D.; Seymen, M.; Suheri, S.; Yavuz, N.; Turkmen, O.; Kurtar, E.S. How do rootstocks of citron watermelon (Citrullus lanatus var. citroides) affect the yield and quality of watermelon under deficit irrigation? Agric. Water Manag. 2020, 241, 106351. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.Q.; Kong, Q.S.; Cheng, F.; Niu, M.L.; Xie, J.J.; Nawaz, M.A.; Bie, Z.L. Comprehensive mineral nutrition analysis of watermelon grafted onto two different rootstocks. Hortic. Plant J. 2016, 2, 105–113. [Google Scholar] [CrossRef]
- Memmott, F.D.; Hassell, R.L. Watermelon (Citrullus lanatus) grafting method to reduce labor cost by eliminating rootstock side shoots. Acta Hortic. 2010, 871, 389–394. [Google Scholar] [CrossRef]
- Hassell, R.L.; Memmott, F.; Liere, D.G. Grafting methods for watermelon production. HortScience 2008, 43, 1677–1679. [Google Scholar] [CrossRef]
- Devi, P.; DeVetter, L.W.; Lukas, S.; Miles, C. Exogenous treatments to enhance splice-grafted watermelon survival. Horticulturae 2021, 7, 197. [Google Scholar] [CrossRef]
- Dabirian, S.; Miles, C.A. Increasing survival of splice-grafted watermelon seedlings using a sucrose application. HortScience 2017, 52, 579–583. [Google Scholar] [CrossRef]
- Devi, P.; Lukas, S.; Miles, C. Fruit maturity and quality of splice-grafted and one-cotyledon grafted watermelon. HortScience 2020, 55, 1090–1098. [Google Scholar] [CrossRef]
- Daley, S.L.; Hassell, R.L. Fatty alcohol application to control meristematic regrowth in bottle gourd and interspecific hybrid squash rootstocks used for grafting watermelon. HortScience 2014, 49, 260–264. [Google Scholar] [CrossRef]
- Choi, D.C.; Kwon, S.W.; Ko, B.R.; Choi, J.S. Using chemical controls to inhibit axillary buds of Lagenaria as rootstock for grafted watermelon (Citrullus lanatus). Acta Hortic. 2002, 588, 43–48. [Google Scholar] [CrossRef]
- El-Gazzar, T.; Dawa, K.; Ibrahim, E.; El-Banna, M.; Mohamed, A. Anatomical study on watermelon grafting. J. Plant Prod. 2017, 8, 999–1009. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Fang, C.; Krishnan, S.; Chen, J.M.; Yu, H.; Murphy, A.S.; Merewitz, E.; Katin-Grazzini, L.; McAvoy, R.J.; Deng, Z.N.; et al. Elevated auxin and reduced cytokinin contents in rootstocks improve their performance and grafting success. Plant Biotechnol. J. 2017, 15, 1556–1565. [Google Scholar] [CrossRef] [PubMed]









| Treatment | Detailed Information |
|---|---|
| T1 | New “hole insertion grafting” without regrown rootstock removal |
| T2 | New “one cotyledon grafting” without regrown rootstock removal |
| T3 | Traditional “hole insertion grafting” with regrown rootstock removal manually three times |
| S1 | Traditional “one cotyledon grafting” with regrown rootstock removal manually three times |
| S2 | Traditional “hole insertion grafting” without regrown rootstock removal |
| S3 | Traditional “one cotyledon grafting” without regrown rootstock removal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Lin, W.; Feng, C.; Wu, X.; Fu, X.; Xiong, M.; Bie, Z.; Huang, Y. A New Grafting Method for Watermelon to Inhibit Rootstock Regrowth and Enhance Scion Growth. Agriculture 2021, 11, 812. https://doi.org/10.3390/agriculture11090812
Liu C, Lin W, Feng C, Wu X, Fu X, Xiong M, Bie Z, Huang Y. A New Grafting Method for Watermelon to Inhibit Rootstock Regrowth and Enhance Scion Growth. Agriculture. 2021; 11(9):812. https://doi.org/10.3390/agriculture11090812
Chicago/Turabian StyleLiu, Changjin, Weiguo Lin, Chongran Feng, Xiangshuai Wu, Xiaohu Fu, Mu Xiong, Zhilong Bie, and Yuan Huang. 2021. "A New Grafting Method for Watermelon to Inhibit Rootstock Regrowth and Enhance Scion Growth" Agriculture 11, no. 9: 812. https://doi.org/10.3390/agriculture11090812
APA StyleLiu, C., Lin, W., Feng, C., Wu, X., Fu, X., Xiong, M., Bie, Z., & Huang, Y. (2021). A New Grafting Method for Watermelon to Inhibit Rootstock Regrowth and Enhance Scion Growth. Agriculture, 11(9), 812. https://doi.org/10.3390/agriculture11090812