A Polysaccharide of Ganoderma lucidum Enhances Antifungal Activity of Chemical Fungicides against Soil-Borne Diseases of Wheat and Maize by Induced Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates, Culture Media, Fungicides and Varieties Tested
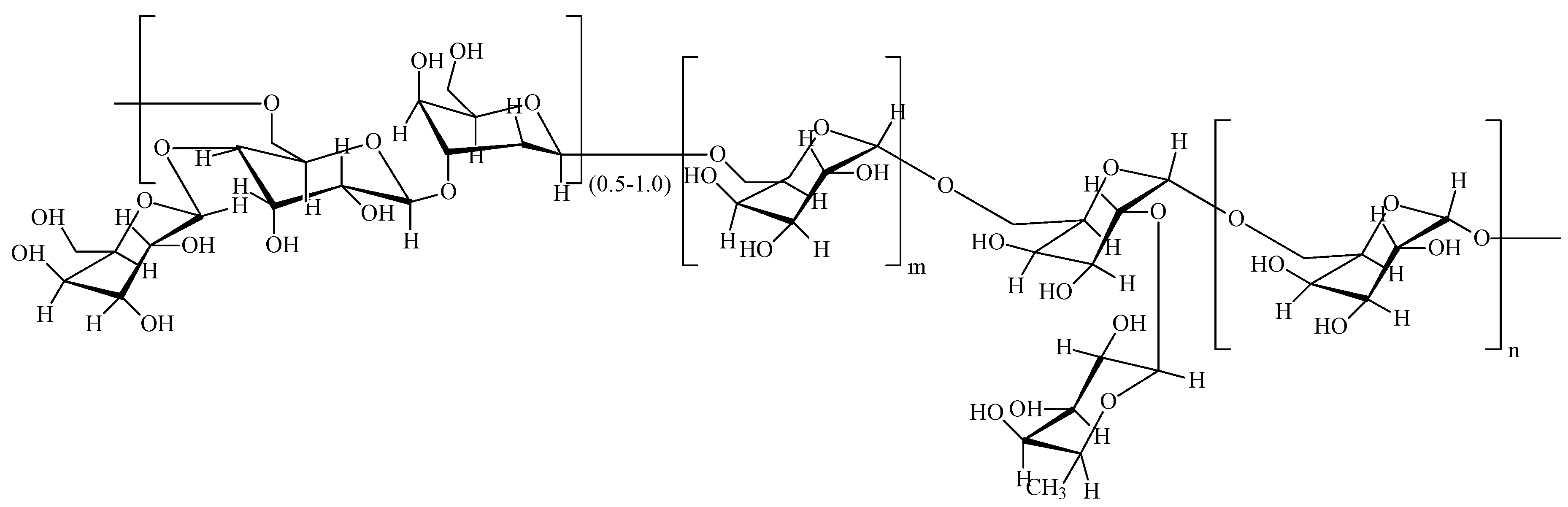
2.2. Extraction and Purification of GLP
2.3. Fungicide Amended-Agar Assay
2.4. Effects of GLP Seed Dressing on Wheat and Maize Germination and Seedling Growth
2.5. Effects of GLP on Wheat Sharp Eyespot, Wheat Root Rot and Maize Stem Rot
2.6. Field Trials
2.7. RNA Extraction and Real-Time Quantitative RT-PCR
2.8. Data Processing and Analysis
3. Results
3.1. In Vivo Assays Test of GLP and Fungicides against Fusarium graminearum, Drechslera sorokiniana and Rhizoctonia cerealis
3.2. Effects of Seed Dressing on Wheat and Maize Germination
3.3. Effects of Seed Dressing on the Growth of Wheat and Maize Seedlings
3.4. Control Effect of The Seed Treatment on 3 Soil-Borne Diseases in Wheat and Maize Continuous Cropping Areas
3.5. Field Control Effect of Seed Treated on Wheat Sharp Eyespot, Wheat Root Rot and Maize Stalk Rot
3.6. Effects of Chemical Seed Treatment on the Yield of Wheat and Maize
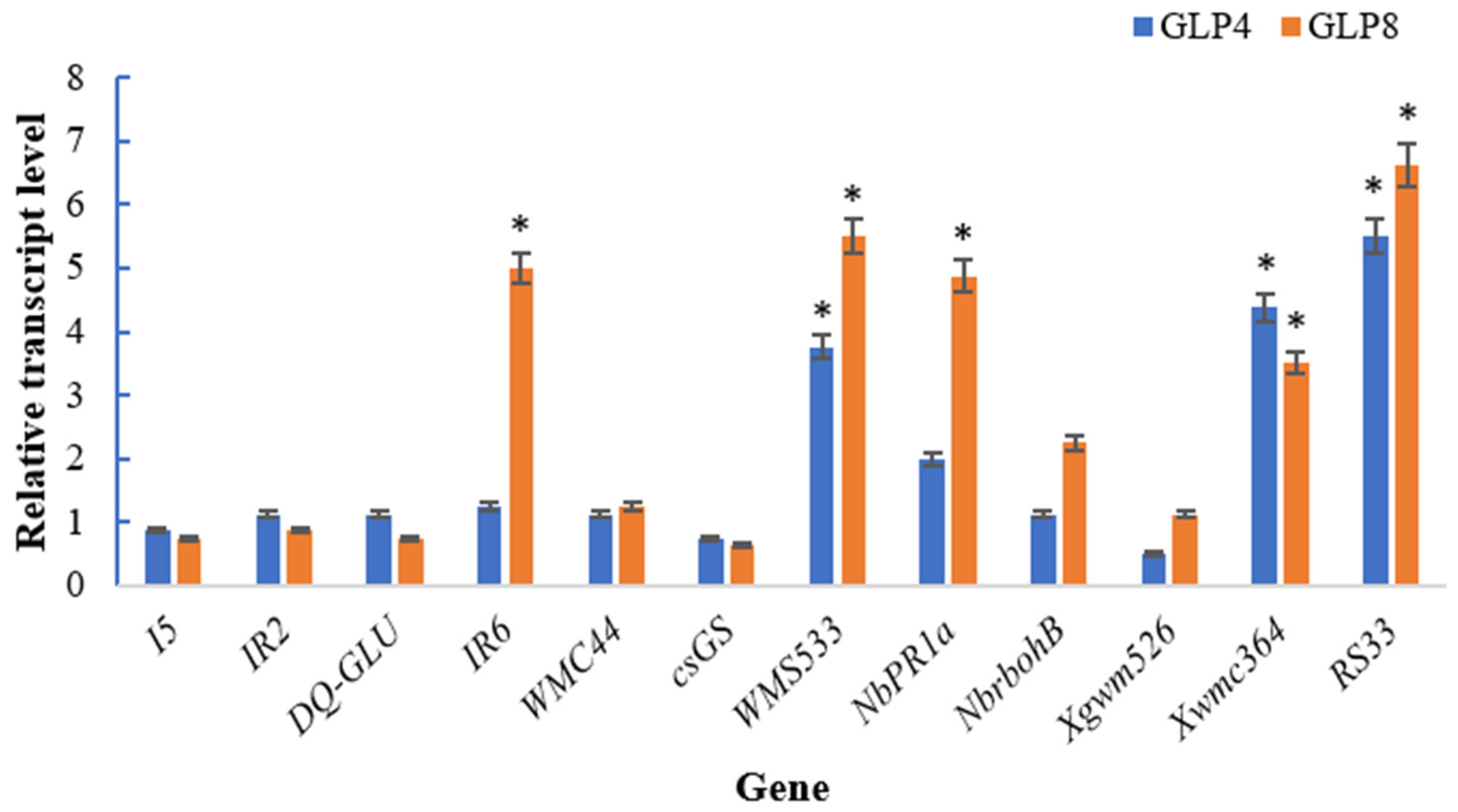
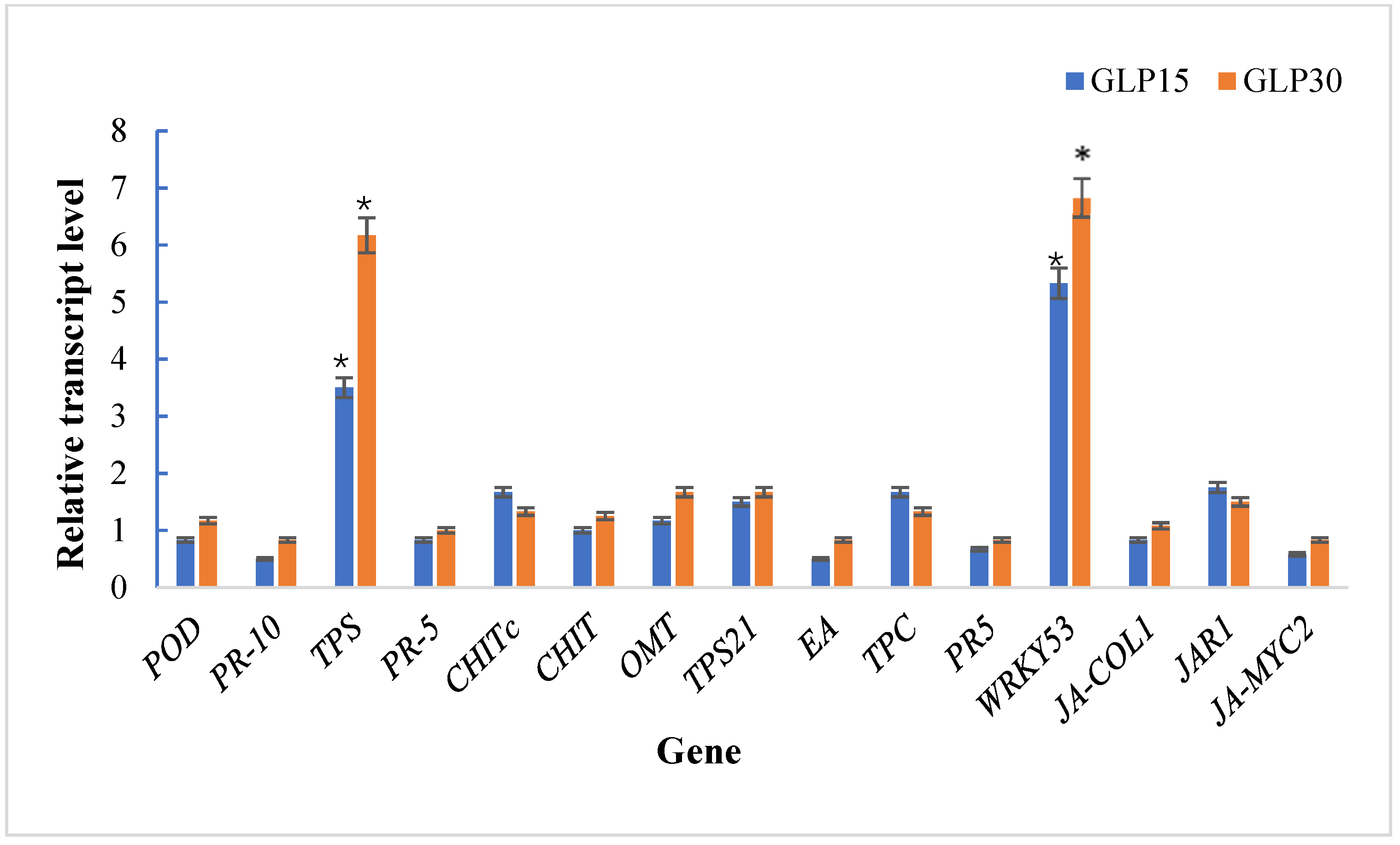
3.7. Effect of Seed Treated GLP on Transcript Quantity of Wheat and Maize Disease Resistance-Related Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.T.; Fournier, J. The Algal Polysaccharide Carrageenans Can Act as an Elicitor of Plant Defence. New Phytol. 2010, 149, 43–51. [Google Scholar] [CrossRef]
- Presello, D.A.; Botta, G.; Iglesias, J.; EyhéRabide, G.H. Effect of disease severity on yield and grain fumonisin concentration of maize hybrids inoculated with Fusarium verticillioides. Crop Prot. 2008, 27, 572–576. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y. The impacts of climate change on water resources and agriculture in China. Nature 2011, 467, 43–51. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Chen, M. Economic impacts of climate change on agriculture: The importance of additional climatic variables other than temperature and precipitation. J. Environ. Econ. Manag. 2017, 83, 8–31. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotox. Environ. Safe 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-X.; Chen, C.; Ye, Z.-H.; Su, X.-Y.; Xiao, J.-J.; Liao, M.; Cao, H.-Q. Development and Application of Seed Coating Agent for the Control of Major Soil-Borne Diseases Infecting Wheat. Agronomy 2019, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Mundt, C.C. Genetic structure and population diversity in the wheat sharp eyespot pathogen Rhizoctonia cerealis in the Willamette Valley, Oregon, USA. Plant Pathol. 2020, 69, 101–111. [Google Scholar] [CrossRef]
- Daval, S.; Lebreton, L.; Gazengel, K.; Boutin, M.; Guillermerckelboudt, A.Y.; Sarniguet, A. The biocontrol bacterium Pseudomonas fluorescens Pf29Arp strain affects the pathogenesis-related gene expression of the take-all fungus Gaeumannomyces graminis var. tritici on wheat roots. Mol. Plant Pathol. 2011, 12, 839. [Google Scholar] [CrossRef]
- Wang, A.; Wei, X.; Rong, W.; Dang, L.; Du, L.P.; Qi, L.; Xu, H.J.; Shao, Y.; Zhang, Z. GmPGIP3 enhanced resistance to both take-all and common root rot diseases in transgenic wheat. Funct. Integr. Genom. 2015, 15, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.V.d.; Simon, J.; Cota, L.V.; Silva, D.D.d.; Almeida, R.E.M.d.; Lanza, F.E.; Lago, B.C.; Pereira, A.A.; Campos, L.J.M.; Figueiredo, J.E.F. Yield losses in off-season corn crop due to stalk rot disease. Pesqui. Agropecu. Bras. 2019, 54, 00283. [Google Scholar] [CrossRef] [Green Version]
