Abstract
Neonicotinoid-based real control of aphids in sugar beet permitted the effective management of associated phytoviruses. However, the prohibition on their usage has prompted an urgent search for viable replacements. The development of sugar beet varieties with aphid and/or virus resistance and/or tolerance has a huge potential to reduce aphids and the harm caused by transmitted viruses. Semiochemicals also play a significant part in determining intra- and inter-specific interactions, which directly affect aphid fitness, feeding activity, and ultimately their capacity to spread viruses. Another method of aphid management involves the use of plant volatile organic compounds (VOCs) in conjunction with an attract and kill strategy. Entomopathogenic fungi could also be used to manage aphids without endangering helpful entomofauna. Finally, soil bacteria are particularly effective biocontrol agents because they induce systemic resistance (ISR) as plant growth promoting rhizobacteria (PGPR). The sugar beet-aphid virus model would be a perfect place to test these microbial players. The adoption of complementing eco-compatible techniques in the sugar beet crop will be ensured by the application of a variety of biocontrol opportunities connected to creative aphid control strategies. This should make it possible to create technical itineraries for a comprehensive approach to controlling aphids and related viruses depending on the situation.
1. Introduction
Sugar beet, Beta vulgaris L., is an economically important crop, providing about 25% of the sugar supply, mainly in Europe [1]. This highly productive sector is especially threatened by insect pests such as aphids, which are vectors of economically important phytoviruses. That means most of these viruses are transmitted from plant to plant by aphids. These different viruses infect sugar beets and cause important damage due to intense yellowing, reducing photosynthetic areas of leaves, resulting in yield loss and reduction of sugar content. Seldom are studies dealing with sugar beet-aphid interactions and the distribution of associated yellowing virus species. A preliminary investigation of the occurrence and distribution of sugar beet-associated viruses has been conducted on around 260 infected sugar beet leaves sampled from 10 countries belonging to three continents (Europe, North America, and South America) from where typical symptoms of virus infection have been examined [2]. A similar study has highlighted the occurrence of the Beet Mild Yellow Virus (BMYV), mainly found in the northern and western regions of Europe, the Beet Chlorosis Virus (BChV) observed in the southern areas of Europe and Chile and the beet yellow virus (BYV), mostly detected in southern Europe, Turkey, and the USA, whereas BWYV, has, so far, not been detected in Europe [3]. Another 2-year investigation conducted from 2017 to 2019 has shown that the closterovirus BYV was widely spread in northern Europe, while the poleroviruses BMYV and BChV mostly occurred in the northern and western European regions [3]. Field experiments revealed more damage to sugar beet crops when inoculated early in the growing season, corresponding to lower yields. Plant infection with the different yellowing viruses and their spread into the sugar beet field can be observed within 4 weeks [4].
Neonicotinoids are systemic neurotoxic insecticides acting as agonists on the insect nicotinic acetylcholine receptors [5]. These characteristics were the main reason for their broad applications, either in seed treatments or foliar applications, against economically important agricultural pests [6]. In sugar beet, neonicotinoids were used in seed treatments on all the European conventional cultivated sites against aphids and associated viruses [6,7]. However, their adverse effects on pollinators and other non-target organisms have been reported and intensively discussed after the damage of 11,000 bee colonies during neonicotinoid maize seed treatments in Germany [7,8,9]. Following these side effects, the European Commission has banned the outdoor use of the three neonicotinoid insecticides: clothianidin, imidacloprid, and thiamethoxam. However, after multiple derogations, several EU member states have allowed the use of seed coating with neonicotinoids, but changes for the next few years are under discussion.
The ban on the use of these chemicals has created a real vacuum in the context of the sanitary control of virus vectors in sugar beet crops and is supposed to induce 11% to 50% yield loss depending on the crop locations [3]. Actually, no alternate method is available to fight against the different beet mild yellow viruses.
Therefore, there is an urgent need to search for novel and effective alternatives, used either solely or in combination, to control aphids and, therefore, virus transmission (Figure 1). Within this context, considering the underground microbial community and their direct and indirect impact on the multitrophic plant–aphid–predator interactions is a novel way to disturb virus transmission. Both direct and indirect effects occurring within these players have to be investigated. For example, E-β-farnesene (EβF) is emitted by most aphid species upon sensing danger [10], and also plays numerous additional roles in aphid ecology, including key foraging cues for many aphid predators [11]. Being a behavior-related semiochemical, EβF plays a primary role in the intraspecific and interspecific interactions of aphids. Moreover, changes in aphid behavior interfere with virus transmission [12]. Indeed, aphids often display a preference for foliage showing disease symptoms, and this choice may increase the number of vectors acquiring the pathogen to enhance the spread of the virus [12]. The repelling effect of EβF will contribute to a decrease in virus transmission by aphids, coupled with the attraction of predators and parasitoids to be efficient aphidophagous agents to control aphids. The EβF, acting as a semiochemical attractant to these beneficials, is seen to provide an interesting strategy to improve the biological control of aphids. However, a deep investigation showed that in the presence of virosed aphids, the attraction of a higher abundance of predators has led to high mobility of the aphids and, therefore, increased virus transmission.
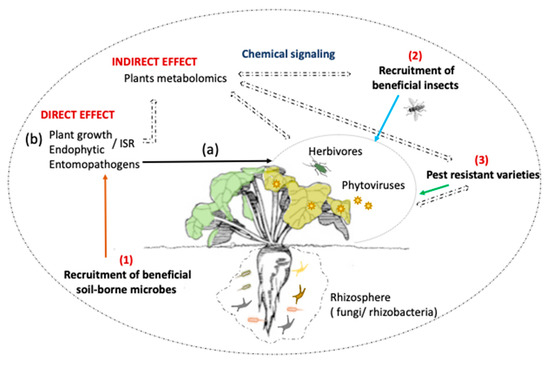
Figure 1.
