Abstract
Lodging is one of the major constraints in attaining high yield in crop production. Major factors associated with stalk lodging involve morphological traits and anatomical features along with the chemical composition of the stem. However, little relevant research has been carried out in sorghum, particularly on the anatomical aspects. In this study, with a high-throughput procedure newly developed by our research group, the nine parameters related to stem regions and vascular bundles were generated in 58 sorghum germplasm accessions grown in two successive seasons. Correlation analysis and principal component analysis were conducted to investigate the relationship between anatomical aspects and stalk mechanical traits (breaking force, stalk strength and lodging index). It was found that most vascular parameters were positively associated with breaking force and lodging index with the correlation coefficient r varying from −0.46 to 0.64, whereas stalk strength was only associated with rind area with the r = 0.38. The germplasm resources can be divided into two contrasting categories (classes I with 23 accessions and II with 30 accessions). Compared to class II, the class I was characterized by a larger number (+40.7%) and bigger vascular bundle (+30%), thicker stem (+19.6%) and thicker rind (+36.0%) but shorter internode (plant) (−91.0%). This study provides the methodology and information for the studies of the stem anatomical parameters in crops and facilitates the selective breeding of sorghum.
1. Introduction
Lodging is defined as the permanent displacement of plant shoots from an upright position due to internal and external factors [1]. Crop lodging reduces grain yield and deteriorates grain quality [2,3,4]. Sorghum (Sorghum bicolor L.) is a staple food source and the fifth most important major cereal, with end-uses as diverse as food, feed, fuel and forage [5,6]. In forage sorghum, yield losses of about 40% have been reported due to stalk lodging [7]. The severity of lodging depends upon both plant characteristics and environmental factors. Studies have shown that crop lodging mainly depends on plant morphology and stalk characteristics [8,9,10,11]. Genetic studies have further indicated that the improvement in the stem or root properties provides a promising strategy for lodging resistance [12,13,14]. It is of great significance to assess the risks of lodging and stem characteristics for breeding new varieties of sorghum.
Studies on the relationship between the culm characteristics and lodging resistance have mainly been focused on some staple crops such as wheat (Triticum aestivum L.), maize (Zea mays L.) and rice (Oryza sativa L.) [15,16,17,18]. Previous studies have clearly indicated that stalk strength is a complex trait which includes the mechanical elasticity and rigidity of the stalk and is closely associated with stem morphological, cell wall chemical and anatomical traits [19,20,21,22,23]. The thickness of sclerenchyma, the quantity, the shape and even the distribution of vascular bundles could affect the lodging resistance of a stalk [18,24,25]. Several previous studies have focused on the anatomical features associated with lodging resistance in maize [26,27]. The cross-sectional morphology of stalks including rind thickness and diameter were reported as the key determinants of stalk bending strength and lodging resistance in maize [19,28,29,30]. The thickness of sclerenchyma is positively correlated with lodging resistance [31,32,33]. A larger proportion of fiber bundle area to stem area will benefit the higher lodging resistance. In addition, the importance of chemical composition and fine anatomical structure in determining stem mechanical properties has been recognized [34,35,36]. However, it should be noted that most results were based on C3 plants such as wheat and rice which have hollow stems differing with C4 plants [18]. Few studies have been reported on the analysis of lodging resistance from the stem structure in sorghum due to the lack of simple techniques for cross-sectional phenotyping on a large scale. Fortunately, a simple and cost-effective method for measuring cross-sectional morphology has recently been developed and applied to several plant samples including sorghum (Sorghum bicolor) [37]. However, only three parameters were investigated in the study and relationship of these parameters and stalk mechanical properties remains unexplored.
In this study, 58 sorghum germplasm accessions with rich genetic diversity assessing two different planting seasons were selected. Three stalk traits, including breaking force, stalk strength and lodging index, were used to evaluate the mechanical properties of the sorghum stalks. In addition, facilitated with a novel method, the parameters associated with vascular bundles and sclerenchyma of stem slices were generated. Thereafter, the relationship between stalk mechanical properties and the anatomical parameters of stems was explored, which provides the reference for selecting lodging resistance superior germplasm resources.
2. Materials and Methods
2.1. Plant Materials
A total of 58 sorghum germplasm accessions collected from China (Sichuan, Guizhou, Hunan, Jilin and Liaoning), United States of America and India were used in this study (Table 1). The materials were planted at the experimental farm on the campus of Guangxi University for two successive growing seasons in 2021. In the first season, the sorghum germplasm accessions were direct-seeded in the field on 17 March 2021 and were harvested on 1 August 2021. In the second season, the seeds of germplasm accessions were sowed in plots at green house first, then 15 days later the seedlings were transplanted into the field on 12 September 2021 and were harvested on 10 January 2022. Twenty individuals from each genotype were grown in two rows with a distance of 30 cm between plants in each row and 60 cm between rows. Field management essentially followed the local sorghum cropping practices. The lines were harvested individually at maturity to prevent seed contamination among lines.

Table 1.
Germplasm resources used in this study.
2.2. The Measurement of Plant Morphological Traits and Stem Mechanical Properties
At the stage of the wax ripening, 10 whole plants of each sorghum germplasm accessions were harvested and measured for stalk traits, including plant height, plant fresh weight, sixth internode length and sixth internode diameter (Figure 1). Plant height (cm) was measured as the length from the ground to the apex of the plant panicle. The fresh weight per plant (g) was measured as the weight of aerial part of a whole plant including the weight of the panicle, stalk and seeds. Stem breaking force refers to the force (N) when the indenter is pressed against the middle of the internode at a slow and uniform speed perpendicular to the stalk axis direction until it breaks. The sixth internodes of three plants from each sorghum germplasm accessions were subjected to a three-point bending test (3PBT) according to previous studies measuring the breaking force (BF) by using a prostrate tester (YYD-1, China) [38,39,40]. In detail, the distance between the two fulcra was set to the distance of two nodes of the sixth stem segments and the breaking site was arranged at the center of the sixth internode.
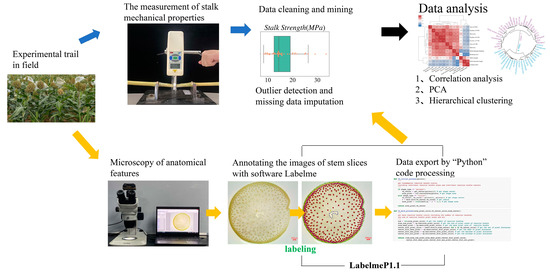
Figure 1.
The pipeline for dataset collection, image processing and data analysis.
The following formula was used to calculate the stalk strength (STR, reported in MPa). STR was considered as the maximum stress required to break the structural integrity of the stem [41] and is given by , where F2 is the force required to induce breakage (N, breaking force), Lin is internode length (mm), γ is internode radius (mm) and I is the second moment of an area (with units of mm4) quantifies the resistance to bending provided by cross-sectional geometry and size. For beams with a solid circular cross-sectional geometry, I was given by the formula , where D is the diameter of the internode. Since precondition of the formula of STR is that the ratio of the length to the diameter should be above 10, the population size of the first season and second season were reduced to 27 and 40, respectively.
The lodging index (LI) was proposed to measure the lodging susceptibility of the lower culm internodes [23,37,42,43]. LI has been defined by calculating the moment of bending divided by the breaking resistance (here calculated as plant height × plant fresh weight/breaking force) according to a previous study [44].
2.3. Microscopy of Anatomical Features
A total of 116 images of stem cross-section slices were collected from the sorghum at booting stage (Figure 1). The middle parts of the second internodes were cut into slices with 0.2 to 0.4 mm thickness by hand. Subsequently, the fresh slice samples were dipped into a solution of 5% phloroglucinol [ethyl alcohol: water = 95:5, (v/v)] and concentrated hydrochloric acid for 15 s for staining. All slices were photographed with an OLYMPUS SZ61 stereoscopic microscope with the magnification is 6.7× to 15×, The resolution is 1790 pixels × 1370 pixels, and the images were saved as BMP files.
2.4. Annotating the Images of Stem Slices from Sorghum Stalks
We used the software “Labelme” to draw contours and to label the classes of vascular bundles and functional zones as annotations in digital images (Figure 1). The images are annotated by “Labelme” to form corresponding json format files. This file holds the attributes associated with each labeled object, including the object label, the object shape and the object contour points. In this study, the object labels included five categories: large vascular bundle, small vascular bundle, stem, rind and cavity. Two kinds of object shapes, polygon and circle, were adopted. The object contour point is the coordinate point set corresponding to the manual annotation point. Each object is a closed curve.
2.5. “Python” Code Processing to Extract Stem Anatomical Parameters
After importing data with “Python”, the scale of the image was firstly identified and the number of pixels in scale was counted in horizontal direction. The fixed physical length of the scale was divided by the number of pixels to obtain the ratio of the physical size of the picture to the pixel size, which will be used for subsequent unit conversion. After that, OpenCV was called to process the json file. The functions in OpenCV library were used to get the parameters of the objects. The perimeter of an object was calculated with cv2.arclength () in pixel scale, the area of an object was calculated with cv2.contourArea(). Cv2.minenclosingcircle () was used to fit the minimum enclosing circle of an object to calculate the center and radius of the shape. The primary traits of vascular bundle were obtained, and then the traits including the number, area and distance of vascular bundle were obtained by unit conversion (Figure 1). The Python codes were provided as Supplementary Material in PDF format (Figure S1).
2.6. Statistical Analysis and Visualization Methods
Python (version 3.9.12) and Excel were used for data collation and statistical analysis (Figure 1). Thermal maps and boxplots were drawn with Matplotlib (version 3.5.1) and the Seaborn (version 0.11.2) loaded in Python. Principal components were calculated with the PRCOMP loaded in R (version 4.1.0). FactoMineR (version 2.4) was used to extract the variance contribution rates of the principal component and the variable contribution rate to each principal component. Ape (version 5.6–2) was used to draw circular cluster maps.
3. Results
3.1. Statistics of the Stem Mechanical Properties of Sorghum Germplasm Resources
The average diameter of the sixth internode was 12.6 mm in the first season (S1), and 11.5 mm in the second season (S2) (Table 2). The average length of the sixth internode was 132.8 mm (S1) and 151.1 mm (S2). The average breaking force was 99.2 N (S1) and 76.4 (S2). The average stalk strength was 17 MPa (S1) and 16 MPa (S2). The average lodging index (LI) was 620.0 cm·g·N−1 (S1) and 886.9 cm·g·N−1 (S2). The diameter and strength had relatively small variation, while the other traits had larger variation with the coefficients of variation (CV) ranging from 20% to 63% (S1) and from 18% to 77% (S2) in two seasons, respectively (Table 2). Despite some slight differences in the values of the traits between two seasons, the correlations of the same traits were all highly significant (r = 0.48 * to 0.86 **), indicating the similarity in the phenotypes of plants in two seasons (Table 2). Most traits were in normal distribution except the average area of small vascular bundles in the first season (Table 2).

Table 2.
Statistics of mechanical properties and relevant stalk traits of sorghum germplasm resources.
3.2. Statistics of Stem Cross-Sectional Parameters of Sorghum Germplasm Resources
The number of large vascular bundle (LVB) ranged from 56–349 in the first season (S1), and 89–390 in the second season (S2) (Table 3). The number of small vascular bundle (SVB) was ranged at 64–274 in S1 and at 78–282 in S2. Obviously, the number of LVB was much higher than SVL. The total number of vascular bundles (TVB) was ranged at 120–570 in S1 and at 167–672 in S2. The average area of LVB and SVB was 0.015 mm2 in S1 (0.023 mm2 in S2) and was 0.006 mm2 in S1 (0.009 mm2 in S2), respectively. The stem area was 33.7 mm2 in S1 and 66.9 mm2 in S2. The average rind area was 4.3 mm2 in S1 and 7.6 mm2 in S2. With the exception of stem diameter (CV = 20.8% in S1 and CV = 18.6% in S2), significant phenotypic variation was detected in the vascular bundle traits among accessions with the CVs ranging from 30% to 50% in S1 and 24% to 47.4% in S2 (Table 3).

Table 3.
Statistics of stem cross-sectional parameters of sorghum germplasm resources.
Compared with the mean values of all vascular bundle parameters in the first season, the parameters in second were larger, whereas the CV of the second season was slightly smaller. The correlation of the same cross-sectional parameters between two seasons was significant (r values mostly about 0.5), essentially indicating the data from the two seasons had the same tendency (Table 3). The average area of large (small) vascular bundle was right-skewed distribution, and the others were normal (Table 3).
3.3. Correlation of Stalk Mechanical Properties with the Stem Cross-Sectional Parameters in Sorghum Germplasm
The relationship of stalk mechanical properties with multiple stem-related traits is of great interest. Here, in the first season, it was found that the breaking force (BF) was positively associated with most vascular bundle parameters, especially significant was the number of LVB, TVB and SVB, but not significantly correlated with the average area of vascular bundles (Figure 2). In addition, the BF was positively correlated with stalk internode diameter (r = 0.75 in S1 and r = 0.71 in S2), but negatively correlated with internode length. Stalk strength is a pressure per unit of cross-sectional geometry and size. In contrast with the BF, the stalk strength is not significantly correlated with most vascular bundle parameters. Interestingly, it was positively correlated with the rind area in both seasons (r = 0.38 in S1 and r = 0.32 in S2) and negatively with the internode diameter (r = −0.45 in S1 and r = −0.6 in S2). It can be preliminarily concluded that increased number of vascular bundles will not increase the stem strength, but increased rind (peripheral sclerenchyma) thickness will increase the stem strength. Lodging index (LI) reflects the genotype being prone to lodging, which was negatively correlated with most vascular indexes, but was positively correlated with internode length (r = 0.85). Since internode length and height are significantly and positively correlated, it is understood that higher plant height has a greater risk of lodging in sorghum germplasm. Stem diameter was positively correlated with all vascular traits, indicating that the number and area of vascular bundles increased with thicker stem. The thicker the stems were, the more vascular bundle number and area were in sorghum germplasm. Internode length and LI were negatively correlated with the number of vascular bundles and stem area. The correlation of traits in two seasons was basically the same with each other, despite the r values being slightly varied. Similarly, BF were positively correlated with all cross-sectional parameters in both seasons. Salk strength was positively and significantly correlated with the sixth internode length (r = 0.51 *) in the second season, and the positive correlation was also observed but did not reach the significant level in the first season (r = 0.32). LI was negatively correlated with all vascular traits, indicating that the increase in vascular bundle will decrease the lodging risk (Figure 3).
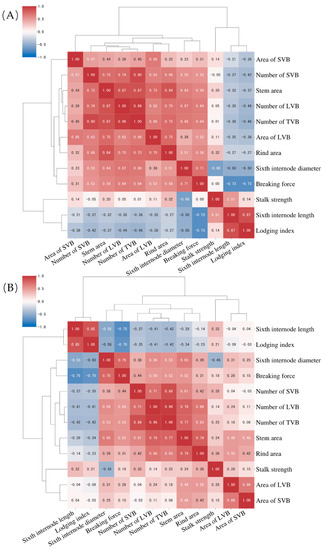
Figure 2.
Heat-map showing the correlation of stalk mechanical properties with the stem cross-sectional parameters in sorghum germplasm: (A) first season (df = 51, r0.05 = 0.271); (B) second season (df = 56, r0.05 = 0.269).
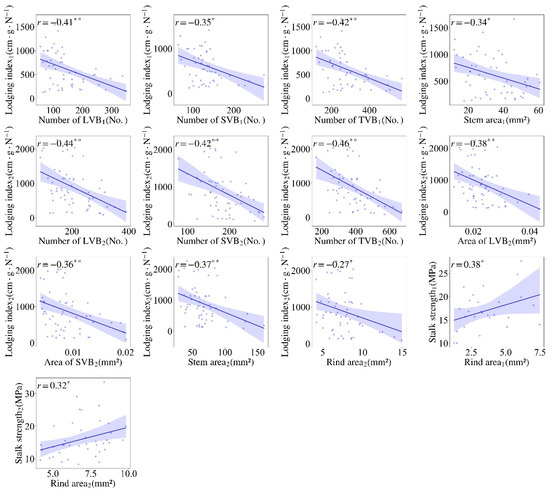
Figure 3.
Scatter plots to illustrate the significant correlation between the stem cross-sectional parameters and stalk mechanical properties in sorghum (the subscript numbers “1” and “2” after the traits indicated season 1 and season 2, respectively). * and ** significant at p ≤ 0.05 and ≤0.01.
3.4. The Principal Components Analysis of the Stalk Mechanical Properties and Stem Cross-Sectional Parameters
The variance contributions of the top three principal components were 51.5%, 17.2% and 11.8% (totaling 80.5%) in S1, and 60.4%, 13.6% and 9% (totaling 83%) in S2 (Table 4). The stem area, the rind area, the sixth internode diameter and the number of vascular bundles were the variables with high contributions to the first principal component (PC1) in both seasons. It was speculated that the PC1 reflects the stem thickness and the resulting changes in the number of vascular bundles. The sixth internode length, the average area of large or small vascular bundles and the lodging index counted more than other traits to the second principal component (PC2) in S1. It might reflect the correlation effect brought by the internode length. The variables that contributed more to the third principal component (PC3) in S1 were the number of vascular bundles, the average area of vascular bundles, the sixth internode length and the mechanical traits including the breaking force and the lodging index which depend on these aforesaid variables. The sixth internode length, the breaking force and the lodging index weighed more to the PC2 in S2. The sixth internode diameter and the average area of large or small vascular bundles contributed more to the PC3 in S2. Compared with S1, the contribution of traits to the principal components in S2 was clearer. The principal component analysis showed the differences among the sorghum germplasm resources were firstly in the number of vascular bundles and stem thickness and secondly by the internode length and the lodging index.

Table 4.
The variance contributions of sorghum germplasm resources.
3.5. Two Contrast Categories Were Obtained and Compared
After normalization and integration of the traits and parameters from two seasons, the sorghum germplasm resources can be divided into two contrasting categories. Class I, consisting of 23 germplasm accessions, is characterized by a larger number of vascular bundles (LVB, SVB and TVB), larger vascular bundle area, larger stem area, thicker stem, thicker rind but shorter internode, which is lodging resistant with both higher breaking force and higher stalk strength (Figure 4). These materials were collected from India, North China, Sichuan and Guizhou. Class II was contrary to class I in terms of the above traits and parameters, with a total of 30 accessions, of which 24 (73%) were local varieties in Guizhou.
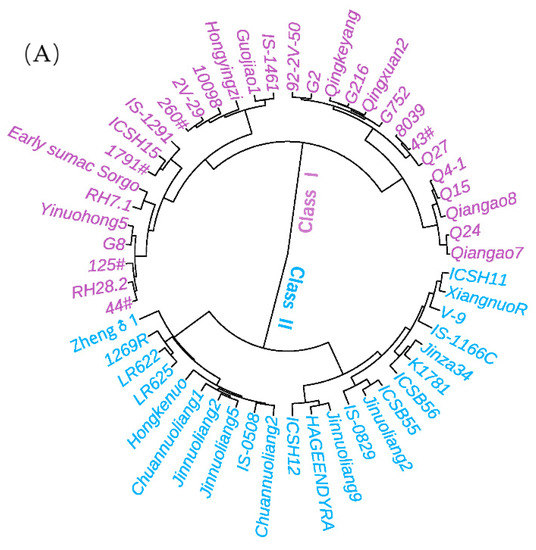
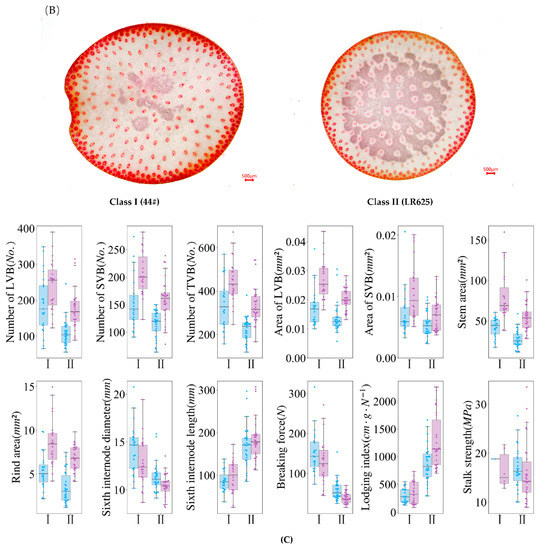
Figure 4.
The differences in two classes of sorghum resources: (A) clustering analysis of sorghum resources (pooled data of two seasons); (B) anatomical sections of the two representative genotypes from class I (left) and class II (right); (C) box-plots to illustrate the differences between class I and class II (season 1 is in blue, season 2 is in purple).
4. Discussion
4.1. The Development of a Novel Procedure Allowed the High-Throughput Extraction of the Anatomical Parameters
Many studies have indicated that stem-related traits, such as basal internode length and thickness, stem wall thickness, leaf sheath covering and thickness, contribute to culm strength and lodging resistance [38,45]. In maize, it was reported that the cross-sectional morphology of stalks, such as rind thickness and diameter are key determinants of stalk bending strength and lodging resistance [19,28,29,30]. Efforts to understand the mechanism of lodging and to improve stem strength and its related components should be an important focus in future sorghum breeding for lodging resistance. However, few studies have been reported in sorghum due to the lack of suitable analysis methods on a large scale. Until recently, a new phenotyping methodology was developed to quantify two-dimensional plant cross sections in a range of plant samples including sorghum (Sorghum bicolor) (maize (Zea mays L.), wheat (Triticum aestivum L.) and Arabidopsis (Arabis thaliana L.) [37]. The new methodology employs an inexpensive stereoscope and a semi-automated image processing algorithm which can be used to produce specimen specific, dimensionally accurate computational models of plant stalks. In this study, we reported a novel procedure by integrating the image annotation software “Labelme” and code processing language “Python” to produce the parameters of vascular bundles and regions of stem slices. The technique is simple, cost-effective and in a high-throughput manner, can be applied to a range of plant samples to investigate the relationship of the vascular bundles and mechanical properties in a sorghum germplasm population. Moreover, compared with the previous study which only generated three types of parameters (diameter, rind thickness and number of vascular bundles), our procedure can generate more parameters including the area of each region and the number, size, location, proportion and density of vascular bundle. For example, our procedure can be extended to produce the distance of each vascular bundles from the central point of a stem slice which might not differ much among the plant species. Our procedure can accurately measure the circumference and the areas of a stem even that the shape of stem circle is irregular. Using a common office laptop (macOS version 10.14.6, processor 2.7 GHZ Intel Core i7), the method only needed 16 s to calculate nine parameters for 58 samples in this study and took 4.7 min for 1000 samples to export 26 parameters traits (simulation experiment). The speed of method mainly depends on the number of plant samples (annotated images).
4.2. The Relationship of the Traits and Parameters of the Sorghum Stem and the Inspiration for Breeding
One of the highlights in this study was that it adapted the three mechanics traits to evaluate the lodging related properties, including breaking force (BF), stalk strength and lodging index (LI). The differences in these indicators can be inferred from their calculation formulas. The BF is intuitive, simple, with the mechanical factor being measured directly with a dynamometer. BF as an index of lodging resistance was used in some previous studies [18,22,46]. However, we know it is affected by many factors, as was found in this study. These factors could include the morphological feature of a stalk as a whole (length, diameter and area etc.) in addition to the chemical composition (material), texture and inner structure (geometric) of a stalk [22,23,47,48,49,50]. It is of interest to investigate the per unit force of a material or tissue (stalk strength here) which is independent of the size (diameter and area) of items and is valuable in breeding programs. In this study, we found that the variation of stalk strength is not large among the germplasm accessions, indicating the homogeneous material properties of the sorghum stalk. This means that the increased breaking force largely depends on the larger size of tissues and organs. It is reasonable that the thicker stem together with the larger number of vascular bundles benefits the stalk breaking force. The result was similar to the finding in maize, whereby maximum stresses were much more sensitive to the changes in dimensions of the stalk cross-section than they were to changes in material properties of stalk components [19]. These results suggested that the breeding of bioenergy sorghum (or feed sorghum) varieties, where the increased digestibility is typically associated with low structural strength and a propensity for lodging, can be counterbalanced by the increases in stalk diameter. One of the significant findings in this study was that stalk strength was significantly correlated with the thickness of rind (peripheral sclerenchyma), composed of a thickened cell wall with great mechanical strength. The result was similar with the findings in wheat [18,51] and maize [25,37]. Therefore, these results suggested that the rind thickness is a critical trait for preventing lodging in crops including grain sorghum. Since lodging is also considered to result from the imbalance between the weight of the upper plant parts and the sturdiness of the basal parts, a lodging index was proposed to measure the lodging susceptibility of the lower culm internodes [23,37,42,43]. Compared to the breaking force and stalk strength, the LI combined the effects of major internal forces of plants and was widely used in both field crop production and academic research. The data of the LI obtained in this study were effective for the evaluation of the lodging resistant performance of the sorghum germplasm resources. As a result, we found that the resources in class I have stronger lodging resistance and the in-depth investigation of these resources will benefit the breeding of the elite sorghum varieties.
5. Conclusions
With the “LabelmeP1.1” method, which combined the image-annotation function of a commercial software “Labelme” with popular coding language “Python“, this study achieved a high-throughput extraction of nine parameters related to sorghum stalk regions and vascular bundles. The diversity of vascular bundle parameters and their relationships in 58 sorghum germplasm accessions grown in two seasons were evaluated. It is clear that vascular traits (number, area and density) were closely related to stalk mechanical traits (breaking force, stalk strength and lodging index) with the r values varying from −0.46 to 0.64. One of the striking findings was that stalk strength was significantly correlated with the rind thickness, suggesting a new approach for preventing lodging in sorghum. Based the morphology and vascular bundle parameters, sorghum germplasm accessions can be divided into two contrasting categories, which will be helpful to understand the stem properties of materials for breeding purpose. In conclusion, high-throughput phenotyping of cross-sectional morphology developed in this study (16 s for 58 annotated images) will increase our ability to investigate the stem anatomical parameters and thus will facilitate the structural engineering of the stalk mechanical strength in crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12101696/s1, Figure S1: The Python code developed in this study to extract stem anatomical parameters.
Author Contributions
Conceptualization, C.M. and L.W.; data curation, R.X. and L.W.; formal analysis, H.F., R.X. and L.W.; funding acquisition, S.L., C.M. and L.W.; investigation, H.F., S.S. and M.Y.; methodology, H.F. and L.W.; project administration, B.F.; resources, M.Y., S.L., R.Z., C.M. and L.W.; software, R.X.; supervision, L.W.; validation, J.W. (Jibin Wang), B.F., J.W. (Jihong Wang) and L.W.; visualization, R.X. and L.W.; writing—original draft, H.F., J.W. (Jibin Wang), J.W. (Jihong Wang), R.X. and L.W.; writing—review and editing, J.W. (Jibin Wang), J.W. (Jihong Wang), R.X. and L.W. All authors will be informed about each step of manuscript processing including submission, revision and revision reminders via emails from our system or assigned by our Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Guangxi (No. 2020GXNSFDA238027), and by Guangxi innovation driven development project (No. AA20302020-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berry, P.; Sylvester-Bradley, R.; Berry, S. Ideotype design for lodging-resistant wheat. Euphytica 2007, 154, 165–179. [Google Scholar] [CrossRef]
- Setter, T.; Laureles, E.; Mazaredo, A. Lodging reduces yield of rice by self-shading and reductions in canopy photosynthesis. Field Crops Res. 1997, 49, 95–106. [Google Scholar] [CrossRef]
- Berry, P.; Spink, J. Predicting yield losses caused by lodging in wheat. Field Crops Res. 2012, 137, 19–26. [Google Scholar] [CrossRef]
- Rutto, L.K.; Xu, Y.; Brandt, M.; Ren, S.; Kering, M.K. Juice, ethanol, and grain yield potential of five sweet sorghum (Sorghum bicolor [L.] Moench) cultivars. J Sustain. Bioenergy Syst. 2013, 3, 113. [Google Scholar] [CrossRef]
- Byrt, C.S.; Grof, C.P.; Furbank, R.T. C4 plants as biofuel feedstocks: Optimising biomass production and feedstock quality from a lignocellulosic perspective. J. Integr. Plant Biol. 2011, 53, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Li, Z.; Leng, C.; Lu, C.; Luo, H.; Liu, Y.; Wu, X.; Liu, Z.; Shang, L.; Jing, H.C. Sorghum breeding in the genomic era: Opportunities and challenges. Theor. Appl. Genet. 2021, 134, 1899–1924. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Sayre, K.; Kaul, J.; Narang, R. Lodging behavior and yield potential of spring wheat (Triticum aestivum L.): Effects of ethephon and genotypes. Field Crops Res. 2004, 87, 207–220. [Google Scholar] [CrossRef]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Haberer, G.; Hellsten, U.; Mitros, T.; Poliakov, A.; et al. The Sorghum bicolor genome and the diversification of grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Y.-h.; Wang, C.-y.; Yin, Y.-p.; Ning, T.-y.; Shi, C.-y.; Li, Y.; Wang, Z.-l. Effects of Nitrogen and PP333 Application on the Lignin Synthesis of Stem in Relation to Lodging Resistance of Wheat (in chinese). Sci. Agric. Sin. 2011, 44, 3529–3536. [Google Scholar]
- Li, G.-h.; Zhong, X.-h.; Tian, K.; Huang, N.-r.; Pan, J.-f.; He, T.-h. Effect of Nitrogen Application on Stem Lodging Resistance of Rice and Its Morphological and Mechanical Mechanisms (in chinese). Sci. Agric. Sin. 2013, 46, 1323–1334. [Google Scholar]
- Liu, C.; Zheng, S.; Gui, J.; Fu, C.; Yu, H.; Song, D.; Shen, J.; Qin, P.; Liu, X.; Han, B. Shortened basal internodes encodes a gibberellin 2-oxidase and contributes to lodging resistance in rice. Mol. Plant 2018, 11, 288–299. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Ishimaru, K. Identification and functional analysis of a locus for improvement of lodging resistance in rice. Plant Physiol. 2004, 134, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.; Guo, H.; Xiao, S.; Jiang, G.; Zhang, X.; Yan, C.; Xin, Z.; Jia, J. Quantitative trait loci (QTL) of stem strength and related traits in a doubled-haploid population of wheat (Triticum aestivum L.). Euphytica 2005, 141, 1–9. [Google Scholar] [CrossRef]
- Huang, X.; Cloutier, S.; Lycar, L.; Radovanovic, N.; Humphreys, D.; Noll, J.; Somers, D.; Brown, P. Molecular detection of QTLs for agronomic and quality traits in a doubled haploid population derived from two Canadian wheats (Triticum aestivum L.). Theor. Appl. Genet. 2006, 113, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-H. The expression of caffeic acid 3-O-methyltransferase in two wheat genotypes differing in lodging resistance. J. Exp. Bot. 2009, 60, 2763–2771. [Google Scholar] [CrossRef]
- Yang, Q.; Ran, W.; Li, L.; Chen, J.; Zheng, H.; Ma, Y.; Mao, J.; Zheng, B.; Shao, R. Correlation and path analysis of lodging resistance with maize stem characters (in chinese). J. Henan Agric. Univ. 2016, 50, 167–170. [Google Scholar]
- Hu, W.; Zhang, Y.e.; Zhao, H.; Wang, X.; Cao, T.; Cao, Y.; Chen, Y.; Yang, J. Comparison and Analysis of the Methods for Evaluating Wheat Lodging Resistance (in chinese). Acta Agric. Boreali-Occident. Sin. 2018, 27, 1780–1788. [Google Scholar]
- Muhammad, A.; Hao, H.; Xue, Y.; Alam, A.; Bai, S.; Hu, W.; Sajid, M.; Hu, Z.; Samad, R.A.; Li, Z.; et al. Survey of wheat straw stem characteristics for enhanced resistance to lodging. Cellulose 2020, 27, 2469–2484. [Google Scholar] [CrossRef]
- Forell, G.V.; Robertson, D.; Lee, S.Y.; Cook, D.D. Preventing lodging in bioenergy crops: A biomechanical analysis of maize stalks suggests a new approach. J. Exp. Bot. 2015, 66, 4367–4371. [Google Scholar] [CrossRef]
- Yao, J.; Ma, H.; Zhang, P.; Ren, L.; Yang, X.; Yao, G.; Zhang, P.; Zhou, M. Inheritance of stem strength and its correlations with culm morphological traits in wheat (Triticum aestivum L.). Can. J. Plant Sci. 2011, 91, 1065–1070. [Google Scholar] [CrossRef]
- Kong, E.; Liu, D.; Guo, X.; Yang, W.; Sun, J.; Li, X.; Zhan, K.; Cui, D.; Lin, J.; Zhang, A. Anatomical and chemical characteristics associated with lodging resistance in wheat. Crop J. 2013, 1, 43–49. [Google Scholar] [CrossRef]
- Li, F.; Zhang, M.; Guo, K.; Hu, Z.; Zhang, R.; Feng, Y.; Yi, X.; Zou, W.; Wang, L.; Wu, C. High-level hemicellulosic arabinose predominately affects lignocellulose crystallinity for genetically enhancing both plant lodging resistance and biomass enzymatic digestibility in rice mutants. Plant Biotechnol. J. 2015, 13, 514–525. [Google Scholar] [CrossRef]
- Li, F.; Xie, G.; Huang, J.; Zhang, R.; Li, Y.; Zhang, M.; Wang, Y.; Li, A.; Li, X.; Xia, T. Os CESA 9 conserved-site mutation leads to largely enhanced plant lodging resistance and biomass enzymatic saccharification by reducing cellulose DP and crystallinity in rice. Plant Biotechnol. J. 2017, 15, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Song, Y.; Wu, Q.; Ma, C.; Zhao, J.; Wan, Y.; Zhao, G. Relationship between stem characteristics and lodging resistance of Tartary buckwheat (Fagopyrum tataricum). Plant Prod. Sci. 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Wang, X.; Mace, E.; Tao, Y.; Cruickshank, A.; Hunt, C.; Hammer, G.; Jordan, D. Large-scale genome-wide association study reveals that drought-induced lodging in grain sorghum is associated with plant height and traits linked to carbon remobilisation. Theor. Appl. Genet. 2020, 133, 3201–3215. [Google Scholar] [CrossRef]
- Qunying, W.; Changhao, H. Studies on the anatomical structures of the stalks of maize with different resistance to lodging (in chinese). Acta Agron. Sin. 1991, 17, 72–75. [Google Scholar]
- Zhou, H.-Y.; Jiang, Y.-F.; Yang, M.-C.; Cheng, W.-D.; Qin, L.-Q.; Xie, X.-D.; Xie, H.-X.; Qin, B.-X.; Wang, L.-Q. Evaluation of Stalk Strength, Vascular Bundle and Fiber in Maize. J. Plant Genet. Resour. 2022, 1–8. (In Chinese) [Google Scholar] [CrossRef]
- Robertson, D.J.; Julias, M.; Lee, S.Y.; Cook, D.D. Maize stalk lodging: Morphological determinants of stalk strength. Crop Sci. 2017, 57, 926–934. [Google Scholar] [CrossRef]
- Stubbs, C.J.; McMahan, C.; Seegmiller, W.; Cook, D.D.; Robertson, D.J. Integrated Puncture Score: Force–displacement weighted rind penetration tests improve stalk lodging resistance estimations in maize. Plant Methods 2020, 16, 113. [Google Scholar] [CrossRef]
- Stubbs, C.J.; Seegmiller, K.; McMahan, C.; Sekhon, R.S.; Robertson, D.J. Diverse maize hybrids are structurally inefficient at resisting wind induced bending forces that cause stalk lodging. Plant Methods 2020, 16, 67. [Google Scholar] [CrossRef]
- van Heerden, P.D.; Donaldson, R.A.; Watt, D.A.; Singels, A. Biomass accumulation in sugarcane: Unravelling the factors underpinning reduced growth phenomena. J. Exp. Bot. 2010, 61, 2877–2887. [Google Scholar] [CrossRef]
- Xiong, S.; Wu, Y.; Wang, X.; Yu, X.; Meng, X.; Zhang, J.; Ma, X. Differences of Stem Main Inclusion Analysis of Wheat Varieties with Different Height and Lodging Resistance (in chinese). J. Triticeae Crops 2017, 37, 1187–1194. [Google Scholar]
- Gomez, F.E.; Mullet, J.E.; Muliana, A.H.; Niklas, K.J.; Rooney, W.L. The genetic architecture of biomechanical traits in sorghum. Crop Sci. 2020, 60, 82–99. [Google Scholar] [CrossRef]
- Tanaka, K.; Murata, K.; Yamazaki, M.; Onosato, K.; Miyao, A.; Hirochika, H. Three distinct rice cellulose synthase catalytic subunit genes required for cellulose synthesis in the secondary wall. Plant Physiol. 2003, 133, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, Y. Rice brittleness mutants: A way to open the ‘Black Box’of monocot cell wall biosynthesis free access. J. Integr. Plant Biol. 2011, 53, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xia, T.; Li, G.; Li, X.; Li, Y.; Wang, Y.; Wang, Y.; Chen, Y.; Xie, G.; Bai, F.-W. Overproduction of native endo-β-1, 4-glucanases leads to largely enhanced biomass saccharification and bioethanol production by specific modification of cellulose features in transgenic rice. Biotechnol. Biofuels 2019, 12, 11. [Google Scholar] [CrossRef]
- Oduntan, Y.A.; Stubbs, C.J.; Robertson, D.J. High throughput phenotyping of cross-sectional morphology to assess stalk lodging resistance. Plant Methods 2022, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Peng, S.; Visperas, R.M.; Ereful, N.; Bhuiya, M.S.U.; Julfiquar, A. Lodging-related morphological traits of hybrid rice in a tropical irrigated ecosystem. Field Crops Res. 2007, 101, 240–248. [Google Scholar] [CrossRef]
- Gomez, F.E.; Carvalho, G., Jr.; Shi, F.; Muliana, A.H.; Rooney, W.L. High throughput phenotyping of morpho-anatomical stem properties using X-ray computed tomography in sorghum. Plant Methods 2018, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.-N.; Yan, J.-Q.; Hai-Bo, L.; Zhao, H.-C.; Huang, Z.-H. Response of Stem Microstructure of Different Spring Maize Varieties to Bending Strength. J. Maize Sci. 2020, 28, 53–59. (In Chinese) [Google Scholar]
- Niklas, K.J. Plant Biomechanics: An Engineering Approach to Plant Form and Function; University of Chicago Press: Chicago, IL, USA, 1992. [Google Scholar]
- Crook, M.; Ennos, A. Stem and root characteristics associated with lodging resistance in four winter wheat cultivars. J. Agric. Sci. 1994, 123, 167–174. [Google Scholar] [CrossRef]
- Fan, C.; Feng, S.; Huang, J.; Wang, Y.; Wu, L.; Li, X.; Wang, L.; Tu, Y.; Xia, T.; Li, J. AtCesA8-driven OsSUS3 expression leads to largely enhanced biomass saccharification and lodging resistance by distinctively altering lignocellulose features in rice. Biotechnol. Biofuels 2017, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Zhu, Q.; Wang, Y.; Inoue, N.; Tanaka, H. Case studies on high yields of paddy rice in Jiangsu Province, China: I. Characteristics of grain production. Jpn. J. Crop Sci. 1993, 62, 267–274. [Google Scholar]
- Zhu, L.; Zhong, D.; Xu, J.; Yu, S.; Li, Z. Differential expression of lodging resistance related QTLs in rice (Oryza sativa L.). Plant Sci. 2008, 175, 898–905. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, F.; Wang, M.; Li, X.; Zhang, M.; Huang, J. Accurate Evaluation and Mechanism Analysis of Mechanical Strength of Sugarcane Stalk. Chin. J. Trop. Crops 2021, 43, 207. (In Chinese) [Google Scholar]
- Lemloh, M.-L.; Pohl, A.; Weber, E.; Zeiger, M.; Bauer, P.; Weiss, I.M.; Schneider, A.S. Structure-property relationships in mechanically stimulated Sorghum bicolor stalks. Bioinspired Mater. 2014, 1, 1–11. [Google Scholar] [CrossRef]
- Gomez, F.E.; Muliana, A.H.; Rooney, W.L. Predicting Stem Strength in Diverse Bioenergy Sorghum Genotypes. Crop Sci. 2018, 58, 739–751. [Google Scholar] [CrossRef]
- Yan, D. Genome-Wide Association Analysis of Lodging-Related Traits in Wheat. Master’s Thesis, Shandong Agricultural University, Taian, China, 2019. [Google Scholar]
- Nan, M. Study Expression of Genes in Stem Lignin Synthesis and Physiological Mechanism of Lodging Resistance in Oat. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2021. [Google Scholar]
- Shah, L.; Yahya, M.; Shah, S.M.A.; Nadeem, M.; Ali, A.; Ali, A.; Wang, J.; Riaz, M.W.; Rehman, S.; Wu, W.; et al. Improving Lodging Resistance: Using Wheat and Rice as Classical Examples. Int. J. Mol. Sci. 2019, 20, 4211. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).