- Venturini, G.; Assante, G.; Toffolatti, S.L.; Vercesi, A. Mating behavior of a Northern Italian population of Fusarium verticillioides associated with maize. J. Appl. Genet. 2011, 52, 367–370. [Google Scholar] [CrossRef] [Green Version]
- Subedi, S.; Subedi, H.; Neupane, S. Status of maize stalk rot complex in western belts of Nepal and its integrated management. J. Maize Res. Dev. 2016, 2, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Gai, X.T.; Xuan, Y.H.; Gao, Z.G. Diversity and pathogenicity of Fusarium graminearum species complex from maize stalk and ear rot strains in northeast China. Plant Pathol. 2017, 66, 1267–1275. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, G.; Guo, Y.; Zhang, D.; Chen, S.; Xu, M. A major QTL for resistance to Gibberella stalk rot in maize. Theor. Appl. Genet. 2010, 121, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Han, J.H.; Ju, K.L.; Kim, K.S. Characterization of the Maize Stalk Rot Pathogens Fusarium subglutinans and F. temperatum and the Effect of Fungicides on Their Mycelial Growth and Colony Formation. Plant Pathol. J. 2014, 30, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Song, F.J.; Xiao, M.G.; Duan, C.X.; Li, H.J.; Zhu, Z.D.; Liu, B.T.; Sun, S.L.; Wu, X.F.; Wang, X.M. Two genes conferring resistance to Pythium stalk rot in maize inbred line Qi319. Mol. Genet. Genom. 2015, 290, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, R.; Shi, G.; Jin, Y.; Robson, M.G.; Huang, X. Overuse or underuse? An observation of pesticide use in China. Sci. Total Environ. 2015, 538, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kunz, S.; Deising, H.B.; Mendgen, K. Acquisition of Resistance to Sterol Demethylation Inhibitors by Populations of Venturia inaequalis. Phytopathology 1997, 87, 1272–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Nes, W.D. Sterol Biosynthesis Inhibitors: Potential for Transition State Analogs and Mechanism-Based Inactivators Targeted at Sterol Methyltransferase. Lipids 2007, 42, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Van, W.H.; Tiefenbacher, A.; König, N.; Dorn, V.M.; Hagenguth, J.F.; Prah, U.; Widhalm, T.; Wiklicky, V.; Koller, R.; Bonkowski, M. Single and Combined Effects of Pesticide Seed Dressings and Herbicides on Earthworms, Soil Microorganisms, and Litter Decomposition. Front. Plant Sci. 2017, 8, 215. [Google Scholar]
- Zaller, J.G.; König, N.; Tiefenbacher, A.; Muraoka, Y.; Querner, P.; Ratzenböck, A.; Bonkowski, M.; Koller, R. Pesticide seed dressings can affect the activity of various soil organisms and reduce decomposition of plant material. BMC Ecol. 2016, 16, 37. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Zhou, F. Zero Growth of Chemical Fertilizer and Pesticide Use: China’s Objectives, Progress and Challenges. J. Resour. Ecol. 2018, 9, 50–58. [Google Scholar]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, S.H.; Dai, Y.C. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Divers. 2012, 56, 49–62. [Google Scholar] [CrossRef]
- Habijanic, J.; Berovic, M.; Boh, B.; Plankl, M.; Wraber, B. Submerged cultivation of Ganoderma lucidum and the effects of its polysaccharides on the production of human cytokines TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. New Biotechnol. 2015, 32, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Takasuka, N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydr. Res. 1976, 47, 99–104. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.M.; Young, J.D. Corrigendum: Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Shuai, X.; Jia, Z.; Li, H.; Liang, X.; Su, D.; Guo, W. Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J. Transl. Med. 2015, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Dou, Y.; Ye, B.; Wu, Q.; Wang, Y.; Hu, M.; Ma, F.; Rong, X.; Guo, J. Ganoderma lucidum polysaccharides improve insulin sensitivity by regulating inflammatory cytokines and gut microbiota composition in mice. J. Funct. Foods 2017, 38, 545–552. [Google Scholar] [CrossRef]
- Chen, X.P.; Yan, C.; Li, S.B.; Chen, Y.G.; Lan, J.Y.; Liu, L.P. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohyd. Polym. 2009, 77, 389–393. [Google Scholar]
- Liu, Y.; Liu, Z.; Hamada, M.S.; Yin, Y.N.; Ma, Z.H. Characterization of laboratory pyrimethanil-resistant mutants of Aspergillus flavus from groundnut in China. Crop Prot. 2014, 60, 5–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Wang, K.; Jiang, L.; Wang, D. Use of Lentinan To Control Sharp Eyespot of Wheat, and the Mechanism Involved. J. Agric. Food Chem. 2017, 65, 10891–10898. [Google Scholar] [CrossRef]
- McDonald, H.; Rovira, A. Development of an Inoculation Technique for Rhizoctonia Solani and Its Application to Screening Cereal Cultivars for Resistance; Parker, C.A., Rovira, A.D., Moore, K.J., Wong, P.T.W., Eds.; Ecology and Management of Soilborne Plant Pathogens, American Phytopathological Society Press: St. Paul, MN, USA, 1985; pp. 174–176. [Google Scholar]
- Peng, D.; Li, S.; Chen, C.; Zhou, M. Combined application of Bacillus subtilis NJ-18 with fungicides for control of sharp eyespot of wheat. Biol. Control 2014, 70, 28–34. [Google Scholar] [CrossRef]
- Guo, R.; Lin, Z.; Mo, X.; Yang, C. Responses of crop yield and water use efficiency to climate change in the North China Plain. Agric. Water Manag. 2009, 97, 1185–1194. [Google Scholar] [CrossRef]
- Hamada, M.S.; Yin, Y.; Chen, H.; Ma, Z. The escalating threat of Rhizoctonia cerealis, the causal agent of sharp eyespot in wheat. Pest Manag. Sci. 2011, 67, 1411–1419. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Li, D.; Teng, M.; Zhang, R.; Zhou, Z.; Zhu, W. Enantioselective bioaccumulation of hexaconazole and its toxic effects in adult zebrafish (Danio rerio). Chemosphere 2015, 138, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Knörr, S.; Keiter, S.; Nagel, T.; Segner, H.; Braunbeck, T. Prochloraz causes irreversible masculinization of zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2015, 22, 16417–16422. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef] [Green Version]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant-Microbe Interact. 2003, 16, 1118. [Google Scholar] [CrossRef] [Green Version]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed Polysaccharides and Derived Oligosaccharides Stimulate Defense Responses and Protection Against Pathogens in Plants. Mar. Drugs 2011, 9, 2514. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- El-Mougy, N.S.; Abd-El-Kareem, F.A.; El-Gamal, N.G.; Fa-Tooh, Y.O. Application of Fungicides Alternatives for Controlling Cowpea Root Rot Disease under Greenhouse and Field Conditions. Egypt J. Phytopathol. 2004, 32, 23–35. [Google Scholar]
- Oostendorp, M.; Kunz, W.; Dietrich, B.; Staub, T. Induced disease resistance in plants by chemicals. Eur. J. Plant Pathol. 2001, 107, 19–28. [Google Scholar] [CrossRef]

| Accession No. | Genes | Left Primer 5′-3′ | Right Primer 5′-3′ | |
|---|---|---|---|---|
| Maize | GRMZM2G103342 | POD | AGTAGATGATCCCTGTCCGC | GCCGCCACCCTCTTATTAGA |
| GRMZM2G112488 | PR-10 | TGAAGGTGGACTCGACGTAC | CGATCGCATGCATGGTCTAC | |
