Insights into Agricultural-Waste-Based Nano-Activated Carbon Fabrication and Modifications for Wastewater Treatment Application
Abstract
:1. Introduction
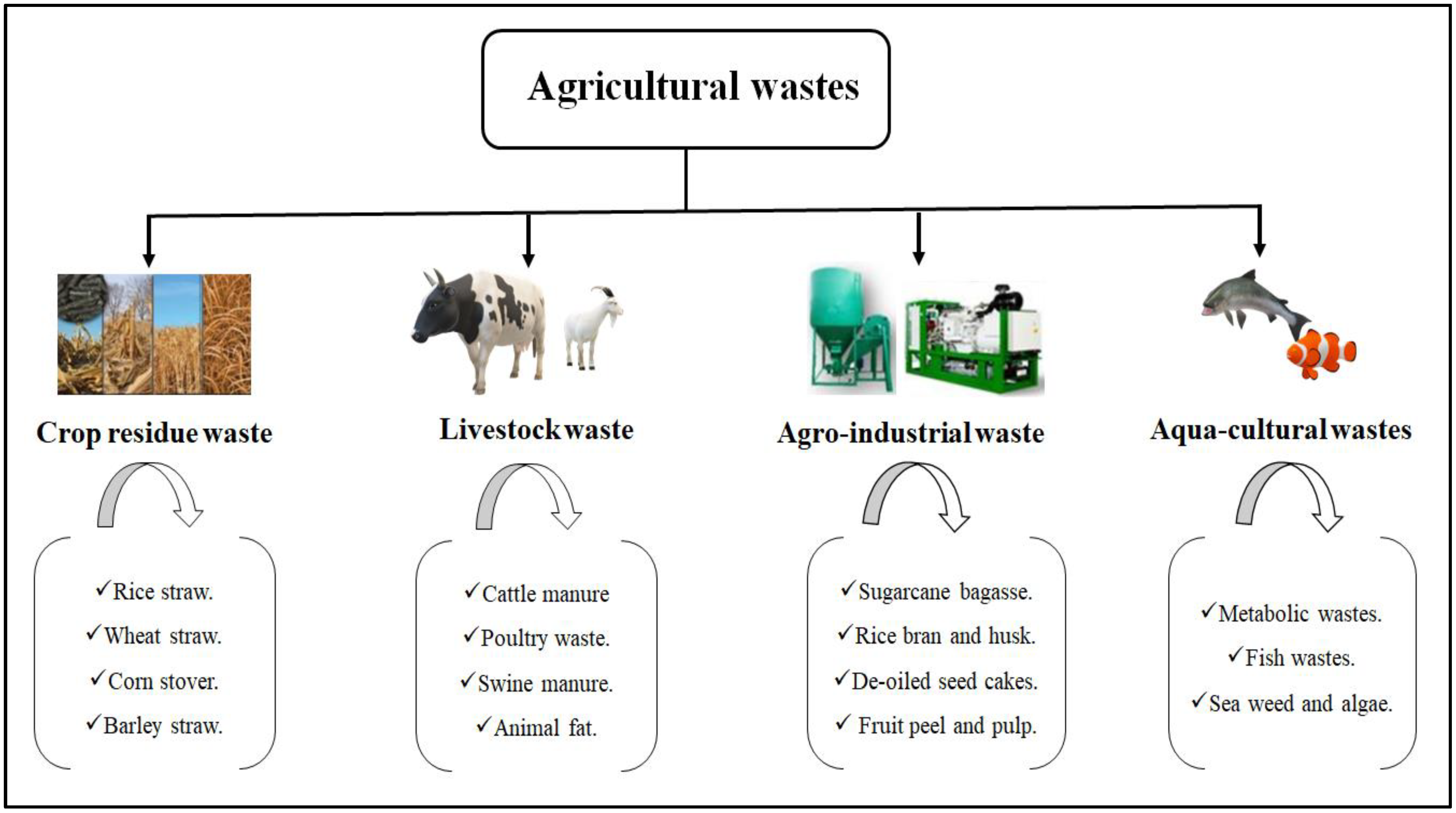
2. Agricultural Waste: Types, Yield and Properties
2.1. Crop Residues Waste
2.2. Livestock Waste
2.3. Agro-Industrial Waste
2.4. Aquaculture Waste
3. Agricultural-Waste-Based Nano-Activated Carbon: Fabrication and Properties
4. Modifications of Agricultural-Waste-Based Nano-Activated Carbon
4.1. Incorporation of Nano-Activated Carbon with Metal Oxides
4.2. Incorporation with a Specific Chemical Compound
4.3. Enhanced Porosity and Surface Area
4.4. Other Modification Techniques
5. Agricultural-Waste-Based Nano-Activated Carbon for Wastewater Treatment Applications
5.1. Drinking Water Filtration
5.2. Removal of Dyes and Organic Compounds
5.3. Removal of Heavy Metals
5.4. Oil Spill Separation
5.5. Removal of Toxins and Pharmaceutical Compounds
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cheng, Z.; Reisner, A.; Liu, Y. Compliance with household solid waste management in rural villages in developing countries. J. Clean. Prod. 2018, 202, 293–298. [Google Scholar] [CrossRef]
- Raheja, S.; Obaidat, M.S.; Kumar, M.; Sadoun, B.; Bhushan, S. A hybrid MCDM framework and simulation analysis for the assessment of worst polluted cities. Simul. Model. Pract. Theory 2022, 118, 102540. [Google Scholar] [CrossRef]
- Nasution, H.; Yahya, E.B.; Abdul Khalil, H.P.S.; Shaah, M.A.; Suriani, A.; Mohamed, A.; Alfatah, T.; Abdullah, C. Extraction and Isolation of Cellulose Nanofibers from Carpet Wastes Using Supercritical Carbon Dioxide Approach. Polymers 2022, 14, 326. [Google Scholar] [CrossRef]
- Khounani, Z.; Hosseinzadeh-Bandbafha, H.; Moustakas, K.; Talebi, A.F.; Goli, S.A.H.; Rajaeifar, M.A.; Khoshnevisan, B.; Jouzani, G.S.; Peng, W.; Kim, K.-H. Environmental life cycle assessment of different biorefinery platforms valorizing olive wastes to biofuel, phosphate salts, natural antioxidant, and an oxygenated fuel additive (triacetin). J. Clean. Prod. 2021, 278, 123916. [Google Scholar] [CrossRef]
- Rizal, S.; Olaiya, F.G.; Saharudin, N.; Abdullah, C.; NG, O.; Mohamad Haafiz, M.; Yahya, E.B.; Sabaruddin, F.; Abdul Khalil, H.P.S. Isolation of textile waste cellulose nanofibrillated fibre reinforced in polylactic acid-chitin biodegradable composite for green packaging application. Polymers 2021, 13, 325. [Google Scholar] [CrossRef]
- Azamzam, A.A.; Rafatullah, M.; Yahya, E.B.; Ahmad, M.I.; Lalung, J.; Alharthi, S.; Alosaimi, A.M.; Hussein, M.A. Insights into Solar Disinfection Enhancements for Drinking Water Treatment Applications. Sustainability 2021, 13, 10570. [Google Scholar] [CrossRef]
- Masilompane, T.M.; Tutu, H.; Etale, A. Cellulose-Based Nanomaterials for Treatment of Acid Mine Drainage-Contaminated Waters. In Application of Nanotechnology in Mining Processes: Beneficiation and Sustainability; Wiley-Scrivener: Hoboken, NJ, USA, 2022; pp. 33–66. [Google Scholar]
- Kim, S.; Nam, S.-N.; Jang, A.; Jang, M.; Park, C.M.; Son, A.; Her, N.; Heo, J.; Yoon, Y. Review of adsorption–membrane hybrid systems for water and wastewater treatment. Chemosphere 2022, 286, 131916. [Google Scholar] [CrossRef]
- Panahi, Y.; Mellatyar, H.; Farshbaf, M.; Sabet, Z.; Fattahi, T.; Akbarzadehe, A. Biotechnological applications of nanomaterials for air pollution and water/wastewater treatment. Mater. Today Proc. 2018, 5, 15550–15558. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul Khalil, H.P.S.; Mistar, E.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification-a critical review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, Y.; Tsang, D.C.; Khan, E.; Yip, A.C.; Ng, Y.H.; Rinklebe, J.; Ok, Y.S. Customised fabrication of nitrogen-doped biochar for environmental and energy applications. Chem. Eng. J. 2020, 401, 126136. [Google Scholar] [CrossRef]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C. Formation, characteristics, and applications of environmentally persistent free radicals in biochars: A review. Bioresour. Technol. 2019, 281, 457–468. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Lakshmi, S.; Avti, P.K.; Hegde, G. Activated carbon nanoparticles from biowaste as new generation antimicrobial agents: A review. Nano-Struct. Nano-Objects 2018, 16, 306–321. [Google Scholar] [CrossRef]
- Obi, F.; Ugwuishiwu, B.; Nwakaire, J. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016, 35, 957–964. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kumar, M.; Alshehri, M.; Keshta, I.; Abugabah, A.; Sharma, S.K. Smart water management framework for irrigation in agriculture. Environ. Technol. 2022, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rizal, S.; Abdul Khalil, H.P.S.; Oyekanmi, A.A.; Gideon, O.N.; Abdullah, C.K.; Yahya, E.B.; Alfatah, T.; Sabaruddin, F.A.; Rahman, A.A. Cotton Wastes Functionalized Biomaterials from Micro to Nano: A Cleaner Approach for a Sustainable Environmental Application. Polymers 2021, 13, 1006. [Google Scholar] [CrossRef] [PubMed]
- Alsharef, A.; Aggarwal, K.; Kumar, M.; Mishra, A. Review of ML and AutoML solutions to forecast time-series data. Arch. Comput. Methods Eng. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Sahu, J.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Jin, Z.; Shah, T.; Zhang, L.; Liu, H.; Peng, S.; Nie, L. Effect of straw returning on soil organic carbon in rice–wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Singh, R.K. Selection of sustainable solutions for crop residue burning: An environmental issue in northwestern states of India. Environ. Dev. Sustain. 2021, 23, 3696–3730. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Liu, X.; Huang, G.; Xiao, W.; Han, L. Characteristics and Non-parametric Multivariate Data Mining Analysis and Comparison of Extensively Diversified Animal Manure. Waste Biomass Valorization 2021, 12, 2343–2355. [Google Scholar] [CrossRef]
- Huang, J.; Qiao, Y.; Liu, H.; Wang, B.; Wang, Z.; Yu, Y.; Xu, M. Effect of torrefaction on physicochemical properties and steam gasification reactivity of chars produced from the pyrolysis of typical food wastes. Energy Fuels 2020, 34, 15332–15342. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, L.; Song, G.; Chen, D. Adsorption of uranium onto modified rice straw grafted with oxygen-containing groups. Environ. Eng. Sci. 2016, 33, 942–950. [Google Scholar] [CrossRef]
- Nam, H.; Choi, W.; Genuino, D.A.; Capareda, S.C. Development of rice straw activated carbon and its utilizations. J. Environ. Chem. Eng. 2018, 6, 5221–5229. [Google Scholar] [CrossRef]
- Xu, J.; Zong, M.-H.; Fu, S.-Y.; Li, N. Correlation between physicochemical properties and enzymatic digestibility of rice straw pretreated with cholinium ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 4340–4345. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, K.; He, L.; Peng, L. Reinforcement of the bio-gas conversion from pyrolysis of wheat straw by hot caustic pre-extraction. Biotechnol. Biofuels 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Topcu, N.S.; Duman, G.; Olgun, H.; Yanik, J. Evaluation of Poultry Manure: Combination of Phosphorus Recovery and Activated Carbon Production. ACS Omega 2022. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Ducey, T.; Ro, K.S.; Hunt, P.G. Livestock waste-to-bioenergy generation opportunities. Bioresour. Technol. 2008, 99, 7941–7953. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Huang, P.-C.; Lin, Y.-Q. Reusing cow manure for the production of activated carbon using potassium hydroxide (KOH) activation process and its liquid-phase adsorption performance. Processes 2019, 7, 737. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Xin, Y.; Yuan, Q. Prediction of biochar yield from cattle manure pyrolysis via least squares support vector machine intelligent approach. Bioresour. Technol. 2016, 202, 158–164. [Google Scholar] [CrossRef]
- Pattanaik, L.; Pattnaik, F.; Saxena, D.K.; Naik, S.N. Biofuels from agricultural wastes. In Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–142. [Google Scholar]
- Sukiran, M.A.; Abnisa, F.; Daud, W.M.A.W.; Bakar, N.A.; Loh, S.K. A review of torrefaction of oil palm solid wastes for biofuel production. Energy Convers. Manag. 2017, 149, 101–120. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Jaramillo-Martínez, D.; Buitrago-Sierra, R.; López, D. Use of Palm Oil Waste for Activated Carbons Production and Its Application in Methylene Blue Removal. ChemistrySelect 2022, 7, e202200791. [Google Scholar] [CrossRef]
- Akinwole, A.; Dauda, A.; Ololade, O. Growth performance of african catfish (Clarias gariepinus) juveniles reared in wastewater treated with alum and Moringa oleifera seed. J. Aquac. Res. Dev. 2016, 7, 1000460. [Google Scholar]
- Ferreira, C.I.; Calisto, V.; Otero, M.; Nadais, H.; Esteves, V.I. Comparative adsorption evaluation of biochars from paper mill sludge with commercial activated carbon for the removal of fish anaesthetics from water in Recirculating Aquaculture Systems. Aquac. Eng. 2016, 74, 76–83. [Google Scholar] [CrossRef]
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Omoniyi, T.; Salami, H. Yield, characterization and potential application of activated carbon produced from Co–Pyrolysis of wood and plastic wastes as adsorbent for aquaculture wastewater treatment. In Proceedings of the 12th CIGR Section VI International Symposium, Ibadan, Nigeria, 22–25 October 2018; p. 25. [Google Scholar]
- Yang, H.; Yu, X.; Liu, J.; Wang, L.; Guo, M. Preparation of magnetic Fe3O4/activated carbon fiber and a study of the tetracycline adsorption in aquaculture wastewater. Mater. Technol. 2019, 53, 505–510. [Google Scholar] [CrossRef]
- Ramírez-Montoya, L.A.; Hernández-Montoya, V.; Montes-Morán, M.A.; Cervantes, F.J. Correlation between mesopore volume of carbon supports and the immobilization of laccase from Trametes versicolor for the decolorization of Acid Orange 7. J. Environ. Manag. 2015, 162, 206–214. [Google Scholar] [CrossRef]
- Om Prakash, M.; Gujjala, R.; Panchal, M.; Ojha, S. Mechanical characterization of arhar biomass based porous nano activated carbon polymer composites. Polym. Compos. 2020, 41, 3113–3123. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, A.; Zhao, Y. Preparation and characterisation of activated carbon from waste tea by physical activation using steam. J. Air Waste Manag. Assoc. 2018, 68, 1269–1277. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul Khalil, H.P.S.; Yahya, E.B.; Olaiya, N.; Alfatah, T.; Suriani, A.; Mohamed, A. Recent trends and future prospects of nanostructured aerogels in water treatment applications. J. Water Process Eng. 2022, 45, 102481. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A.R. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Gan, Y.X. Activated carbon from biomass sustainable sources. C 2021, 7, 39. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Roufegarinejad, L.; Amarowicz, R. Recent advances in the use of walnut (Juglans regia L.) shell as a valuable plant-based bio-sorbent for the removal of hazardous materials. RSC Adv. 2020, 10, 7026–7047. [Google Scholar] [CrossRef]
- Danish, M.; Pin, Z.; Ziyang, L.; Ahmad, T.; Majeed, S.; Yahya, A.N.A.; Khanday, W.A.; Abdul Khalil, H.P.S. Preparation and characterization of banana trunk activated carbon using H3PO4 activation: A rotatable central composite design approach. Mater. Chem. Phys. 2022, 282, 125989. [Google Scholar] [CrossRef]
- Lam, S.S.; Su, M.H.; Nam, W.L.; Thoo, D.S.; Ng, C.M.; Liew, R.K.; Yuh Yek, P.N.; Ma, N.L.; Nguyen Vo, D.V. Microwave pyrolysis with steam activation in producing activated carbon for removal of herbicides in agricultural surface water. Ind. Eng. Chem. Res. 2018, 58, 695–703. [Google Scholar] [CrossRef]
- Shan, Y.; Yang, W.; Li, Y.; Chen, H.; Liu, Y. Removal of elemental mercury from flue gas using microwave/ultrasound-activated Ce–Fe magnetic porous carbon derived from biomass straw. Energy Fuels 2019, 33, 8394–8402. [Google Scholar] [CrossRef]
- Tuerhong, T.; Kuerban, Z. Preparation and characterization of cattle manure-based activated carbon for hydrogen sulfide removal at room temperature. J. Environ. Chem. Eng. 2022, 10, 107177. [Google Scholar] [CrossRef]
- Jawad, A.H.; Bardhan, M.; Islam, M.A.; Islam, M.A.; Syed-Hassan, S.S.A.; Surip, S.; ALOthman, Z.A.; Khan, M.R. Insights into the modeling, characterization and adsorption performance of mesoporous activated carbon from corn cob residue via microwave-assisted H3PO4 activation. Surf. Interfaces 2020, 21, 100688. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, F.; Xiao, H.-M.; Feng, Q.-P.; Xiong, L.-Y.; Fu, S.-Y. Preparation of pore-size controllable activated carbon fibers from bamboo fibers with superior performance for xenon storage. Chem. Eng. J. 2015, 270, 528–534. [Google Scholar] [CrossRef]
- Guo, J.; Song, Y.; Ji, X.; Ji, L.; Cai, L.; Wang, Y.; Zhang, H.; Song, W. Preparation and characterization of nanoporous activated carbon derived from prawn shell and its application for removal of heavy metal ions. Materials 2019, 12, 241. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, S.G.; Ahmed, S.M.; Amr, A.E.-G.E.; Kamel, A.H. Porous activated carbon from lignocellulosic agricultural waste for the removal of acetampirid pesticide from aqueous solutions. Molecules 2020, 25, 2339. [Google Scholar] [CrossRef]
- Sharma, A.; Jindal, J.; Mittal, A.; Kumari, K.; Maken, S.; Kumar, N. Carbon materials as CO2 adsorbents: A review. Environ. Chem. Lett. 2021, 19, 875–910. [Google Scholar] [CrossRef]
- Prabu, D.; Kumar, P.S.; Rathi, B.S.; Sathish, S.; Anand, K.V.; Kumar, J.A.; Mohammed, O.B.; Silambarasan, P. Feasibility of magnetic nano adsorbent impregnated with activated carbon from animal bone waste: Application for the chromium (VI) removal. Environ. Res. 2022, 203, 111813. [Google Scholar] [CrossRef]
- Legrouri, K.; Khouya, E.; Hannache, H.; El Hartti, M.; Ezzine, M.; Naslain, R. Activated carbon from molasses efficiency for Cr (VI), Pb (II) and Cu (II) adsorption: A mechanistic study. Chem. Int 2017, 3, 301. [Google Scholar]
- Guo, X.; Chen, F. Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater. Environ. Sci. Technol. 2005, 39, 6808–6818. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef]
- Li, B.; Yin, W.; Xu, M.; Tan, X.; Li, P.; Gu, J.; Chiang, P.; Wu, J. Facile modification of activated carbon with highly dispersed nano-sized α-Fe2O3 for enhanced removal of hexavalent chromium from aqueous solutions. Chemosphere 2019, 224, 220–227. [Google Scholar] [CrossRef]
- Khalil, A.M.; Eljamal, O.; Amen, T.W.; Sugihara, Y.; Matsunaga, N. Optimized nano-scale zero-valent iron supported on treated activated carbon for enhanced nitrate and phosphate removal from water. Chem. Eng. J. 2017, 309, 349–365. [Google Scholar] [CrossRef]
- Sabermahani, F.; Mahani, N.M.; Noraldiny, M. Removal of thallium (I) by activated carbon prepared from apricot nucleus shell and modified with rhodamine B. Toxin Rev. 2017, 36, 154–160. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb (II), Cd (II) and Cu (II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Fang, J.; Sun, Y.; Cao, X. Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem. Eng. J. 2013, 231, 512–518. [Google Scholar] [CrossRef]
- Tang, J.; Lv, H.; Gong, Y.; Huang, Y. Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal. Bioresour. Technol. 2015, 196, 355–363. [Google Scholar] [CrossRef]
- Shang, M.-R.; Liu, Y.-G.; Liu, S.-B.; Zeng, G.-M.; Tan, X.-F.; Jiang, L.-H.; Huang, X.-X.; Ding, Y.; Guo, Y.-M.; Wang, S.-F. A novel graphene oxide coated biochar composite: Synthesis, characterization and application for Cr (VI) removal. Rsc Adv. 2016, 6, 85202–85212. [Google Scholar] [CrossRef]
- Patra, C.; Gupta, R.; Bedadeep, D.; Narayanasamy, S. Surface treated acid-activated carbon for adsorption of anionic azo dyes from single and binary adsorptive systems: A detail insight. Environ. Pollut. 2020, 266, 115102. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, S.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of CO2 on activated carbons prepared by chemical activation with cupric nitrate. ACS Omega 2020, 5, 10423–10432. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Peng, L.; Liu, Y.; Zhao, G.; Chen, J.Y.; Yu, G. Biobased nano porous active carbon fibers for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 15205–15215. [Google Scholar] [CrossRef]
- Abuzalat, O.; Wong, D.; Elsayed, M.A. Nano-porous composites of activated carbon–metal organic frameworks (Fe-BDC@ AC) for rapid removal of Cr (VI): Synthesis, adsorption, mechanism, and kinetics studies. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1924–1934. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr (VI), Cu (II) and Cd (II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Reçber, Z.B.; Burhan, H.; Bayat, R.; Nas, M.S.; Calimli, M.H.; Demirbas, Ö.; Şen, F.; Hassan, K.-M. Fabrication of activated carbon supported modified with bimetallic-platin ruthenium nano sorbent for removal of azo dye from aqueous media using enhanced ultrasonic wave. Environ. Pollut. 2022, 302, 119033. [Google Scholar] [CrossRef]
- Deng, J.; Li, B.; Yin, W.; Bu, H.; Yang, B.; Li, P.; Zheng, X.; Wu, J. Enhanced bacterial inactivation by activated carbon modified with nano-sized silver oxides: Performance and mechanism. J. Environ. Manag. 2022, 311, 114884. [Google Scholar] [CrossRef]
- Yahya, E.B.; Jummaat, F.; Amirul, A.; Adnan, A.; Olaiya, N.; Abdullah, C.; Rizal, S.; Mohamad Haafiz, M.; Abdul Khalil, H.P.S. A review on revolutionary natural biopolymer-based aerogels for antibacterial delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, W.; Shen, G.; Ji, G.; Zhang, Y.; Gao, C.; Han, L. Carbonization and ball milling on the enhancement of Pb (II) adsorption by wheat straw: Competitive effects of ion exchange and precipitation. Bioresour. Technol. 2019, 273, 70–76. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Fu, J.; Yuan, L.; Li, Z.; Liu, C.; Zhao, D.; Wang, X. A novel magnetic biochar/MgFe-layered double hydroxides composite removing Pb2+ from aqueous solution: Isotherms, kinetics and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 278–287. [Google Scholar] [CrossRef]
- Huang, D.; Liu, C.; Zhang, C.; Deng, R.; Wang, R.; Xue, W.; Luo, H.; Zeng, G.; Zhang, Q.; Guo, X. Cr (VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresour. Technol. 2019, 276, 127–132. [Google Scholar] [CrossRef]
- Lin, P.; Wu, J.; Ahn, J.; Lee, J. Adsorption characteristics of Cd (II) and Ni (II) from aqueous solution using succinylated hay. Int. J. Miner. Metall. Mater. 2019, 26, 1239–1246. [Google Scholar] [CrossRef]
- Liang, S.; Shi, S.; Zhang, H.; Qiu, J.; Yu, W.; