Dwarf Interstocks Improve Aroma Quality of ‘Huahong’ Apple (Malus × domestica)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Enrichment of Apple VOCs by Solid-Phase Microextraction (SPME)
2.3. Test Instrument and Conditions of GC-MS
2.4. Identification and Quantitation of VOCs
2.5. Data Analysis
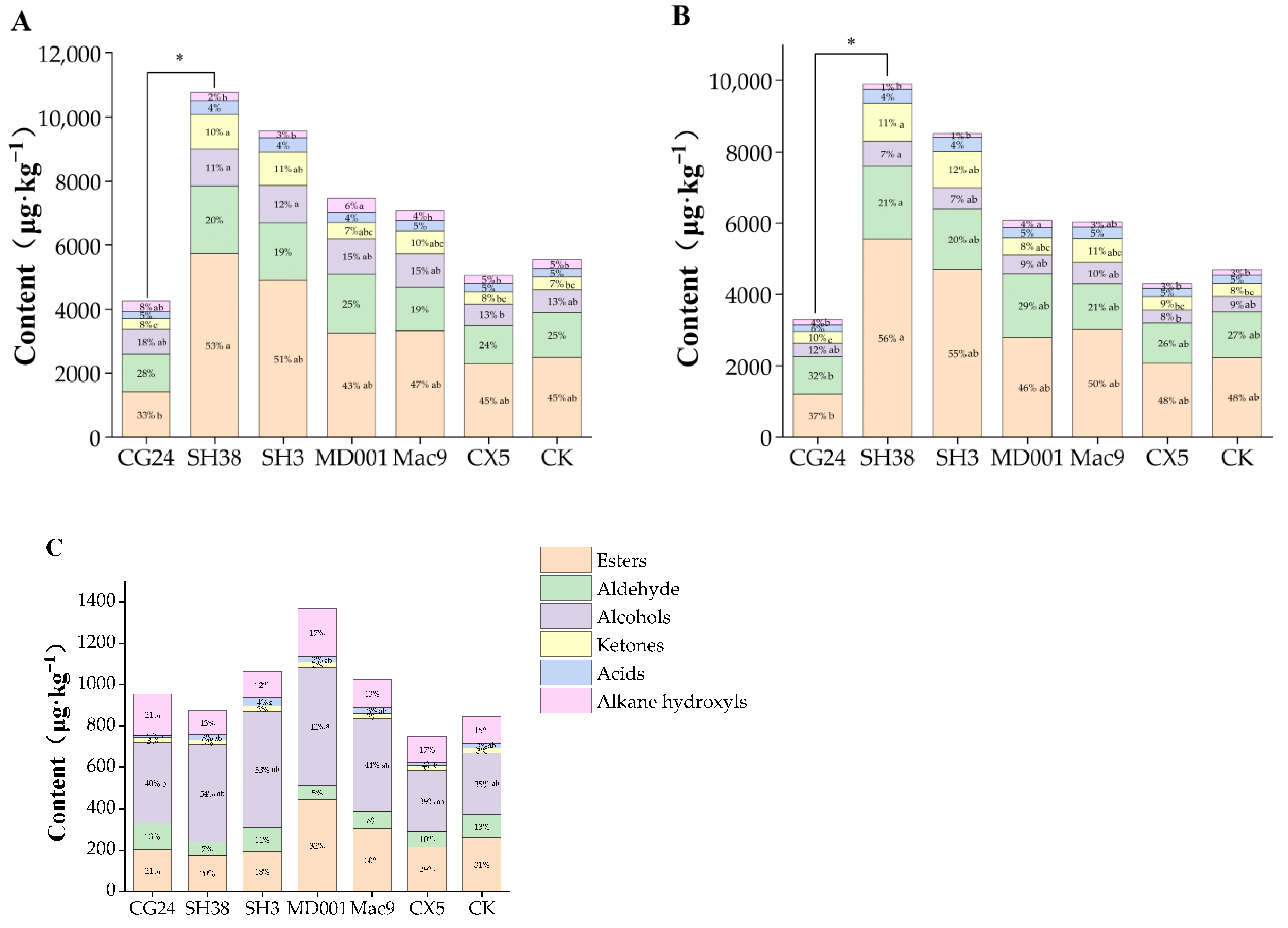
3. Results
3.1. VOCs in ‘Huahong’ Apples
3.1.1. Aroma Profile of ‘Huahong’ Apple Fruits
3.1.2. Difference of VOCs between Skin and Pulp of ‘Huahong’ Apple
3.2. Effects of Dwarf Interstock on VOCs in ‘Huahong’ Apple
3.2.1. Content Differences in Interstock/Scion Combinations
3.2.2. Differences in VOCs in Apple Skin and Pulp of Different Interstock/Scion
Combinations
3.3. Principal Component Analysis
4. Discussion
4.1. Aroma Profile of ‘Huahong’ Apple
4.2. Effects on Aroma of ‘Huahong’ Apples of Different Interstocks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef] [PubMed]
- Tandon, K.S.; Baldwin, E.A.; Shewfelt, R.L. Aroma perception of individual volatile compounds in fresh tomatoes as affected by the medium of evaluation. Postharvest Biol. Technol. 2000, 20, 261–268. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.M.; Tao, R.; Zhang, T.H.; Wang, H.; Wang, S.; Sun, L.L.; Gao, H. Effect of different fruit growing bags on fruit quality of ‘Ruixue’ apple. J. Fruit Sci. 2020, 37, 1326–1335. [Google Scholar] [CrossRef]
- Liang, X.F.; Zhang, R.; Gleason, M.L.; Sun, G.Y. Sustainable apple disease management in China: Challenges and future directions for a transforming industry. Plant Dis. 2022, 106, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, Y.H.; Wang, Z.C.; Li, M.Y. Production efficiency and change characteristics of China’s apple industry in terms of planting scale. PLoS ONE 2021, 16, e0254820. [Google Scholar] [CrossRef]
- Hoying, S.A.; Robinson, T.L. The apple orchard planting systems puzzle. Acta Hortic. 2000, 513, 257–260. [Google Scholar] [CrossRef]
- Sadowski, A.; Mackiewicz, M.; Dziuban, R. Growth and early bearing of apple trees as affected by the type of nursery trees used for planting. Acta Hortic. 2007, 732, 447–455. [Google Scholar] [CrossRef]
- Han, M.Y.; Ma, F.W.; Li, B.Z.; Zhang, L.S.; Li, X.J.; Zhang, L.G. The state of apple development in Italy and France. Northwest Hortic. 2008, 2, 49–50. [Google Scholar]
- Du, M. Isolation and Functional Analysis of MbMYB4 and MbMYB108 in Malus baccata (L.) Borkh. Master’s Thesis, Northeast Agricultural University, Shanxi, China, 2021. [Google Scholar] [CrossRef]
- Maria, L.M. Influence of planting and training systems on fruit yield in apple orchard. J. Fruit Ornam. Plant Res. 2004, 12, 97–104. [Google Scholar]
- Robinson, T.L. Recent advances and future directions in orchard planting systems. Acta Hortic. 2004, 732, 367–381. [Google Scholar] [CrossRef]
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Gili, B.; Zipora, T.; Ron, P. Effects of rootstock/scion combinations on the flavor of citrus fruit. J. Agric. Food Chem. 2013, 61, 11286–11294. [Google Scholar] [CrossRef]