| GRMZM2G127087 | TPS | GACCAGACAAGAGCTACCGA | CCCATTCCAACAAACGCAGA | |
| GRMZM2G402613 | PR-5 | CGGCAACAGCAACTACCAAG | GCACACAAATCCAGCTACGT | |
| GRMZM2G453805 | CHCTc | CGTGCAGAACAACTACAGCA | ATGCGTCTTTGTCTCCCGAT | |
| GRMZM2G162505 | CHIT | ACCTCCACCAATCCAACCAA | CAACAGCCGCTACAAGTACC | |
| GRMZM2G336824 | OMT | CCACCCTTCTCCAGATCCTC | AGACTCACAAAGGGAGCCAA | |
| AC205502.4_FG004 | TPS21 | AGAGGTCACACGCTTCATGA | GCCATGCTCACTGATGTAGC | |
| GRMZM2G148904 | EA | CACGTACCCCATCTTCCTCA | GATCATCAATCGTCGGAGCG | |
| GRMZM2G028306 | TPC | AGAGAAAAGGCTGGAGTGCT | GATTTAGTCGCTGCAGGGTG | |
| GRMZM2G136372 | PR5 | CTCCAGCCAACTTCTTTCTTTG | GGGGTGAACATCATCGTCTTAT | |
| GRMZM2G449681 | WRKY53 | GACATTGTGGTCCCATCTGAT | GAGAGGGGGAGAGAAGAGAT | |
| GRMZM2G079112 | JA-COL1 | TGGAACCCCTAAAAATTTCCCT | GATCTGTTTCCCTCCTCGAC | |
| GRMZM2G091276 | JAR1 | TCCTCTCTATTGCGAAAAGGTT | AGTGCCAGTAAGAAAGTTCAGA | |
| GRMZM2G001930 | JA-MYC2 | AATCCGCATTCTGAACCATTTC | GAAAGAGGAGGAGAAATGGTGG | |
| U76259 | EF1A | GCCTGGTATGGTTGTTACT | CATACCCACGCTTCAGATCC | |
| Wheat | AK455337.1 | IR5 | GCCGTTCGCATAGTCAATC | CGCACCATTATTCGCTTGT |
| AK332579.1 | IR2 | GACATCCATTTCCAGGGGC | GCGGTCTGGGCATTCATC | |
| JF718349.1 | DQ-GLU | GCTGGAAAGGATGTTGCT | TGCCCGTTACACTTGGAT | |
| HG670306.1 | IR6 | CACTGGGTCGTGACACTTCT | CCTCCTCTTCCTTGTATGCTG | |
| HG670306.1 | WMC44 | GGTCTTCTGGGCTTTGATCCTG | TGTTGCTAGGGACCCGTAGTGG | |
| AK446945.1 | csGS | AAGATTGTTCACAGATCCATGTCA | GAGTATTCCGGCTCAAAAAGG | |
| AK448410.1 | WMS533 | AAGGCGAATCAAACGGAATA | GTTGCTTTAGGGGAAAAGCC | |
| XM_016633336.1 | NbPR1a | CGTTGAGATGTGGGTCAATG | CCTAGCACATCCAACACGAA | |
| AK449901.1 | NbrbohB | GTGATGCTCGTTCTGCTCTT | CTTTAGCCTCAGGGTGGTTG | |
| XM_013385118.1 | Xgwm526 | CAATAGTTCTGTGAGAGCTGCG | CCAACCCAAATACACATTCTCA | |
| AK449144.1 | Xwmc364 | ATCACAATGCTGGCCCTAAAAC | CAGTGCCAAAATGTCGAAAGTC | |
| HG670306.1 | RS33 | TGGAGAGGACAGCCCATGGAGTTGGTAGTAGGTGC | GCCCTTGCTCACCATGCTGCTGATAACATGATCCA | |
| AK450528.1 | Actin | CACTGGAATGGTCAAGGCTG | CTCCATGTCATCCCAGTTG |
| Treatments | Regression Equation | R2 | EC50 Value (mg·L−1) | 95%Confidence Interval (mg·L−1) |
|---|---|---|---|---|
| Prochloraz | Y = 5.794 + 0.750 X | 0.974 | 0.088 | 0.072~0.106 |
| Fludioxonil | Y = 5.170 + 0.750 X | 0.962 | 0.593 | 0.442~0.795 |
| Hexaconazole | Y = 5.095 + 0.814 X | 0.980 | 0.764 | 0.594~0.981 |
| Metalaxyl | Y = 3.102 + 1.014 X | 0.960 | 5.927 | 4.419~7.650 |
| Fluopimomide | Y = 5.195 + 1.045 X | 0.969 | 6.137 | 5.643~6.983 |
| GLP | / | / | / | / |
| Treatments | Regression Equation | R2 | EC50 Value (mg·L−1) | 95%Confidence Interval (mg·L−1) |
|---|---|---|---|---|
| Hexaconazole | Y = 1.29 + 1.54 X | 0.988 | 0.144 | 0.111~0.176 |
| Prochloraz | Y = 1.06 + 1.40 X | 0.983 | 0.182 | 0.125~0.229 |
| Fludioxonil | Y = 0.28 + 1.98 X | 0.981 | 0.766 | 0.624~0.908 |
| Difenoconazole | Y = 0.92 + 1.57 X | 0.968 | 3.259 | 2.635~3.913 |
| Thiram | Y = −1.74 + 2.51 X | 0.973 | 6.915 | 5.526~8.314 |
| Fluopimomide | Y = −5.28 + 3.22 X | 0.985 | 43.33 | 38.21~48.45 |
| GLP | / | / | / | / |
| Treatments | Regression Equation | R2 | EC50 Value (mg·L−1) | 95%Confidence Interval (mg·L−1) |
|---|---|---|---|---|
| Hexaconazole | Y = 2.79 + 2.30 X | 0.952 | 0.094 | 0.032~1.255 |
| Fludioxonil | Y = 0.76 + 1.47 X | 0.985 | 0.312 | 0.081~0.574 |
| Fluopimomide | Y = −1.00 + 1.62 X | 0.986 | 4.169 | 3.125~4.852 |
| Prochloraz | Y = −1.17 + 1.54 X | 0.983 | 5.738 | 5.112~6.312 |
| GLP | / | / | / | / |
| Wheat Cultivated Variety | Treatment | Dosage (mL/100 kg Seed) | Germination Rate (%) | ||
|---|---|---|---|---|---|