Biocontrol strategies team up with sugar beet in a multitrophic interaction context to control aphids and associated plant viruses. Depending on the control strategy, direct and indirect impacts have been highlighted. Direct effect (solid arrows) is assessed on both aphids and plants. (a) aphids: entomopathogenic effect (black arrow) and trophic interaction (blue arrow) are caused by EPF and aphid predators’ (i.e., hoverfly larvae) recruitment, respectively. The selection of aphid–sugar beet resistance varieties also plays a role in the limitation of aphid infestation and therefore virus infection (green arrow). (b) host plant: increment of plant growth parameters and induced systemic resistance against aphids are the consequences of endophytic fungi and/or bacteria and rhizobacteria recruitment (orange arrow). Indirect effect (dash arrow) refers to a series of semiochemicals induced by different sources (microbial treatment and plants following infestation with herbivores) and their impact on higher tropic levels (beneficial insects).
New research directions with a focus on the interactions between sugar beets, viruses, and their transmitting aphid vectors are in progress. It is important to understand the mechanisms beyond these interactions in order to identify new targets to develop alternatives to neonicotinoids. Screening available sugar beet genotypes for the selection of virus resistance/tolerance and testing the interactions with vector aphids might serve as a first perspective of control. According to the sugar beet seed providers, effective resistance to yellowing viruses and their vectoring aphids is not available so far and needs to be investigated. Other promising alternatives to neonicotinoids aim to develop biocontrol measures such as semiochemicals, entomopathogenic fungi, and plant growth promoting rhizobacteria (PGPR), make use of virus manipulation processes, and cross-protection. A global approach has to be thought of, including the different trophic levels before applying new crop protection strategies.
2. Manipulation of Virus Processes
Virus manipulation processes exerted on plants and vectors to promote vector transmission could be reversed to inhibit virus acquisition by aphid vectors. Virus infection is known to alter plant phenotype (color, size, and texture) and plant metabolism (primary and secondary metabolites), affecting odor emission (volatiles) and sap composition. These virus-induced alterations of plants can impact the aphid vector and therefore the virus transmission efficiency [13]. Viruses strictly relying on aphids for their survival will induce plant changes and vector behavior modifications to foster their transmission and spread [14]. Although the molecular processes behind these virus manipulation processes are still unclear, some viral proteins [15] and metabolic pathways seem to be involved [16,17]. In particular, disrupting metabolic pathways affected by plant viruses to attract their vectors and facilitate their acquisition could be an alternate way to block virus propagation. On potyvirus-infected plants, the use of ethylene perception inhibitors has already been shown to induce callose deposition, a plant defense reaction against aphids, which could be responsible for the reduced aphid fecundity on the treated plants [18]. Interestingly, by inhibiting ethylene signaling, aphid settling on infected plants was reduced and virus spread was inhibited in laboratory conditions [19]. This pioneering research shows that metabolic pathways could be the targets for management of aphid-borne viruses. In general, research should be done to identify plant pathways that can be targeted to inhibit aphid development and virus acquisition.
3. Cross-Protection in Multi-Infections
Another alternative is to analyze whether the cross-protection approach can be developed for sugar beets. Like other agricultural crops, sugar beets are often infected by different viruses (“multi-infection”). It is suspected that a virus that infects the host first may gain a competitive advantage over viruses that infect plants later on. The most intense form of competition between plant viruses is cross-protection, where previous infection with one virus prevents secondary infection with genetically similar viruses or even viruses belonging to different families [20]. Cross-protection is efficient when using non-aggressive virus strains to protect plants from a subsequent infection with a more severe strain. For example, changes in the amino acid composition of potyivral protein (HC-Pro) were shown to result in mild strains, conferring cucumber plants protection against aggressive strains [21,22]. The molecular mechanisms behind cross protection involve different mechanisms such as RNA silencing [23] or blocking of virus entry into a cell already infected by a virus [24].
Multi- or co-infection of sugar beet has been reported in the field and the percentage composition of these different “virus cocktails” differs from year to year [25]. The same authors found increased transmission rates of BMYV to healthy test plants when it was simultaneously acquired by aphids from polerovirus co-infected (BMYV and BChV) source plants compared to source plants that were infected by BMYV. The consequences of such multi-infections on viral loads, virus transmissibility, symptom development, aphid behavior, and fecundity have yet to be explored, in particular, whether these multi-infections have a potential for cross-protection and could be useful for biocontrol and to replace neonicotinoids. To this end, it would be necessary to identify virus species, strains, or isolates in sugar beets, which may have an antagonistic effect on the accumulation of related or distant viruses in co-infected plants.
4. Breeding for Resistant Varieties
Breeding for new resistant/tolerant sugar beet genotypes is also an important way to protect sugar beets from yellowing viruses. Sources of resistance genes to yellowing viruses were identified [26], such as quantitative trait loci for resistance to BYV [27] and BMYV [28], resulting in the development of molecular markers for selection. Beet varieties resistant to vector aphids have also been identified [29], but focusing on breeding for direct resistance to viruses has been evaluated as more efficient because of gene-to-gene interactions and less emergence of counter resistance.
A promising strategy is the breeding for recessive resistance in sugar beet against aphid-transmitted yellowing poleroviruses, a program currently funded by the Federal Office of Agriculture and Food (BLE) in Germany and put into practice at IfZ, Goettingen. About half of the 200 known virus resistance genes in plants are recessively inherited, suggesting that this form of resistance is more common for viruses than for other plant pathogens [30]. Sugar beet infecting poleroviruses have a viral-genome-linked protein (VPg) [31], with a function for translation initiation. VPg is a hub protein that controls many processes leading to virus production and spread in the host plant and interacts with many proteins, notably host factors involved in protein synthesis within viral replication factories or within the nucleus [32]. For potyviruses, VPg interacts with different translation initiation factors (eIFs) of their host plants. Non-functional and/or interrupted VPg-interaction leads to recessive resistance. A transfer of results is not feasible because even close viruses need different eIFs for plant interaction [33]. In addition, the same virus might need different eIFs for interaction with different host plants [32]. Sugar beet-elFs were tested in studies of protein–protein interactions such as yeast two-hybrid assays (YTH) and bimolecular fluorescence complementation (BiFC) for interaction with VPgs of BMYV, BChV, and BtMV, resulting in multiple eIF–VPg interactions with sugar beet hosts. VPgs react with functionally redundant eIF (iso)4E, eIF4E, and eIF4E-like sugar beet [34]. Domain characterization will enable us to implement natural variations of eIFs in the breeding process.