- Jukić Špika, M.; Dumičić, G.; Brkić Bubola, K.; Soldo, B.; Goreta Ban, S.; Vuletin Selak, G.; Ljubenkov, I.; Mandušić, M.; Žanić, K. Modification of the sensory profile and volatile aroma compounds of tomato fruits by the scion × rootstock interactive effect. Front. Plant Sci. 2021, 11, 616431. [Google Scholar] [CrossRef]
- Jin, Z.X.; Sun, H.; Sun, T.Y.; Wang, Q.J.; Yao, Y.X. Modifications of ‘gold finger’ grape berry quality as affected by the different rootstocks. J. Agric. Food Chem. 2016, 64, 4189–4197. [Google Scholar] [CrossRef]
- Marioli, C.Q.; Ana, M.M.G.; Gastón, G.G.; Yerko, M.S. Effect of rootstocks on volatile composition of Merlot wines. J. Sci. Food Agric. 2020, 100, 3517–3524. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Miguel, T.; Alvarez, K.; José, M.O. Rootstock effect on volatile composition of Albariño wines. Appl. Sci. 2021, 11, 2135. [Google Scholar] [CrossRef]
- Seker, M.; Ekinci, N.; Gur, E. Effects of different rootstocks on aroma volatile constituents in the fruits of peach (Prunus persica L. Batsch cv. ‘cresthaven’). N. Z. J. Crop Hortic. Sci. 2016, 45, 1–13. [Google Scholar] [CrossRef]
- Riccardo, L.B.; Vittorio, F.; Giuseppe, A.; Felice, F.; Pasquale, A. Fruit quality and volatile fraction of ‘Pink Lady’ apple trees in response to rootstock vigor and partial rootzone drying. J. Sci. Food Agric. 2008, 88, 1325–1334. [Google Scholar] [CrossRef]
- Gur, E. The effects of different rootstocks on aroma volatile constituents in the fruits of ‘Fuji’ apples (Malus domestica Borkh.). Appl. Ecol. Environ. Res. 2019, 17, 11745–11756. [Google Scholar] [CrossRef]
- Man, S.D.; Cong, P.H. Introduction to three new apple cultivars. North. Fruits 1994, 1, 018. [Google Scholar] [CrossRef]
- Man, S.D.; Niu, J.Z.; Cong, P.H.; Qin, H.L. Breeding of late ripening new apple variety ‘Huahong’. China Fruits 1999, 1, 13–14. [Google Scholar] [CrossRef]
- Meng, H.Z.; Jiang, X.; Chen, X.D.; Li, Z.Y.; Xu, J.Z. Effects of SH40 interstocks and scion-roots on apple root growth and content of endogenous hormones. Acta Hortic. Sin. 2018, 45, 1193–1203. [Google Scholar] [CrossRef]
- Zhou, J.T.; Zhao, D.Y.; Cheng, C.G.; Yan, S. Effects of different dwarfing interstocks on mineral contents in flower, fruitlets and fruit, and fruit quality of ‘Huahong’ apple. China Fruits 2020, 03, 23–27+33. [Google Scholar] [CrossRef]
- Li, Q.S.; Gao, Y.; Wang, K.; Sun, S.M.; Lu, X.; Li, L.W.; Feng, J.R.; Wang, D.J. Effects of different dwarfing interstocks on the total phenols and compounds of polyphenols in ‘Huahong’ apple. J. Fruit Sci. 2022, 39, 1191–1202. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Harker, F.R.; Kupferman, E.M.; Marin, A.B.; Gunson, F.A.; Triggs, C.M. Eating quality standards for apples based on consumer preferences. Postharvest Biol. Technol. 2008, 50, 70–78. [Google Scholar] [CrossRef]
- Lu, X.; Gao, Y.; Wang, K.; Sun, S.M.; Li, L.W.; Li, H.F.; Li, Q.S.; Feng, J.R.; Wang, D.J. Analysis of aroma characteristics in different cultivated apple strains. Sci. Agric. Sin. 2022, 55, 543–557. [Google Scholar] [CrossRef]
- Nikfardjam, M.P.; Maier, D. Development of a headspace trap HRGC/MS method for the assessment of the relevance of certain aroma compounds on the sensorial characteristics of commercial apple juice. Food Chem. 2011, 126, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.H.; Soukoulis, C.; Fisk, I. Atmospheric pressure chemical ionisation mass spectrometry analysis linked with chemometrics for food classification—A case study: Geographical provenance and cultivar classification of monovarietal clarified apple juices. Food Chem. 2014, 146, 149–156. [Google Scholar] [CrossRef]
- Schiller, D.; Contreras, C.; Vogt, J.; Dunemann, F.; Defilippi, B.G.; Beaudry, R.; Schwab, W. A dual positional specific lipoxygenase functions in the generation of flavor compounds during climacteric ripening of apple. Hortic. Res. 2015, 2, 323–351. [Google Scholar] [CrossRef] [PubMed]
- Salas, N.A.; Gonzalez-Aguilar, G.A.; Jacobo-Cuellar, J.L.; Espino, M.; Sepulveda, D.; Guerrero, V.; Olivas, G.I. Volatile compounds in golden delicious apple fruit (Malus domestica) during cold storage. Rev. Fitotec. Mex. 2016, 39, 159–173. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Moreno-Peris, E.; Cortés-Olmos, C.; Díez-Díaz, M.; González-Mas, M.C.; de Luis-Margarit, A.; Fita, A.; Rodríguez-Burruezo, A. Hybridization in peppers (Capsicum spp.) to improve the volatile composition in fully ripe fruits: The effects of parent combinations and fruit tissues. Agronomy 2020, 10, 751. [Google Scholar] [CrossRef]
- Yang, S.B.; Hao, N.N.; Meng, Z.P.; Li, Y.J.; Zhao, Z.Y. Identifification, comparison and classifification of volatile compounds in peels of 40 apple cultivars by HS-SPME with GC-MS. Foods 2021, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Meng, Z.P.; Fan, J.; Yan, L.Y.; Yang, Y.Z.; Zhao, Z.Y. Evaluation of the volatile profiles in pulp of 85 apple cultivars (Malus domestica) by HS-SPME combined with GC-MS. J. Food Meas. Charact. 2021, 15, 4215–4225. [Google Scholar] [CrossRef]
- Sanz, C.; Olias, J.M.; Perez, A.G. Aroma biochemistry of fruits and vegetables. In Proceedings of the Phytochemical Society of Europe; Tomás-Barberán, F.A., Robins, R.J., Eds.; Oxford University Press Inc.: New York, NY, USA; Oxford, UK, 1996; pp. 125–156. [Google Scholar]
- Hao, J.H.; Qi, Z.Y.; Li, J.R.; Liu, C.J.; Wang, L.; Jin, W.T.; Li, X.; Qi, H.Y. Effects of grafting on free fatty acid contents and related synthetic enzyme activities in peel and flesh tissues of oriental sweet melon during the different development period. IOP Conf. Ser. Earth Environ. Sci. 2018, 185, 012009. [Google Scholar] [CrossRef] [Green Version]
- Echeverra, G.; Graell, J.; López, M.L.; Lara, I. Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’ apples. Postharvest Biol. Technol. 2004, 31, 217–227. [Google Scholar] [CrossRef]
- Du, X.M.; Yang, T.Z.; Gao, J.D.; Wang, Q.; Cai, H.C.; Li, C.Y. Advances of effect of apple rootstocks on grafted varieties. Acta Agric. Boreali-Occident. Sin. 2020, 29, 487–495. [Google Scholar]
- Possner, D.R.E.; Kliewer, W.M. The localization of acids, sugars, potassium and calcium in developing grape berries. Vitis 1985, 24, 229–240. [Google Scholar]
- Harada, T. Grafting and RNA transport via phloem tissue in horticultural plants. Sci. Hortic. 2010, 125, 545–550. [Google Scholar] [CrossRef]
- Wang, C.; Han, J.; Liu, C.; Kibet, K.; Kayesh, E.; Shangguan, L.; Li, X.; Fang, J. Identification of microRNAs from Amur grape (Vitis amurensis Rupr.) by deep sequencing and analysis of microRNA variations with bioinformatics. BMC Genom. 2012, 13, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albacete, A.; Martínez-Andújar, C.; Martínez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; PérezAlfocea, F. Unravelling rootstock × scion interactions to improve food security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef]
- Qiu, L.; Jiang, B.; Fang, J.; Shen, Y.; Fang, Z.; Kumar, S.R.M.; Yi, K.; Shen, C.; Yan, D.; Zheng, B. Analysis of transcriptome in hickory (Carya cathayensis), and uncover the dynamics in the hormonal signaling pathway during graft process. BMC Genom. 2016, 17, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.Y.; Yan, S.; Xu, K.; Yuan, J.C.; Zhou, J.T.; Chen, C.G.; Zhao, L.L.; Zhang, S.Y.; Hou, G.X. Effects of dwarfing interstocks on nursery tree morphology and carbohydrates and nitrogen nutrition in “Yanfu No. 3” apple. J. Fruit Sci. 2022, 11, 17. Available online: https://doi.org/10.13925/j.cnki.gsxb.20220179 (accessed on 19 July 2022).
- Kviklys, D. Rootstock genotype determines phenol content in apple fruits. Plant Soil Environ. 2014, 60, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Li, H.Q.; Wang, W.R.; Duan, C.Q.; Wang, J.; He, F. The influence of rootstocks on the scions’ aromatic profiles of Vitis vinifera L. cv. Chardonnay. Sci. Hortic. 2020, 272, 109517. [Google Scholar] [CrossRef]
- Huang, L.; Grosser, J.W.; Gmitter, F.G., Jr.; Sims, C.A.; Wang, Y. Effects of scion/rootstock combination on flavor quality of orange juice from huanglongbing (HLB)–affected trees: A two–year study of the targeted metabolomics. J. Agric. Food Chem. 2020, 68, 3286–3296. [Google Scholar] [CrossRef]
- Muna, E.H.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Kanehira, A.; Yamada, K.; Iwaya, T.; Tsuwamoto, R.; Kasai, A.; Nakazono, M.; Harada, T. Apple phloem cells contain some mrnas transported over long distances. Tree Genet. Genomes 2010, 6, 635–642. [Google Scholar] [CrossRef]
- Rubio, B.; Stammitti, L.; Cookson, S.J.; Cookson, S.J.; Teyssier, E.; Gallusci, P. Small RNA populations reflect the complex dialogue established between heterograft partners in grapevine. Hortic. Res. 2022, 9, uhab067. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; Leeuwen, C.V.; Tominaga, T.; Soyer, J.P.; Gaudillere, J.P.; Denis, D. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L cv Sauvignon blanc in field conditions. J. Sci. Food Agric. 2005, 85, 73–85. [Google Scholar] [CrossRef]
- Yan, D.; Shi, J.R.; Ren, X.L.; Tao, Y.S.; Ma, F.W.; Li, R.; Liu, X.R.; Liu, C.H. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus × domestica). Food Chem. 2020, 3227, 127074. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Hao, N.N.; Feng, R.F.; Meng, Z.P.; Li, Y.A.; Zhao, Z.Y. Transcriptome and metabolite profiling analyses provide insight into volatile compounds of the apple cultivar ‘Ruixue’ and its parents during fruit development. BMC Plant Biol. 2021, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Weather Spark. Available online: https://zh.weatherspark.com (accessed on 10 October 2022).



| Code | Interstock/Scion Combinations | Scion/Interstock Value | Tree Height (m) | Breeding Unit |
|---|---|---|---|---|
| CG24 | Huahong/CG24/M. baccata | 0.52 ± 0.05 | 2.89 ± 0.04 | Cornell University and New York State Agricultural Experiment Station, Geneva |
| SH38 | Huahong/SH38/M. baccata | 0.99 ± 0.02 | 2.97 ± 0.09 | Pomology Institute, Shanxi Academy of Agricultural Sciences |
| SH3 | Huahong/SH3/M. baccata | 1.02 ± 0.02 | 2.95 ± 0.07 | Pomology Institute, Shanxi Academy of Agricultural Science |
| MD001 | Huahong/MD001/M. baccata | 0.76 ± 0.02 | 3.20 ± 0.10 | Mudanjiang Branch of Heilongjiang Academy of Agricultural Sciences |
| Mac9 | Huahong/Mac9/M. baccata | 0.79 ± 0.05 | 2.63 ± 0.04 | Michigan State University (USA) |
| CX5 | Huahong/CX5/M. baccata | 0.81 ± 0.02 | 3.30 ± 0.12 | Research Institute of Pomology, Chinese Academy of Agricultural Sciences |
| CK | Huahong/M. baccata | - | 4.20 ± 0.00 |
| Code | Compound | CG24 | SH38 | SH3 | MD001 | Mac9 | CX5 | CK |
|---|---|---|---|---|---|---|---|---|
| E1 | Butyl acetate | nd | 45.74 ± 26.46 | 27.05 ± 2.55 | 28.56 ± 3.1 | nd | 13.11 ± 0.48 | 21.37 ± 2.31 |
| E2 | 2-Methyl butyl acetate | 284.49 ± 51.15 | 709.52 ± 355.62 | 340.53 ± 129.65 | 612.7 ± 208.25 | nd | 488.3 ± 14.31 | 524.06 ± 35.19 |
| E3 | Isoamyl propionate | 39.86 ± 5.08 | nd | 53.77 ± 9.18 | 88.02 ± 43.1 | 50.41 ± 7.18 | 45.83 ± 4.12 | 38.29 ± 1.27 |
| E4 | Ethyl acetate | 160.29 ± 4.91b | 487.33 ± 161.46a | 199.33 ± 21.66b | 293.05 ± 81.2ab | 239.04 ± 11.6ab | 164.72 ± 7.67b | 210.23 ± 27.56b |
| E5 | 2-Methyl butyl 2-methyl butyrate | 34.05 ± 1.16 | 105.7 ± 56.31 | 99.73 ± 16.99 | 75.17 ± 40.26 | 83.72 ± 21.65 | 72.14 ± 19.21 | 64.97 ± 13.58 |
| E6 | Hexyl 2-methyl butyrate | 352.9 ± 90.13c | 1695.17 ± 704.76ab | 2161.38 ± 393.29a | 687.29 ± 383.93bc | 930.68 ± 108.17bc | 421.8 ± 97.97c | 473.34 ± 156.53bc |
| E7 | Butyl propionate | 14.72 ± 2.31 | 45.99 ± 24.94 | 30.75 ± 1.15 | 38.27 ± 2.09 | 23.38 ± 1.22 | 12.01 ± 1.15 | 11.47 ± 1.22 |
| E8 | 4-Pentene-1-acetate | 10.28 ± 1.22b | 23.36 ± 2.31a | nd | 16.73 ± 4.5ab | nd | 15.94 ± 0.66ab | 13.96 ± 1.22b |
| E9 | Propyl 2-methyl butyrate | nd | 43.16 ± 11.07 | 24.75 ± 2.6 | nd | 20.39 ± 1.37 | nd | nd |
| E10 | Butyl butyrate | 15.14 ± 2.25 | 85.19 ± 48.41 | 57.13 ± 11.77 | 46.16 ± 9.26 | 51.93 ± 3.14 | 24.14 ± 5.72 | 32.53 ± 4.13 |
| E11 | Ethyl 3-methylvalerate | nd | 49.84 ± 17.83 | 41.33 ± 7.66 | 42.74 ± 14.71 | 24.98 ± 1.61 | 29.24 ± 12.72 | 26.88 ± 5.78 |
| E12 | Butyl 2-methyl butyrate | 41.69 ± 11.8b | 259.21 ± 137.31a | 257.04 ± 50.83a | 146.64 ± 41.85ab | 140.01 ± 6.62ab | 56.26 ± 0.65ab | 63.61 ± 19.75ab |
| E13 | Caproic acid propyl ester | 19.79 ± 1.96 | 101 ± 60.36 | 55.62 ± 12.07 | 54.39 ± 19.11 | 60 ± 9.68 | 43.14 ± 7.27 | 38.68 ± 11.14 |
| E14 | Propionic acid ester | 31.86 ± 5.8 | 168.92 ± 92.74 | 114.58 ± 17.72 | 68.58 ± 47.36 | 70.48 ± 15.82 | 32.89 ± 6.46 | 36.57 ± 12.27 |
| E15 | Octylic acid methyl ester | 9.72 ± 0.25 | 59.38 ± 24.87 | 47.15 ± 8.6 | 37.5 ± 22.61 | 35.32 ± 3.39 | 23.52 ± 6.99 | 24.3 ± 6.49 |
| E16 | Amyl 2-methyl butyrate | 7.81 ± 0.83b | 36.3 ± 17.19a | 33.27 ± 5.81ab | 23.28 ± 1.21ab | 16.71 ± 3.48ab | 12.24 ± 1.07ab | 19.35 ± 1.81ab |
| E17 | Hexyl butyrate | nd | nd | 47.33 ± 1.15a | nd | 20.09 ± 3.61b | 11.87 ± 1.15b | 15.37 ± 2.95b |
| E18 | Valerate-3-methyl-2-butenyl ester | 14.48 ± 0.22b | 42.49 ± 10.85a | 24.93 ± 1.17ab | 28.39 ± 11.89ab | 17.67 ± 2.3b | 21.29 ± 3.32ab | 16.51 ± 1.75b |
| E19 | Caproic acid butyl ester | 66.87 ± 17.38b | 802.98 ± 456.36a | 558.67 ± 95.39ab | 226.43 ± 108.49ab | 535.44 ± 57.81ab | 224.8 ± 60.09ab | 254.99 ± 98.49ab |
| E20 | Octanoic acid ethyl ester | 12.48 ± 1.33 | 53.76 ± 21.31 | nd | 38.64 ± 15.41 | 19.14 ± 1.94 | 28.95 ± 5.47 | 20.54 ± 5.58 |
| E21 | Hexanoic acid-2-methyl butyl ester | 13.96 ± 2.77 | 74.68 ± 43.53 | 61.94 ± 8.33 | 24.08 ± 5.87 | 85.27 ± 12.64 | 59.14 ± 15.05 | 50.33 ± 16.17 |
| E22 | Caproic acid butyl ester | 8.61 ± 0.9 | 75.63 ± 43.9 | nd | 25.19 ± 11.13 | 53.24 ± 1.13 | 27.11 ± 6.63 | 25.99 ± 10.07 |
| E23 | Hexanoic acid-3-methyl-2-butenyl ester | 5.95 ± 0.75b | 29.69 ± 12.56a | 22.33 ± 1.24ab | 26.91 ± 1.21ab | 22.23 ± 1.42ab | 24.31 ± 4.86ab | 17.95 ± 5.65ab |
| E24 | Caproic acid ester | 72.32 ± 20.16c | 569.99 ± 268.32a | 432.97 ± 58.69abc | 159.76 ± 68.7bc | 497.13 ± 15.39ab | 210.14 ± 43.57abc | 225.13 ± 65.03abc |
| E25 | Octanoic acid-2-methyl butyl ester | nd | nd | 16.34 ± 1.1ab | 8.57 ± 0.52b | 18.98 ± 1.82a | 12.08 ± 0.74ab | 13.61 ± 4.55b |
| A1 | Hexanal | 398.65 ± 38.45b | 788.46 ± 197.49a | 609.3 ± 105.75ab | 682.01 ± 66.07ab | 511.76 ± 34.78ab | 458.62 ± 19.39ab | 432.32 ± 37.99b |
| A2 | 2-Hexene aldehyde | 44.2 ± 6.74 | 86.87 ± 24.03 | 63.18 ± 9.72 | 54.04 ± 6.57 | 59.9 ± 6.14 | 46.81 ± 4.34 | 46.99 ± 7 |
| A3 | Z-2-Heptene aldehyde | 155.44 ± 8.97bc | 216.64 ± 34.31abc | 236.08 ± 49.47ab | 157.85 ± 31.67bc | 120.68 ± 3.59c | 147.53 ± 15.01bc | 297.56 ± 23.11a |
| A4 | E-2-Octene aldehyde | 114.75 ± 5.17b | 295.36 ± 80.51a | 229.35 ± 34.95ab | 184.93 ± 47.06ab | 150 ± 8.32b | 138.65 ± 17.12b | 154.55 ± 13.66ab |
| A5 | 2-Methylbutyraldehyde | 17.26 ± 2.54 | 35.17 ± 11.59 | 35.82 ± 3.32 | 34.26 ± 13.67 | 19.84 ± 1.79 | 20.56 ± 1.83 | 15.18 ± 2.04 |
| A6 | Butyl aldehyde | 22.88 ± 1.85b | 54.49 ± 16.01a | 40.22 ± 6.25ab | 41.58 ± 9.13ab | 31.13 ± 1.56ab | 27.32 ± 0.75ab | 21.28 ± 3.65b |
| A7 | Caprylic aldehyde | 97.88 ± 39.52b | 109.7 ± 23.7ab | 97.62 ± 34.25ab | 154.84 ± 12.99a | 85.44 ± 16.28ab | 82.63 ± 11.96ab | 79.25 ± 30.83ab |
| A8 | Nonanal | 160.77 ± 31.76 | 381.73 ± 124.42 | 344.72 ± 88.87 | 427.74 ± 55.91 | 276.3 ± 31.47 | 177.76 ± 15.9 | 175.48 ± 70.02 |
| A9 | 2-Nonene aldehyde | 15.16 ± 3.36 | 44.74 ± 15.88 | nd | 28.41 ± 7.51 | nd | 14.88 ± 1.95 | 16.81 ± 2.7 |
| A10 | E-2-Decyl olefine aldehyde | 19.8 ± 3.66 | 30.11 ± 7.47 | 27.67 ± 8.12 | 28.74 ± 2.87 | 28.79 ± 4.8 | 20.35 ± 2.65 | 28.45 ± 7.91 |
| AL1 | Methanol | 113.36 ± 6.98 | 167.67 ± 30.22 | 132.53 ± 25.32 | 120 ± 8.93 | 128.35 ± 8.21 | 113.92 ± 9.5 | 112.96 ± 10.24 |
| AL2 | Butanol | 35.01 ± 4.04b | 72.33 ± 1.35a | nd | 33.92 ± 0.33b | 43.5 ± 7.78b | 17.45 ± 2.17c | 18.74 ± 1.28c |
| AL3 | 2-Methyl-1-butanol | 89.7 ± 18.4 | 121.88 ± 30.93 | 148.91 ± 11.6 | 123.58 ± 31.1 | 164.84 ± 10.11 | 137.37 ± 2.91 | 113.45 ± 12.97 |
| AL4 | Hexanol | 89.12 ± 20.45bc | 191.09 ± 67.43ab | 227.16 ± 35.1a | 81.82 ± 25.58bc | 151.04 ± 7.53abc | 77 ± 10.36c | 87.27 ± 2.29bc |
| AL5 | 1-Pentene-3-ol | 14.57 ± 2.32 | nd | 0 ± 1.21 | 21.43 ± 4.11 | 14.96 ± 0.84 | 15.03 ± 1.03 | 24.02 ± 4.43 |
| AL6 | 2-Octene-1-ol | 41.32 ± 13.39 | 131.59 ± 56.62 | 91.67 ± 47.83 | 149.46 ± 34.81 | 98.27 ± 1.64 | nd | 82.08 ± 3.53 |
| AH1 | Hexane | 127.2 ± 8.52 | 137.1 ± 16.34 | 117.95 ± 10.11 | 168.26 ± 26.28 | 138.81 ± 12.97 | 127.62 ± 12.65 | 131.94 ± 20.89 |
| AH2 | Tetradecane | 12.09 ± 0.51 | nd | nd | 28.02 ± 5.84 | nd | nd | nd |
| AH3 | 7-Methyl-pentadecane | nd | 5.79 ± 0.98c | nd | 18.6 ± 0.55a | 16.19 ± 2.02ab | nd | 14.16 ± 1.04b |
| AC1 | 2-Methylbutyric acid | 154.72 ± 16.63 | 305.56 ± 107.74 | 290.39 ± 28 | 203.72 ± 45.57 | 243.32 ± 19.22 | 187.62 ± 17.45 | 192.61 ± 17.48 |
| AC2 | Caproic acid | 42.25 ± 10.56 | 92.44 ± 31.12 | 87.04 ± 13.71 | 73.84 ± 7.86 | 66.69 ± 14.04 | 44.9 ± 2.1 | 51.42 ± 11.06 |
| K1 | 6-Methyl-5-heptene-2-ketone | 291.15 ± 46.92c | 1006.83 ± 393.99a | 971.28 ± 40.35ab | 449.29 ± 232.85abc | 649.51 ± 84.46abc | 372.75 ± 51.19abc | 341.22 ± 81.47bc |
| K2 | 3,4,5-Trimethyl-2-cyclopenten-1-one | 22.96 ± 4.16ab | 55.14 ± 18.7a | 55.53 ± 7.77a | 28.46 ± 8.84ab | 27.9 ± 2.51ab | nd | 17.79 ± 1.81b |
| Code | Compound | CG24 | SH38 | SH3 | MD001 | Mac9 | CX5 | CK |
|---|---|---|---|---|---|---|---|---|
| E1 | Butyl acetate | 10.2 ± 1.15b | 15.36 ± 2.46ab | 13.03 ± 3.52ab | 23.01 ± 6.2a | 16.3 ± 2.95ab | 8.04 ± 1.58b | 14.95 ± 2.16ab |
| E2 | 2-Methyl butyl acetate | 143.29 ± 52.07 | 93.6 ± 36.13 | 110.09 ± 17.38 | 355.4 ± 203.25 | 206.31 ± 19.71 | 172.94 ± 11.96 | 195.13 ± 57.51 |
| E4 | Ethyl acetate | 38 ± 5.35 | 45.39 ± 12.36 | 47.82 ± 9.99 | 43.81 ± 15.3 | 60.14 ± 7.69 | 27.96 ± 2.38 | 39.76 ± 5.03 |
| E6 | Hexyl 2-methyl butyrate | 11.99 ± 2.75ab | 21.63 ± 8.38ab | 22.48 ± 4.18a | 22.33 ± 4.09a | 20.03 ± 1.01ab | 7.14 ± 0.95b | 10.73 ± 1.52ab |
| A1 | Hexanal | 45.56 ± 17.27 | 21.7 ± 1.55 | 31.91 ± 3.34 | 53.19 ± 16.19 | 38.9 ± 14.1 | 28.18 ± 7.33 | 31.77 ± 8.84 |
| A2 | 2-Hexene aldehyde | 15.37 ± 3.49 | 10.07 ± 1.34 | 14.94 ± 3.09 | nd | 11.56 ± 1.85 | 11.04 ± 0.42 | 11.18 ± 0.76 |
| A3 | Z-2-Heptene aldehyde | 17.72 ± 0.95ab | 11.51 ± 1.98a | 21.19 ± 7.98ab | nd | 8.66 ± 0.76a | 15.56 ± 2.58ab | 27.6 ± 4.28a |
| A4 | Benzaldehyde | 16.59 ± 6.8 | nd | 9.67 ± 1.96 | nd | 10.48 ± 1.19 | nd | 12.04 ± 0.34 |
| A11 | E-2-Octene aldehyde | 9.88 ± 0.98 | 9.09 ± 1.45 | 11.65 ± 1.96 | nd | nd | 9.55 ± 0.52 | 10.89 ± 0.57 |
| A12 | β-Cyclocitral | 4.77 ± 0.59ab | 4.01 ± 1.35ab | 5.42 ± 0.31a | nd | nd | 3.62 ± 0.37ab | 2.24 ± 1.01b |
| A13 | 3,4-Dimethylphenylacetaldehyde | 7.18 ± 0.25 | 7.77 ± 0.57 | 8.2 ± 0.81 | nd | 6.69 ± 1.09 | 7.61 ± 0.58 | 7.04 ± 1.22 |
| A14 | Pentanal | 10.64 ± 0.54ab | nd | 12.16 ± 0.75ab | 13.44 ± 2.93a | 9.05 ± 0.42ab | nd | 8.16 ± 0.29b |
| AL1 | Methanol | 68.51 ± 6.1 | 62.84 ± 5.27 | 67.89 ± 6.58 | 87.86 ± 28.05 | 62.27 ± 1.83 | 55.21 ± 5.08 | 52.05 ± 1.73 |
| AL2 | Butanol | 27.87 ± 0.57ab | 25.37 ± 7.82ab | 43.42 ± 15.76b | 48.73 ± 17.91a | 29.91 ± 3.2ab | 11.87 ± 1.39ab | 14.76 ± 3.75ab |
| AL3 | 2-Methyl-1-butanol | 128.79 ± 34.02 | 84.19 ± 10.09 | 136.47 ± 7.94 | 256.79 ± 124.98 | 146.85 ± 9.48 | 128.03 ± 4.71 | 112.95 ± 21.46 |
| AL4 | Hexanol | 147.6 ± 42.7bc | 273.3 ± 9.48ab | 298.86 ± 62.54a | 169.74 ± 67.15abc | 198.58 ± 28.91abc | 84.21 ± 12.13c | 103.62 ± 24.27c |
| AL7 | 2-Methyl-1-hexanol | 13.66 ± 3.99 | 13.56 ± 3.2 | nd | nd | nd | 5.51 ± 0.69 | 5.92 ± 0.52 |
| AL8 | cis-α,α-5-Trimethyl-5-vinyltetrahydrofuran-2-methanol | nd | 9.67 ± 0.61bc | 14.03 ± 0.61a | 7.56 ± 0.03d | 10.06 ± 0.6b | 8.02 ± 0.27cd | 8.7 ± 0.94bcd |
| AH1 | Hexane | 200 ± 53.23 | 116.79 ± 14.21 | 126.2 ± 2.6 | 233.44 ± 88.55 | 136.46 ± 7.6 | 125.65 ± 9.99 | 129.14 ± 11.43 |
| AC1 | 2-Methylbutyric acid | 12.95 ± 4.11b | 24.67 ± 7.34ab | 40.04 ± 10.83a | 25.83 ± 6.58ab | 29 ± 0.92ab | 15.26 ± 1.75b | 22.37 ± 7.62ab |
| K3 | 2-Octanone | 25.16 ± 1.07 | 23.38 ± 0.63 | 27.13 ± 2.45 | 28.1 ± 3.17 | 23.19 ± 1.77 | 23.23 ± 0.25 | 23.57 ± 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Gao, Y.; Wang, K.; Sun, S.; Liu, Z.; Yan, P.; Feng, J.; Li, Q.; Li, L.; Wang, D. Dwarf Interstocks Improve Aroma Quality of ‘Huahong’ Apple (Malus × domestica). Agriculture 2022, 12, 1710. https://doi.org/10.3390/agriculture12101710
Lu X, Gao Y, Wang K, Sun S, Liu Z, Yan P, Feng J, Li Q, Li L, Wang D. Dwarf Interstocks Improve Aroma Quality of ‘Huahong’ Apple (Malus × domestica). Agriculture. 2022; 12(10):1710. https://doi.org/10.3390/agriculture12101710
Chicago/Turabian StyleLu, Xiang, Yuan Gao, Kun Wang, Simiao Sun, Zhao Liu, Peng Yan, Jianrong Feng, Qingshan Li, Lianwen Li, and Dajiang Wang. 2022. "Dwarf Interstocks Improve Aroma Quality of ‘Huahong’ Apple (Malus × domestica)" Agriculture 12, no. 10: 1710. https://doi.org/10.3390/agriculture12101710