| Control Germination 50% | Control Total Germination | 3 Days after Control Total Germination | |||
| Jimai 22 | CK | 100 | 50.00 b | 95.00 bc | 97.00 b |
| 4% GLP | 100 | 52.25 a | 97.25 a | 98.25 a | |
| 8% GLP | 100 | 53.25 a | 96.25 ab | 97.25 ab | |
| 0.5% Hexaconazole | 100 | 50.00 b | 95.00 bc | 95.75 c | |
| 1% Hexaconazole | 100 | 46.75 c | 94.00 c | 95.75 c | |
| 2% Hexaconazole | 100 | 44.75 d | 91.75 d | 95.50 c | |
| 0.5% H + 4% GLP | 100 | 50.25 b | 96.75 ab | 97.00 b | |
| Shannong 23 | CK | 100 | 48.75 b | 96.00 b | 97.00 b |
| 4% GLP | 100 | 50.00 ab | 96.75 ab | 97.75 ab | |
| 8% GLP | 100 | 51.75 a | 98.25 a | 98.25 a | |
| 0.5% Hexaconazole | 100 | 48.75 b | 95.00 b | 95.75 c | |
| 1% Hexaconazole | 100 | 47.25 b | 92.75 c | 95.75 c | |
| 2% Hexaconazole | 100 | 44.00 c | 90.50 d | 96.75 bc | |
| 0.5% H + 4% GLP | 100 | 49.00 b | 95.00 b | 97.00 b | |
| Luyuan 502 | CK | 100 | 48.00 b | 95.00 bc | 96.50 ab |
| 4% GLP | 100 | 48.25 b | 96.25 ab | 97.00 a | |
| 8% GLP | 100 | 49.25 ab | 97.25 a | 97.25 a | |
| 0.5% Hexaconazole | 100 | 50.25 a | 96.00 ab | 96.00 b | |
| 1% Hexaconazole | 100 | 45.75 c | 94.25 c | 96.00 b | |
| 2% Hexaconazole | 100 | 42.75 d | 91.75 d | 94.75 c | |
| 0.5% H + 4% GLP | 100 | 48.00 b | 96.00 ab | 96.00 b | |
| Maize Cultivated Variety | Treatment | Dosage (mL/100 kg Seed) | Germination Rate (%) | ||
|---|---|---|---|---|---|
| Control Germination 50% | Control Total Germination | 3 Days after Control Total Germination | |||
| Zhengdan 958 | CK | 100 | 49.00 b | 96.75 a | 97.50 b |
| 15% GLP | 100 | 51.25 a | 97.00 a | 97.75 b | |
| 30% GLP | 100 | 51.75 a | 97.25 a | 99.25 a | |
| 20% Prochloraz | 100 | 49.00 b | 96.75 a | 97.75 b | |
| 40% Prochloraz | 100 | 47.75 c | 95.75 b | 98.00 b | |
| 80% Prochloraz | 100 | 47.25 c | 94.50 c | 99.25 a | |
| 20% P + 15% GLP | 100 | 49.75 b | 97.00 a | 97.75 b | |
| Xianyu 335 | CK | 100 | 49.00 b | 95.75 c | 98.75 a |
| 15% GLP | 100 | 52.25 a | 97.00 b | 97.75 b | |
| 30% GLP | 100 | 53.75 a | 98.25 a | 99.00 a | |
| 20% Prochloraz | 100 | 48.00 b | 97.00 b | 97.75 b | |
| 40% Prochloraz | 100 | 46.00 c | 95.75 c | 97.50 b | |
| 80% Prochloraz | 100 | 45.25 c | 94.50 d | 97.50 b | |
| 20% P + 15% GLP | 100 | 49.00 b | 96.75 b | 99.00 a | |
| Luning 202 | CK | 100 | 49.00 b | 96.75 b | 98.75 a |
| 15% GLP | 100 | 50.25 ab | 96.75 b | 99.00 a | |
| 30% GLP | 100 | 51.25 a | 98.25 a | 99.25 a | |
| 20% Prochloraz | 100 | 48.75 b | 98.00 a | 99.00 a | |
| 40% Prochloraz | 100 | 46.50 c | 94.50 d | 97.75 b | |
| 80% Prochloraz | 100 | 46.00 c | 95.75 c | 97.50 b | |
| 20% P + 15% GLP | 100 | 48.75 b | 97.75 a | 99.00 a | |
| Wheat Cultivated Variety | Treatment | Dosage (mL/ 100 kg Seed) | Height (cm) | ||
|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | |||
| Jimai 22 | CK | 100 | 7.35 c | 18.50 b | 27.50 b |
| 4% GLP | 100 | 9.03 b | 20.56 a | 28.02 b | |
| 8% GLP | 100 | 9.81 a | 21.18 a | 30.96 a | |
| 0.5% Hexaconazole | 100 | 7.18 c | 18.95 b | 26.96 c | |
| 1% Hexaconazole | 100 | 6.25 d | 15.94 c | 26.35 c | |
| 2% Hexaconazole | 100 | 5.28 e | 15.29 c | 26.02 c | |
| 0.5% H + 4% GLP | 100 | 7.22 c | 18.75 b | 28.55 b | |
| Shannong 23 | CK | 100 | 6.28 c | 15.50 b | 26.50 b |
| 4% GLP | 100 | 7.50 b | 16.80 a | 27.00 b | |
| 8% GLP | 100 | 8.75 a | 18.06 a | 28.50 a | |
| 0.5% Hexaconazole | 100 | 6.39 c | 15.0 bc | 26.96 b | |
| 1% Hexaconazole | 100 | 5.47 d | 14.33 c | 26.03 c | |
| 2% Hexaconazole | 100 | 4.84 d | 13.91 c | 25.78 c | |
| 0.5% H + 4% GLP | 100 | 6.52 c | 15.85 b | 27.12 b | |
| Luyuan 502 | CK | 100 | 7.01 d | 17.50 c | 26.50 b |
| 4% GLP | 100 | 9.84 b | 20.75 b | 28.22 a | |
| 8% GLP | 100 | 11.96 a | 24.33 a | 27.85 a | |
| 0.5% Hexaconazole | 100 | 6.50 e | 16.96 c | 26.35 b | |
| 1% Hexaconazole | 100 | 6.06 e | 16.34 d | 25.96 b | |
| 2% Hexaconazole | 100 | 5.43 e | 16.02 d | 25.78 b | |
| 0.5% H + 4% GLP | 100 | 7.44 d | 17.69 c | 26.86 b | |
| Maize Cultivated Variety | Treatment | Dosage (mL/100 kg Seed) | Height (cm) | ||
|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | |||
| Zhengdan 958 | CK | 100 | 7.23 c | 28.50 b | 55.50 a |
| 15% GLP | 100 | 7.88 b | 30.56 a | 58.22 a | |
| 30% GLP | 100 | 8.31 a | 31.18 a | 58.56 a | |
| 20% Prochloraz | 100 | 7.18 c | 28.31 b | 56.39 a | |
| 40% Prochloraz | 100 | 7.06 c | 28.25 b | 55.29 a | |
| 80% Prochloraz | 100 | 6.89 c | 27.96 b | 55.18 a | |
| 20% P + 15% GLP | 100 | 7.83 b | 30.47 b | 55.49 a | |
| Xianyu 335 | CK | 100 | 6.52 c | 26.50 b | 55.50 b |
| 15% GLP | 100 | 7.50 b | 27.00 b | 56.78 b | |
| 30% GLP | 100 | 8.57 a | 28.50 a | 58.06 a | |
| 20% Prochloraz | 100 | 6.48 c | 27.12 b | 56.23 b | |
| 40% Prochloraz | 100 | 6.29 c | 26.51 b | 55.85 b | |
| 80% Prochloraz | 100 | 6.37 c | 26.02 b | 54.95 b | |
| 20% P + 15% GLP | 100 | 7.35 b | 26.94 b | 56.17 b | |
| Luning 202 | CK | 100 | 5.36 b | 24.50 c | 47.50 a |
| 15% GLP | 100 | 6.48 a | 25.22 b | 47.75 a | |
| 30% GLP | 100 | 6.96 a | 25.85 b | 48.33 a | |
| 20% Prochloraz | 100 | 5.22 b | 24.35 c | 47.88 a | |
| 40% Prochloraz | 100 | 5.08 b | 24.12 c | 46.89 a | |
| 80% Prochloraz | 100 | 5.11 b | 23.98 c | 46.85 a | |
| 20% P + 15% GLP | 100 | 6.44 a | 25.29 c | 47.69 a | |
| Wheat Cultivated Variety | Agentia | Dosage (mL/100 kg Seed) | Control Effect (%) | ||
|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | |||
| Jimai 22 | 4% GLP | 100 | 28.1 ± 1.7 d | 24.5 ± 2.1 d | 22.9 ± 1.9 d |
| 8% GLP | 100 | 32.7 ± 2.6 b | 31.4 ± 2.4 b | 29.6 ± 3.7 c | |
| 0.5% Hexaconazole | 100 | 50.3 ± 4.9 c | 43.7 ± 1.8 c | 40.1 ± 2.5 b | |
| 1% Hexaconazole | 100 | 79.7 ± 7.4 a | 69.8 ± 7.1 a | 64.3 ± 3.7 a | |
| 0.5% H + 4% GLP | 100 | 75.8 ± 2.7 a | 68.1 ± 1.9 a | 66.6 ± 2.4 a | |
| Shannong 23 | 4% GLP | 100 | 24.5 ± 0.6 d | 21.9 ± 0.4 e | 19.6 ± 1.1 d |
| 8% GLP | 100 | 30.8 ± 1.5 bc | 27.9 ± 1.3 d | 26.2 ± 3.7 c | |
| 0.5% Hexaconazole | 100 | 43.4 ± 3.2 c | 38.6 ± 1.6 c | 30.8 ± 2.2 c | |
| 1% Hexaconazole | 100 | 72.0 ± 4.3 a | 66.5 ± 3.9 a | 59.4 ± 2.5 b | |
| 0.5% H + 4% GLP | 100 | 67.1 ± 5.3 b | 65.7 ± 1.3 a | 65.2 ± 2.8 a | |
| Luyuan 502 | 4% GLP | 100 | 30.5 ± 1.8 d | 27.9 ± 2.7 d | 26.0 ± 1.6 d |
| 8% GLP | 100 | 36.0 ± 3.1 c | 32.4 ± 1.3 c | 29.6 ± 0.8 c | |
| 0.5% Hexaconazole | 100 | 51.6 ± 3.7 b | 47.7 ± 2.5 b | 42.2 ± 1.2 b | |
| 1% Hexaconazole | 100 | 77.3 ± 8.3 a | 71.5 ± 2.9 a | 63.6 ± 2.0 a | |
| 0.5% H + 4% GLP | 100 | 73.4 ± 4.1 a | 68.7 ± 2.6 a | 65.2 ± 1.2 a | |
| Wheat Cultivated Variety | Agentia | Dosage (g a.i./100 kg Seed) | Control Effect (%) | ||
|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | |||
| Jimai 22 | 4% GLP | 100 | 29.8 ± 1.9 d | 26.3 ± 2.7 d | 24.5 ± 1.7 c |
| 8% GLP | 100 | 35.4 ± 3.1 c | 32.7 ± 2.2 c | 30.8 ± 1.7 b | |
| 0.5% Hexaconazole | 100 | 46.9 ± 4.5 b | 40.3 ± 2.9 b | 35.7 ± 3.5 b | |
| 1% Hexaconazole | 100 | 76.3 ± 3.4 a | 62.4 ± 4.6 a | 55.3 ± 4.1 a | |
| 0.5% H + 4% GLP | 100 | 79.4 ± 3.6 a | 70.3 ± 4.1 a | 61.5 ± 3.4 a | |
| Shannong 23 | 4% GLP | 100 | 28.2 ± 1.7 d | 25.7 ± 1.6 d | 22.9 ± 1.8 d |
| 8% GLP | 100 | 38.5 ± 2.1 c | 34.7 ± 1.9 c | 30.5 ± 1.6 c | |
| 0.5% Hexaconazole | 100 | 52.4 ± 2.8 b | 46.8 ± 3.1 b | 42.5 ± 1.7 b | |
| 1% Hexaconazole | 100 | 80.2 ± 7.9 a | 74.5 ± 9.1 a | 68.6 ± 5.7 a | |
| 0.5% H + 4% GLP | 100 | 82.6 ± 3.8 a | 76.7 ± 4.2 a | 74.9 ± 2.5 a | |
| Luyuan 502 | 4% GLP | 100 | 30.5 ± 2.6 d | 29.3 ± 1.8 c | 28.6 ± 2.4 c |
| 8% GLP | 100 | 39.3 ± 2.8 c | 35.4 ± 2.3 b | 34.7 ± 3.1 b | |
| 0.5% Hexaconazole | 100 | 49.2 ± 2.6 b | 41.5 ± 2.5 b | 37.8 ± 3.5 b | |
| 1% Hexaconazole | 100 | 80.4 ± 3.5 a | 71.5 ± 3.5 a | 65.4 ± 2.5 a | |
| 0.5% H + 4% GLP | 100 | 81.5 ± 4.2 a | 73.6 ± 3.2 a | 67.1 ± 2.9 a | |
| Maize Cultivated Variety | Agentia | Dosage (g a.i./100 kg Seed) | Control Effect (%) | ||
|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | |||
| Zhengdan 958 | 15% GLP | 100 | 29.5 ± 3.6 d | 27.8 ± 2.1 d | 26.2 ± 2.4 d |
| 30% GLP | 100 | 37.6 ± 1.9 c | 34.8 ± 1.9 c | 32.2 ± 2.3 c | |
| 20% Prochloraz | 100 | 68.8 ± 3.5 b | 55.6 ± 3.4 b | 49.3 ± 5.6 b | |
| 40% Prochloraz | 100 | 88.8 ± 7.8 a | 75.9 ± 6.9 a | 60.6 ± 7.8 a | |
| 20% P + 15% GLP | 100 | 84.2 ± 7.7 a | 75.6 ± 2.4 a | 67.9 ± 4.1 a | |
| Xianyu 335 | 15% GLP | 100 | 30.2 ± 1.8 d | 26.5 ± 1.7 d | 23.2 ± 1.7 d |
| 30% GLP | 100 | 39.7 ± 2.4 c | 37.6 ± 1.2 c | 34.9 ± 2.6 c | |
| 20% Prochloraz | 100 | 68.2 ± 3.7 b | 53.1 ± 4.2 b | 46.2 ± 3.1 b | |
| 40% Prochloraz | 100 | 82.9 ± 3.7 a | 74.4 ± 2.7 a | 65.2 ± 3.7 a | |
| 20% P + 15% GLP | 100 | 80.8 ± 3.9 a | 73.8 ± 6.7 a | 65.1 ± 3.4 a | |
| Luning 206 | 15% GLP | 100 | 27.3 ± 2.5 d | 23.2 ± 2.1 c | 21.6 ± 2.3 c |
| 30% GLP | 100 | 38.8 ± 2.3 c | 36.4 ± 1.7 b | 35.1 ± 1.2 b | |
| 20% Prochloraz | 100 | 53.5 ± 3.8 b | 37.6 ± 3.6 b | 32.9 ± 3.7 b | |
| 40% Prochloraz | 100 | 76.2 ± 2.1 a | 62.5 ± 3.7 a | 56.2 ± 4.5 a | |
| 20% P + 15% GLP | 100 | 73.7 ± 3.5 a | 70.7 ± 2.6 a | 61.9 ± 3.4 a | |
| Year | Wheat Cultivated Variety | Agentia Treatment (100 mL/100 kg Seed) | Wheat Sharp Eyespot | Wheat Root Rot | ||
|---|---|---|---|---|---|---|
| Before Overwintering | Jointing Stage | Filling Period | ||||
| 2017–2018 | Jimai 22 | CK | / | / | / | |
| 8% GLP | 38.6 ± 1.7 b | 28.4 ± 1.9 c | 21.3 ± 0.6 d | 36.2 ± 2.2 b | ||
| 1% Hexaconazole | 80.6 ± 2.3 a | 58.4 ± 2.1 b | 38.6 ± 2.4 b | 81.3 ± 4.9 a | ||
| 0.5% H + 4% GLP | 78.7 ± 2.5 a | 68.2 ± 4.2 a | 42.4 ± 4.5 a | 75.9 ± 3.7 a | ||
| Shannong 23 | CK | / | / | / | c | |
| 8% GLP | 35.7 ± 1.5 b | 26.9 ± 1.1 d | 19.8 ± 1.7 d | 32.8 ± 11.9 b | ||
| 1% Hexaconazole | 81.3 ± 2.5 a | 62.4 ± 3.2 b | 39.2 ± 2.6 b | 79.6 ± 3.8 a | ||
| 0.5% H + 4% GLP | 78.8 ± 2.4 a | 67.3 ± 2.7 a | 42.8 ± 2.8 a | 75.3 ± 4.1 a | ||
| Luyuan 502 | CK | / | / | / | ||
| 8% GLP | 32.7 ± 1.2 b | 29.3 ± 1.5 c | 20.9 ± 1.2 d | 36.4 ± 3.3 b | ||
| 1% Hexaconazole | 80.5 ± 5.7 a | 63.2 ± 4.4 b | 30.3 ± 1.8 b | 70.6 ± 2.9 a | ||
| 0.5% H + 4% GLP | 79.6 ± 5.2 a | 70.4 ± 1.9 a | 41.9 ± 2.3 a | 73.3 ± 3.1 a | ||
| 2018–2019 | Jimai 22 | CK | / | / | / | |
| 8% GLP | 36.3 ± 1.6 b | 26.2 ± 1.4 d | 20.8 ± 1.7 d | 40.2 ± 1.1 b | ||
| 1% Hexaconazole | 76.9 ± 2.5 a | 56.3 ± 2.1 b | 35.6 ± 2.3 b | 85.8 ± 7.6 a | ||
| 0.5% H + 4% GLP | 75.8 ± 2.8 a | 63.3 ± 2.9 a | 41.7 ± 3.2 a | 80.5 ± 5.2 a | ||
| Shannong 23 | CK | / | / | / | c | |
| 8% GLP | 37.9 ± 0.4 b | 32.2 ± 1.7 d | 24.2 ± 0.9 b | 30.4 ± 2.4 b | ||
| 1% Hexaconazole | 80.3 ± 4.1 a | 64.5 ± 3.5 b | 40.2 ± 3.3 a | 79.2 ± 6.6 a | ||
| 0.5% H + 4% GLP | 78.9 ± 7.2 a | 69.2 ± 4.9 a | 43.7 ± 1.7 a | 72.4 ± 3.3 a | ||
| Luyuan 502 | CK | / | / | / | ||
| 8% GLP | 35.6 ± 2.1 b | 30.2 ± 1.4 c | 22.7 ± 1.7 c | 34.2 ± 2.9 b | ||
| 1% Hexaconazole | 81.4 ± 3.8 a | 61.2 ± 2.2 b | 29.8 ± 1.9 b | 75.8 ± 4.1 a | ||
| 0.5% H + 4% GLP | 77.1 ± 5.1 a | 72.7 ± 3.6 a | 40.6 ± 1.2 a | 70.6 ± 5.2 a | ||
| Year | Maize Cultivated Variey | Agentia | Dosage (mL/100 kg Seed) | Control Effect (%) | |
|---|---|---|---|---|---|
| 30 July | 20 August | ||||
| 2017 | Zhengdan 958 | 30% GLP | 100 | 32.5 ± 2.5 b | 24.7 ± 1.9 b |
| 40% Prochloraz | 100 | 76.4 ± 5.2 a | 59.8 ± 4.3 a | ||
| 20% P + 15%GLP | 100 | 73.2 ± 4.1 a | 56.2 ± 5.7 a | ||
| Xianyu 335 | 30% GLP | 100 | 33.9 ± 2.6 b | 25.7 ± 2.4 b | |
| 40% Prochloraz | 100 | 75.5 ± 1.9 a | 57.2 ± 4.3 a | ||
| 20% P + 15% GLP | 100 | 76.4 ± 2.8 a | 60.1 ± 5.3 a | ||
| Luning 206 | 30% GLP | 100 | 35.9 ± 1.9 b | 34.2 ± 1.4 b | |
| 40% Prochloraz | 100 | 75.3 ± 3.5 a | 54.4 ± 2.8 a | ||
| 20% P + 15% GLP | 100 | 74.1 ± 2.7 a | 52.5 ± 1.6 a | ||
| 2018 | Zhengdan 958 | 30% GLP | 100 | 38.2 ± 1.5 b | 29.7 ± 2.1 b |
| 40% Prochloraz | 100 | 71.6 ± 4.6 a | 55.3 ± 3.1 a | ||
| 20% P + 15% GLP | 100 | 79.5 ± 6.2 a | 61.4 ± 2.8 a | ||
| Xianyu 335 | 30% GLP | 100 | 36.5 ± 2.9 b | 24.7 ± 1.4 b | |
| 40% Prochloraz | 100 | 80.6 ± 7.3 a | 60.5 ± 5.2 a | ||
| 20% P + 15% GLP | 100 | 73.3 ± 4.8 a | 53.2 ± 2.9 a | ||
| Luning 206 | 30% GLP | 100 | 36.1 ± 1.7 b | 25.4 ± 0.6 b | |
| 40% Prochloraz | 100 | 78.2 ± 4.8 a | 56.3 ± 2.2 a | ||
| 20% P + 15% GLP | 100 | 82.6 ± 2.2 a | 50.8 ± 2.7 a | ||
| Cultivated Variety | Treatment | Dosage (mL/100 kg Seed) | Yield (kg·hm−2) | ||
|---|---|---|---|---|---|
| 2017 | 2018 | ||||
| Maize | Zhengdan 958 | CK | 100 | 8233.80 b | 8193.75 c |
| 30% GLP | 100 | 8344.05 ab | 8252.10 bc | ||
| 40% Prochloraz | 100 | 8555.70 a | 8584.35 a | ||
| 20%P + 15% GLP | 100 | 8582.85 a | 8526.45 ab | ||
| Xianyu 335 | CK | 100 | 8252.25 b | 8440.95 b | |
| 30% GLP | 100 | 8406.45 ab | 8549.10 b | ||
| 40% Prochloraz | 100 | 8631.30 a | 8849.10 a | ||
| 20%P + 15% GLP | 100 | 8624.25 a | 8890.20 a | ||
| Luning 202 | CK | 100 | 7596.60 b | 7865.55 b | |
| 30% GLP | 100 | 7652.70 ab | 8125.80 ab | ||
| 40% Prochloraz | 100 | 7931.25 a | 8503.80 a | ||
| 20%P + 15% GLP | 100 | 7953.90 a | 8577.90 a | ||
| Wheat | Jimai 22 | CK | 100 | 7248.60 b | 6253.95 b |
| 8% GLP | 100 | 7344.45 ab | 6604.80 ab | ||
| 1% Hexaconazole | 100 | 7554.25 ab | 6454.05 b | ||
| 0.5% H + 4% GLP | 100 | 7692.84 a | 7128.60 a | ||
| Shannong 23 | CK | 100 | 7362.45 b | 6224.40 b | |
| 8% GLP | 100 | 7492.05 ab | 6610.20 ab | ||
| 1% Hexaconazole | 100 | 7624.35 ab | 6440.55 ab | ||
| 0.5% H + 4% GLP | 100 | 7910.40 a | 6979.35 a | ||
| Luyuan 502 | CK | 100 | 7210.95 b | 5950.95 b | |
| 8% GLP | 100 | 7338.75 ab | 6318.75 ab | ||
| 1% Hexaconazole | 100 | 7522.05 ab | 6277.90 ab | ||
| 0.5% H + 4% GLP | 100 | 7722.90 a | 6535.20 a | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Sun, S.; Chen, Q.; Zhang, Z.; Wang, J.; Liu, Y.; Wang, H. A Polysaccharide of Ganoderma lucidum Enhances Antifungal Activity of Chemical Fungicides against Soil-Borne Diseases of Wheat and Maize by Induced Resistance. Agriculture 2022, 12, 55. https://doi.org/10.3390/agriculture12010055
Yang X, Sun S, Chen Q, Zhang Z, Wang J, Liu Y, Wang H. A Polysaccharide of Ganoderma lucidum Enhances Antifungal Activity of Chemical Fungicides against Soil-Borne Diseases of Wheat and Maize by Induced Resistance. Agriculture. 2022; 12(1):55. https://doi.org/10.3390/agriculture12010055
Chicago/Turabian StyleYang, Xiu, Shoumin Sun, Qiqi Chen, Zhongxiao Zhang, Jie Wang, Yali Liu, and Hongyan Wang. 2022. "A Polysaccharide of Ganoderma lucidum Enhances Antifungal Activity of Chemical Fungicides against Soil-Borne Diseases of Wheat and Maize by Induced Resistance" Agriculture 12, no. 1: 55. https://doi.org/10.3390/agriculture12010055