5. Selection of Resistant Varieties
Although many resistances of sugar beet against diseases and parasites have been developed during the last few years, so far, no resistant/tolerated sugar beet variety against viral yellowing symptoms and their vectoring aphids has been found and validated. This was the consequence of efficient insecticide-based approaches that were applied to control aphids and associated viruses in sugar beet crops since the early 1990s [7]. At the European level, the recent banning of neoniconitoids in seed coating has led to redirecting research interests to investigate virus and aphid varietal resistance. Hence, only a few recent papers are available on the topic of sugar beet—virus–aphid interactions, and the mechanisms involved are not understood, leading to difficulties in identifying new targets to develop alternatives to neonicotinoids. Screening available sugar beet genotypes for the selection of virus resistance/tolerance and testing the interactions with vector aphids might serve as a first perspective of control.
Beet yellow virus (BYV) and beet mild yellowing virus (BMYV) are responsible for reducing sugar beet yields by 50% [35]. Viruses move via the phloem and can colonize mesophyll and epidermal cells [36]. They are persistently transmitted by aphids in a circulative and non-propagative mode throughout the life span of the vectors [37]. The preferential aphid species for the transmission of sugar beet poleroviruses is Myzus persicae (Sulzer) [38,39]. Poleroviruses are strictly limited to the cell types of the host’s phloem, that is parenchyma, sieve elements, and companion cells [40]. They have icosaedric particles containing an RNA genome. In contrast, beet yellow virus (BYV, Closteroviridae family) has long flexuous and filamentous particles containing an RNA genome. BYV is located in phloem and transmitted by two major aphid species, M. persicae and Aphis fabae [41], in a semi-persistent, non-circulative transmission mode where transmitted virus particles are retained in the aphid stylets. These different viruses can infect simultaneously or successively an individual plant. However, the effect of such multi-infections on symptom expression and yield is largely unknown. It is interesting to point out that all these different viruses share a common vector (M. persicae), although they are transported by the aphids using different transmission modes relying on specific interactions with the aphids.
Three levels of variability are associated with investigations of viral transmission, that is the host plant characteristics related to sugar beet varieties, the virus causing the yellowing symptoms, and the aphid vector diversity/variability of vector capacity, at the species (mainly M. persicae and A. fabae) but also clonal levels. There is barely any information on whether different lineages of M. persicae have different transmission capabilities, although it is known that there are adapted lineages to specific hosts due to transcriptional plasticity of duplicated genes [42] and/or gene amplification [43]. Then, different M. persicae clones transmit viruses with variable efficiencies.
A current focus of research in our laboratory is to screen a large number of varieties of cultivated beets for the selection of resistant/tolerant varieties focusing on BMYV and BYV (one Luteoviridae and one Closteroviridae) to account for the possibility that different M. persicae clones transmit viruses with different efficiencies. Collaboration with several beet seed providers, sugar beet research institutes, and laboratories (IRBAB, IRS Netherlands, INRAe Colmar) allowed experiments on potential resistant varieties. Viral transmission efficiency (plant to plant, by aphids) was calculated based on the percentage of infected sugar beet plants for different aphid–virus combinations by ELISA (enzyme-linked immunosorbent assay). Strong reduced transmission rates of up to about 80% were found for both viruses, in contrast to other varieties with 15–30% compared to susceptible varieties (unpublished). These preliminary results are extended to other varieties by ongoing experiments. Moreover, to better characterize the most interesting varieties, dual choice assays for aphids to choose their preferred host plant variety were performed. Susceptible varieties were significantly preferred by aphids at all time points and in both viral systems compared to the resistant varieties (unpublished). Some obviously less resistant varieties were found to be less attractive during the first day, then (day 2–5) were chosen by the aphids similarly to the susceptible variety (BMYV) or even preferred (BYV) (unpublished). In addition, our first data for aphid life span and fecundity showed the lowest survival and reproduction on the resistant varieties, with a decrease after 2 days, with no more aphids left after 4 days. In addition, population growth assays conducted on the same varieties over 10 days showed fresh weight reductions of the youngest aphid larval stages on the varieties with strong resistance (unpublished).
A list of beet varieties with increasing viral transmission rates will have to be established and will correspond to a resistance gradient for the diversity of virus/aphid species combinations. On this basis, the most resistant varieties will have to be investigated for further characterization of the resistance mechanisms and to be selected for future field growing.
6. Use of Semiochemicals
Semiochemicals are signaling chemicals involved in intraspecific and interspecific communication between plants, insect pests, parasitoids, and predators. Their use within an integrated pest management strategy (IPM) is a way for sustainable management due to their moderately non-toxic effect on the non-target fauna and their efficiency at a very small amount [44,45,46]. Semiochemicals can be used for monitoring, mass trapping, mating disruption, attract and kill, and push-pull strategies [44,47]. Despite the positive outcomes and their promising role in IPM and toward diverse agricultural insect pests, few studies have been conducted on aphids using a push-pull strategy [48,49,50,51].
To date, sex pheromones and plant volatiles have been the most widely used semiochemicals for control method development [44,47]. In addition, insect attraction mechanisms by plant volatiles and related compounds are well documented [47]. For example, studies have reported the repellent effects of cis-jasmonate and methyl salicylate on different aphid species, such as Nasonovia ribis-nigri, the lettuce aphid, Phorodon humuli, the damson-hop aphid, Sitobion avenae, the cereal aphid, and A. fabae, the black bean aphid [52,53,54,55]. A previous study conducted in a sugar beet field highlighted that dodecanoic acid had a repellent effect on A. fabae and, therefore, decreased the spread of BYV and BMYV [56]. In contrast, other plant volatiles have been shown to be attractive to insects, for example benzyl acetate, methyl salicylate, limonene, α-pinene, (E)-β-farnesene, β-myrcene, (Z)-3-hexen-1-ol, ethyl acetate, and acetic acid. Blends of volatiles are often more attractive than single compounds [47].
To our knowledge, very seldom are the studies on the sugar beet varietal impact on aphid behavior and virus transmission. Moreover, no VOC analysis was available to compare potential attractive/repellent semiochemicals in shaping the sugar beet–aphid interactions, leading to the suggestion of innovative behavior-modifying approaches for aphids management. Identification of attractive/repellent cues for sugar beet aphids is in progress in our laboratory and will initiate new perspectives by proposing mass trapping or “lure and kill” (or “attract and kill”) according to the association either with trapping devices or entomopathogenic microbials.
7. Entomopathogenic Fungi, a Biorational Control Agent with Wide Spectrum of Activity
Entomopathogenic fungi (EPF) exhibit a complex of fungal species mostly within the Ascomycota (orders Hypocreales and Onygenales) and Entomophthoromycota (orders Entomophthorales and Neozygitales) [57]. Species of the order Hypocreales, such as the genera Beauveria (Cordycipitaceae) and Metarhizium (Clavicipitaceae), are important components of agroecosystems and ubiquitous inhabitants of the rhizosphere, isolated from different agricultural and ecological zones [58]. Several species within both genera have been thoroughly investigated for years due to their promising potential as biocontrol agents against a wide range of insect pest species [59]. Although they have promising potential under controlled conditions, the reliability and efficacy of these pathogenic fungi are often challenged in the field. As a solution to enhance the entomotoxic effect of these fungal biological control agents, the current upsurge of research has focused on the ubiquitous and plant endophytic lifestyle of this specific fungal group since it might provide a solution [60,61]. Recently, a meta-analysis has revealed that EPF inoculations of plants often lead to reduced herbivore fitness [62]. For example, and besides their direct application against herbivores, species such as Beauveria bassiana and several species within Metarhizium spp. have been studied as root colonizers with the potential to improve plant growth and reduce above-ground herbivores [63,64,65]. Recent insights suggest that EPFs are involved in the modulation of the plant’s chemical machinery, leading to the production of bioactive plant defense compounds [62] and that effects of EPF inoculations against herbivores are likely associated with systemic regulation of plant metabolism [65], but these hypotheses require further confirmation and should take into account multiple variables.
Most of the studies dealing with the evaluation of the virulence of different species of Metarhizium against aphids have shown a promising entomotoxic effect with a mortality range of between 60 and 100%, depending on the fungal species, the mode of application, the aphid species, and the development stage [66,67,68,69]. To date, the exact roles of Beauveria and Metarhizium spp. in modulating the plant’s intrinsic defense system and their impact on insect pests are still not fully described [70]. Furthermore, even if plant colonization by EPF is known to vary with fungal species, environmental conditions, and host species [71], further investigations are clearly needed to understand the interactions between endophytic EPF, plants, and insects, more particularly aphids. Except for the model genera Beauveria and Metarhzium, other pathogenic and opportunistic fungi with diverse lifestyles have never been considered for potential endophytic behavior. Likewise, only a few studies have focused on the production of fungal specialized metabolites within host plants. Indeed, the plant metabolome and its changes driven by plants and related fungi, alone or associated, have to be investigated in relation to insect behavior and biology. In addition, the activity of plant signaling pathways and their cross-talk in multitrophic interactions has to be determined at different timescales. The diversity of endophyte EPF leads to various defensive responses, rendering host plants more or less susceptible to insect pests. Hence, data to support the systemic, rather than local, effects of plant-associated EPF should be urgently generated for further use in pest biocontrol. Whereas, the role of the EPF in underpinning plants and insects in terrestrial agroecosystems has recently been reported, there is little information about the effects of these fungi either on aphid-borne diseases or trophic interactions, including those between pests and their insect biological controls. In addition, the mechanisms behind their ecological interactions are still largely unknown. The next crucial step in biological control to manage aphids and associated viruses in sugar beet crops is to investigate the latent potential of EPF at cross-kingdom levels and answer the fundamental question of the mechanisms involved. So far, biological alternatives using EPF have been adopted as a safe alternative to chemicals, but their potential has still not been fully explored and need to be further investigated. For the sugar beet system, there is an urgent need to investigate the role and potential of the plant endophytic microbial communities and defense related metabolites to suppress sugar beet insects and their associated plant viruses.
8. Rhizobacteria and Plant Defenses
Exploiting selected strains of plant-associated and beneficial bacteria (Plant Growth Promoting Rhizobacteria—PGPR) as microbial biological control agents is a promising alternative [72]. Indeed, some soil bacteria have the ability, by inducing systemic resistance (ISR), to improve plant health and mediate host plant resistance against economically important agricultural pests, including fungi, bacteria, viruses, nematodes, and insects [73,74,75,76]. ISR is stimulated by various phylogenetically unrelated bacterial species and is of great interest for biocontrol since this enhanced defensive capacity is expressed in all plant parts and is followed by an increase in resistance against subsequent attack [76]. Bacteria belonging to the Bacillus and Pseudomonas genera are mainly studied [77]. The ISR effect can be provided by applying the living bacteria or, alternatively, the molecules they secrete, which are mainly responsible for the stimulation of host immunity. Following perception of the bacteria or their elicitors (cell surface components, such as flagellin and lipopolysaccharides), iron-regulated metabolites (siderophores and SA produced by the rhizobacteria), and antibiotics [78,79,80]. Treated plants were reported to activate direct and indirect responses against bioagressors. ISR-induced responses include accumulation of hydrolytic enzymes (pathogen related proteins—PRP–with glucanase and chitinase activities, for example), cell wall enhancement, accumulation of defense-related enzymes, production of antimicrobial phytoalexins, stimulation of the lipoxygenase pathway, and production of secondary metabolites (alkaloids, phenols, non-volatile, and volatile organic compounds, including terpenes) [77,81]. These reactions have direct and indirect antibiosis effects on insects, such as decreased insect growth and development, inhibition of reproduction, hydrolysis of chitin, attraction of predators and parasitoids, and behavioral modification [77,82]. However, these defense mechanisms only occur after bioagressor challenge and, globally, no major transcriptional reprogramming is observed in PGPR-treated plants before sensing the invader [83,84].
Interesting results were obtained with a strain of Bacillus velezensis on the behavior and development of sugar beet aphids. Indeed, 31 to 48% reductions in host plant selection by M. persicae aphids were observed from day one to five when PGPR was applied to sugar beets. Moreover, the reduction of M. persicae aphid fecundity was observed on PGPR-treated plants from days 2 to 5, with 22 to 51% decreases in aphid numbers, respectively (unpublished data). Further comprehensive studies that combine ecological, biochemical, and molecular approaches are needed to better characterize PGPR-induced systemic resistance against sugar beet aphids.
The implementation of a practical alternative and innovative control of aphids and associated viruses is possible through the application of PGPR, which leads to the manipulation of multitrophic interactions involving plants, microbials, aphid pests, and aphidophagous beneficials. These interactions focus on the vector role of aphids for associated phytoviruses in sugar beets, since modulation of aphid behavior may also have a strong impact on virus transmission efficiency in crops. The integration of pests, beneficials, and related viral diseases reflects the needed holistic approach to consider such kinds of crop protection in field assays. To determine both direct and indirect defense mechanisms related to PGPR applications, complementary approaches are in progress. This will provide a broad range of applicability for farmers according to proposed technical itineraries coupling different biological agents and molecules to control aphids and associated beet viruses.
9. Conclusions
The implementation of innovative eco-compatible approaches in IPM, namely the use of semiochemicals, EPF, and PGPRs in combination with the use of resistant beet varieties, is a promising approach to ensure sustainable pest management. This has to be done to the benefit of sugar beet producers and to create synergies from experimental data provided by diverse partners, including national research centers, universities, and private societies from the sugar beet industry. The specific goals to be achieved are the validation under field conditions of complementary alternative aphid control methods combining resistant varieties, semiochemical releasers, EPF, and PGPR used to replace neonicotinoïd insecticides in sugar beet virus control. Only the promotion of synergies by the development of multidisciplinary approaches will provide a holistic way for the efficient control of aphids and associated viruses to advise sugar beet producers.
Author Contributions
Conceptualization and methodology, F.F.; validation, I.B.F., L.I. and C.T.; formal analysis, F.F., I.B.F., L.I. and C.T.; investigation, A.F., Y.A.C.G., C.T. and L.I.; data curation, A.F. and Y.A.C.G.; writing—original draft preparation, F.F.; writing—review and editing, all; funding acquisition, F.F. All authors have read and agreed to the published version of the manuscript.
Funding
CT was funded by the Service public de Wallonie economie emploi recherche, Beware program, project AphidVirBeet. IBF had a post-doc grant from Wallonia—Brussels International.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Biancardi, E.; Mcgrath, J.M.; Panella, L.W.; Lewellen, R.T.; Stevanato, P. Sugar Beet. In Root and Tuber Crops; Bradshaw, J.E., Ed.; Springer: New York, NY, USA, 2010; pp. 173–219. [Google Scholar]
- Stevens, M.; Patron, N.J.; Dolby, C.A.; Weekes, R.; Hallsworth, P.B.; Lemaire, O.; Smith, H.G. Distribution and Properties of Geographically Distinct Isolates of Sugar Beet Yellowing Viruses. Plant Pathol. 2005, 54, 100–107. [Google Scholar] [CrossRef]
- Hossain, R.; Menzel, W.; Lachmann, C.; Varrelmann, M. New Insights into Virus Yellows Distribution in Europe and Effects of Beet Yellows Virus, Beet Mild Yellowing Virus, and Beet Chlorosis Virus on Sugar Beet Yield Following Field Inoculation. Plant Pathol. 2021, 70, 584–593. [Google Scholar] [CrossRef]
- Stevens, M.; Hallsworth, P.B.; Smith, H.G. The Effects of Beet Mild Yellowing Virus and Beet Chlorosis Virus on the Yield of UK Field-Grown Sugar Beet in 1997, 1999 and 2000. Ann. Appl. Biol. 2004, 144, 113–119. [Google Scholar] [CrossRef]
- Elbert, A.; Haas, M.; Springer, B.; Thielert, W.; Nauen, R. Applied Aspects of Neonicotinoid Uses in Crop Protection. Pest Manag. Sci. 2008, 64, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Bub, S.; Petschick, L.L.; Stehle, S.; Wolfram, J. Applied Pesticide Toxicity Shifts toward Plants and Invertebrates, Even in GM Crops. Science 2021, 372, 81–84. [Google Scholar] [CrossRef]
- Hauer, M.; Hansen, A.L.; Manderyck, B.; Olsson, Å.; Raaijmakers, E.; Hanse, B.; Stockfisch, N.; Märländer, B. Neonicotinoids in Sugar Beet Cultivation in Central and Northern Europe: Efficacy and Environmental Impact of Neonicotinoid Seed Treatments and Alternative Measures. Crop Prot. 2017, 93, 132–142. [Google Scholar] [CrossRef]
- Goulson, D. Review: An Overview of the Environmental Risks Posed by Neonicotinoid Insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Stuligross, C.; Williams, N.M. Past Insecticide Exposure Reduces Bee Reproduction and Population Growth Rate. Proc. Natl. Acad. Sci. USA 2021, 118, e2109909118. [Google Scholar] [CrossRef]
- Francis, F.; Martin, T.; Lognay, G.; Haubruge, E. Role of (E)-Farnesene in Systematic Aphid Prey Location by Episyrphus Balteatus Larvae (Diptera: Syrphidae). Eur. J. Entomol. 2005, 102, 431–436. [Google Scholar] [CrossRef]
- Francis, F.; Lognay, G.; Haubruge, E. Olfactory Responses to Aphid and Host Plant Volatile Releases: (E)-Beta-Farnesene an Effective Kairomone for the Predator Adalia Bipunctata. J. Chem. Ecol. 2004, 30, 741–755. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Infection of Host Plants by Cucumber Mosaic Virus Increases the Susceptibility of Myzus Persicae Aphids to the Parasitoid Aphidius Colemani. Sci. Rep. 2015, 5, 10963. [Google Scholar] [CrossRef]
- Dader, B.; Then, C.; Berthelot, E.; Ducousso, M.; Ng, J.C.K.; Drucker, M. Insect Transmission of Plant Viruses: Multilayered Interactions Optimize Viral Propagation. Insect Sci. 2017, 24, 929–946. [Google Scholar] [CrossRef]
- Carmo-Sousa, M.; Moreno, A.; Plaza, M.; Garzo, E.; Fereres, A. Cucurbit Aphid-Borne Yellows Virus (CABYV) Modifies the Alighting, Settling and Probing Behaviour of Its Vector Aphis Gossypii Favouring Its Own Spread. Ann. Appl. Biol. 2016, 169, 284–297. [Google Scholar] [CrossRef]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R.; Mauck, K. Evolutionary Determinants of Host and Vector Manipulation by Plant Viruses. In Advances in Virus Research; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 101, pp. 189–250. [Google Scholar]
- Ziegler-Graff, V. Molecular Insights into Host and Vector Manipulation by Plant Viruses. Viruses 2020, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ye, J. Manipulation of Jasmonate Signaling by Plant Viruses and Their Insect Vectors. Viruses 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; De Alwis, M.; Bak, A.; Dong, H.; Whitham, S.A.; Jander, G. Disruption of Ethylene Responses by Turnip Mosaic Virus Mediates Suppression of Plant Defense against the Green Peach Aphid Vector. Plant Physiol. 2015, 169, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Mackenzie, P.F.; Perilla-Henao, L.M.; Aegerter, B.J.; Casteel, C.L. Ethylene Signaling Mediates Potyvirus Spread by Aphid Vectors. Oecologia 2019, 190, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, H.; Carr, J.P. Cross-Protection: A Century of Mystery. Adv. Virus Res. 2010, 76, 211–264. [Google Scholar] [CrossRef]
- Gal-On, A. A Point Mutation in the FRNK Motif of the Potyvirus Helper Component-Protease Gene Alters Symptom Expression in Cucurbits and Elicits Protection Against the Severe Homologous Virus. Phytopathology 2000, 90, 467–473. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Natsuaki, T.; Kosaka Seiichi Okuda, Y.; Wang, W.; Natsuaki, T.; Okuda, S.; Kosaka, Y. Comparison of the Nucleotide and Amino Acid Sequences of Parental and Attenuated Isolates of Zucchini Yellow Mosaic Virus. J. Gen. Plant Pathol. 2006, 72, 52–56. [Google Scholar] [CrossRef]
- Ziebell, H.; Payne, T.; Berry, J.O.; Walsh, J.A.; Carr, J.P. A Cucumber Mosaic Virus Mutant Lacking the 2b Counter-Defence Protein Gene Provides Protection against Wild-Type Strains. J. Gen. Virol. 2007, 88, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Folimonova, S.Y. Developing an Understanding of Cross-Protection by Citrus Tristeza Virus. Front. Microbiol. 2013, 4, 2–9. [Google Scholar] [CrossRef]
- Schlaefli, H.; Marmonier, A.; Chesnais, Q.; Villeroy, C.; Brault, V. La Multi-Infection Virale de La Betterave à Sucre: Effets Sur l’Accumulation Des Virus et Leur Transmission Par Pucerons. In Proceedings of the Journées BAPOA, Nice, France, 17–18 May 2022. [Google Scholar]
- Francis, S.A.; Luterbacher, M.C. Identification and Exploitation of Novel Disease Resistance Genes in Sugar Beet. Pest Manag. Sci. 2003, 59, 225–230. [Google Scholar] [CrossRef]
- Grimmer, M.K.; Bean, K.M.R.; Qi, A.; Stevens, M.; Asher, M.J.C. The Action of Three Beet Yellows Virus Resistance QTLs Depends on Alleles at a Novel Genetic Locus That Controls Symptom Development. Plant Breed. 2008, 127, 391–397. [Google Scholar] [CrossRef]
- James, L.C.; Bean, K.M.R.; Grimmer, M.K.; Barnes, S.; Kraft, T.; Stevens, M. Varieties of the Future: Identification of “broad Spectrum” Genetic Resistance in Sugar Beet. Int. Sugar J. 2012, 114, 164–168. [Google Scholar]
- Zhang, C.L.; Xu, D.C.; Jiang, X.C.; Zhou, Y.; Cui, J.; Zhang, C.X.; Chen, D.F.; Fowler, M.R.; Elliott, M.C.; Scott, N.W.; et al. Genetic Approaches to Sustainable Pest Management in Sugar Beet (Beta Vulgaris). Ann. Appl. Biol. 2008, 152, 143–156. [Google Scholar] [CrossRef]
- Truniger, V.; Aranda, M. Recessive Resistance to Plant Viruses. Adv. Virus Res. 2009, 75, 119–159. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Freeman, B.; Liu, H.Y.; Herrbach, E.; Lemaire, O. Beet Poleroviruses: Close Friends or Distant Relatives? Mol. Plant Pathol. 2005, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Laliberté, J.F. The Genome-Linked Protein VPg of Plant Viruses—A Protein with Many Partners. Curr. Opin. Virol. 2011, 1, 347–354. [Google Scholar] [CrossRef]
- Reinbold, C.; Lacombe, S.; Ziegler-Graff, V.; Scheidecker, D.; Wiss, L.; Beuve, M.; Caranta, C.; Brault, V. Closely Related Poleroviruses Depend on Distinct Translation Initiation Factors to Infect Arabidopsis Thaliana. Mol. Plant-Microbe Interact. 2013, 26, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Rollwage, L.; Hossain, R.; Varrelmann, M. Zuckerrübe Infizierende Poleroviren Interagieren Mit Multiplen Translationsintiationsfaktoren Ihres Wirtes. In Proceedings of the 54. Jahrestagung des DPG ArbeitskreisesViruskrankheit der Pflanzen, Dossenheim, Germany, 21–22 March 2022. [Google Scholar]
- Smith, H.G.; Hallsworth, P.B. The Effects of Yellowing Viruses on Yield of Sugar Beet in Field Trials, 1985 and 1987. Ann. Appl. Biol. 1990, 116, 503–511. [Google Scholar] [CrossRef]
- Dolja, V.V. Beet Yellows Virus: The Importance of Being Different. Mol. Plant Pathol. 2003, 4, 91–98. [Google Scholar] [CrossRef]
- Gray, S.; Gildow, F.E. Luteovirus-Aphid Interactions. Annu. Rev. Phytopathol. 2003, 41, 539–566. [Google Scholar] [CrossRef]
- Heathcote, G.D. Aphids Caught on Sticky Traps in Eastern England in Relation to the Spread of Yellowing Viruses of Sugar-Beet. Bull. Entomol. Res. 1974, 64, 669–676. [Google Scholar] [CrossRef]
- Heathcote, G.D. The Time of Flight and the Relative Importance of Myzus Persicae (Sulz.) and Aphis Fabae Scop. in Relation to the Incidence of Beet Yellows as Shown by Trap Catches at Rothamsted and Broom’s Barn. Bull. Entomol. Res. 1966, 56, 473–480. [Google Scholar] [CrossRef]
- Boissinot, S.; Pichon, E.; Sorin, C.; Piccini, C.; Scheidecker, D.; Ziegler-Graff, V.; Brault, V. Systemic Propagation of a Fluorescent Infectious Clone of a Polerovirus Following Inoculation by Agrobacteria and Aphids. Viruses 2017, 9, 166. [Google Scholar] [CrossRef]
- Thielemann, R.; Nagi, A. Welche Bedeutung Haben Die Zur “Aphis Fabae-Gruppe” Gehörenden Blattlausstämme für die Übertragung des Schwachen Vergilbungsvirus Auf Beta-Rüben? Z. Pflanz. Pflanzenschutz 1979, 86, 161–168. [Google Scholar]
- Mathers, T.C.; Chen, Y.; Kaithakottil, G.; Legeai, F.; Mugford, S.T.; Baa-Puyoulet, P.; Bretaudeau, A.; Clavijo, B.; Colella, S.; Collin, O.; et al. Rapid Transcriptional Plasticity of Duplicated Gene Clusters Enables a Clonally Reproducing Aphid to Colonise Diverse Plant Species. Genome Biol. 2017, 18, 27. [Google Scholar] [CrossRef]
- Bass, C.; Zimmer, C.T.; Riveron, J.M.; Wilding, C.S.; Wondji, C.S.; Kaussmann, M.; Field, L.M.; Williamson, M.S.; Nauen, R. Gene Amplification and Microsatellite Polymorphism Underlie a Recent Insect Host Shift. Proc. Natl. Acad. Sci. USA 2013, 110, 19460–19465. [Google Scholar] [CrossRef]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex Pheromones and Their Impact on Pest Management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Smart, L.E.; Aradottir, G.I.; Bruce, T.J.A. Chapter 6—Role of Semiochemicals in Integrated Pest Management. In Integrated Pest Management: Current Concepts and Ecological Perspective; Abrol, D.P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 93–109. [Google Scholar]
- Sharma, A.; Sandhi, R.K.; Reddy, G.V.P. A Review of Interactions between Insect Biological Control Agents and Semiochemicals. Insects 2019, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Gregg, P.C.; Del Socorro, A.P.; Landolt, P.J. Advances in Attract-and-Kill for Agricultural Pests: Beyond Pheromones. Annu. Rev. Entomol. 2018, 63, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-b.; Chen, J.-l.; Liu, Y.; Francis, F.; Haubruge, E.; Bragard, C.; Sun, J.; Cheng, D. Influence of Garlic Intercropping or Active Emitted Volatiles in Releasers on Aphid and Related Beneficial in Wheat Fields in China. J. Integr. Agric. 2013, 12, 467–473. [Google Scholar] [CrossRef]
- Xu, Q.; Hatt, S.; Lopes, T.; Zhang, Y.; Bodson, B.; Chen, J.; Francis, F. A Push–Pull Strategy to Control Aphids Combines Intercropping with Semiochemical Releases. J. Pest Sci. 2018, 91, 93–103. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, L.; Liu, Y.; Chen, J.; Francis, F. Use of Slow-Release Plant Infochemicals to Control Aphids: A First Investigation in a Belgian Wheat Field. Sci. Rep. 2016, 6, 31552. [Google Scholar] [CrossRef]
- Xu, Q.; Hatt, S.; Han, Z.; Francis, F.; Chen, J. Combining E-β-Farnesene and Methyl Salicylate Release with Wheat-Pea Intercropping Enhances Biological Control of Aphids in North China. Biocontrol. Sci. Technol. 2018, 28, 883–894. [Google Scholar] [CrossRef]
- Hardie, J.; Isaacs, R.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Methyl Salicylate and (−)-(1R,5S)-Myrtenal Are Plant-Derived Repellents for Black Bean Aphid, Aphis Fabae Scop. (Homoptera: Aphididae). J. Chem. Ecol. 1994, 20, 2847–2855. [Google Scholar] [CrossRef]
- Pettersson, J.; Pickett, J.A.; Pye, B.J.; Quiroz, A.; Smart, L.E.; Wadhams, L.J.; Woodcock, C.M. Winter Host Component Reduces Colonization by Bird-Cherry Oat Aphid, Rhopalosiphum Padi (L.) (Homoptera, Aphididae), and Other Aphids in Cereal Fields. J. Chem. Ecol. 1994, 20, 2565–2574. [Google Scholar] [CrossRef]
- Birkett, M.A.; Campbell, C.A.M.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New Roles for Cis-Jasmone as an Insect Semiochemical and in Plant Defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef]
- Ninkovic, V.; Ahmed, E.; Glinwood, R.; Pettersson, J. Effects of Two Types of Semiochemical on Population Development of the Bird Cherry Oat Aphid Rhopalosiphum Padi in a Barley Crop. Agric. Entomol. 2003, 5, 27–34. [Google Scholar] [CrossRef]
- Herrbach, E. Effect of Dodecanoic Acid on the Colonisation of Sugar Beet by Aphids and the Secondary Spread of Virus Yellows. Ann. Appl. Biol. 1987, 111, 477–482. [Google Scholar] [CrossRef]
- Boomsma, J.J.; Jensen, A.B.; Meyling, N.V.; Eilenberg, J. Evolutionary Interaction Networks of Insect Pathogenic Fungi. Annu. Rev. Entomol. 2014, 59, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect Pathogens as Biological Control Agents: Back to the Future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G. Review on Safety of the Entomopathogenic Fungus Metarhizium Anisopliae. Biocontrol. Sci. Technol. 2007, 17, 879–920. [Google Scholar] [CrossRef]
- Berbee, M.L. The Phylogeny of Plant and Animal Pathogens in the Ascomycota. Physiol. Mol. Plant Pathol. 2001, 59, 165–187. [Google Scholar] [CrossRef]
- Vega, F.E.; Meyling, N.V.; Luangsa-Ard, J.J.; Blackwell, M. Fungal Entomopathogens. In Insect Pathology; Vega, F., Kaya, H., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 171–220. ISBN 9780123849847. [Google Scholar]
- Gange, A.C.; Koricheva, J.; Currie, A.F.; Jaber, L.R.; Vidal, S. Meta-Analysis of the Role of Entomopathogenic and Unspecialized Fungal Endophytes as Plant Bodyguards. New Phytol. 2019, 223, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Keyser, C.A.; Jensen, B.; Meyling, N.V. Dual Effects of Metarhizium Spp. and Clonostachys Rosea against an Insect and a Seed-Borne Pathogen in Wheat. Pest Manag. Sci. 2016, 72, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Mantzoukas, S.; Eliopoulos, P.A. Endophytic Entomopathogenic Fungi: A Valuable Biological Control Tool against Plant Pests. Appl. Sci. 2020, 10, 360. [Google Scholar] [CrossRef]
- Rasool, S.; Vidkjær, N.H.; Hooshmand, K.; Jensen, B.; Fomsgaard, I.S.; Meyling, N.V. Seed Inoculations with Entomopathogenic Fungi Affect Aphid Populations Coinciding with Modulation of Plant Secondary Metabolite Profiles across Plant Families. New Phytol. 2021, 229, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Saranya, S.; Ushakumari, R.; Jacob, S.; Philip, B.M. Efficacy of Different Entomopathogenic Fungi against Cowpea Aphid, Aphis Craccivora (Koch). J. Biopestic. 2010, 3, 138–142. [Google Scholar]
- Shan, L.T.; Feng, M.G. Evaluation of the Biocontrol Potential of Various Metarhizium Isolates against Green Peach Aphid Myzus Persicae (Homoptera: Aphididae). Pest Manag. Sci. 2010, 66, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Mweke, A.; Ulrichs, C.; Nana, P.; Akutse, K.S.; Fiaboe, K.K.M.; Maniania, N.K.; Ekesi, S. Evaluation of the Entomopathogenic Fungi Metarhizium Anisopliae, Beauveria Bassiana and Isaria Sp. for the Management of Aphis Craccivora (Hemiptera: Aphididdae). J. Econ. Entomol. 2018, 111, 1587–1594. [Google Scholar] [CrossRef]
- Reingold, V.; Kottakota, C.; Birnbaum, N.; Goldenberg, M.; Lebedev, G.; Ghanim, M.; Ment, D. Intraspecies Variation of Metarhizium Brunneum against the Green Peach Aphid, Myzus Persicae, Provides Insight into the Complexity of Disease Progression. Pest Manag. Sci. 2021, 77, 2557–2567. [Google Scholar] [CrossRef]
- Branine, M.; Bazzicalupo, A.; Branco, S. Biology and Applications of Endophytic Insect-Pathogenic Fungi. PLoS Pathog. 2019, 15, e1007831. [Google Scholar] [CrossRef]
- Lovett, B.; St. Leger, R.J. Stress Is the Rule Rather than the Exception for Metarhizium. Curr. Genet. 2015, 61, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and Exploiting Plant Beneficial Microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting Ready for Battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U. Chapter 9 Priming of Induced Plant Defense Responses. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2009; Volume 51, pp. 361–395. [Google Scholar]
- Zamioudis, C.; Pieterse, C.M.J. Modulation of Host Immunity by Beneficial Microbes. Mol. Plant-Microbe Interact. 2012, 25, 139–150. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Disi, J.; Simmons, J.; Zebelo, S. Plant Growth-Promoting Rhizobacteria-Induced Defense Against Insect Herbivores. In Field Crops: Sustainable Management by PGPR; Springer: Cham, Switzerland, 2019; pp. 385–410. [Google Scholar] [CrossRef]
- Ongena, M.; Thonart, P. Resistance Induced in Plants by Non-Pathogenic Microorganisms: Elicitation and Defense Responses. Floric. Ornam. Plant Biotechnol. 2006, 3, 447–463. [Google Scholar]
- Jourdan, E.; Ongena, M.; Thonart, P. Caractéristiques Moléculaires de l’immunité Des Plantes Induite Par Les Rhizobactéries Non Pathogènes. Biotechnol. Agron. Soc. Environ. 2008, 12, 437–449. [Google Scholar]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 2–19. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Sarafat Ali, M.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K. Bacillus Velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef] [PubMed]
- Karthiba, L.; Saveetha, K.; Suresh, S.; Raguchander, T.; Saravanakumar, D.; Samiyappan, R. PGPR and Entomopathogenic Fungus Bioformulation for the Synchronous Management of Leaffolder Pest and Sheath Blight Disease of Rice. Pest Manag. Sci. 2010, 66, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Cawoy, H.; Mariutto, M.; Henry, G.; Fisher, C.; Vasilyeva, N.; Thonart, P.; Dommes, J.; Ongena, M. Plant Defense Stimulation by Natural Isolates of Bacillus Depends on Efficient Surfactin Production. Mol. Plant-Microbe Interact. 2014, 27, 87–100. [Google Scholar] [CrossRef]
- Debois, D.; Fernandez, O.; Franzil, L.; Jourdan, E.; de Brogniez, A.; Willems, L.; Clément, C.; Dorey, S.; De Pauw, E.; Ongena, M. Plant Polysaccharides Initiate Underground Crosstalk with Bacilli by Inducing Synthesis of the Immunogenic Lipopeptide Surfactin. Environ. Microbiol. Rep. 2015, 7, 570–582. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).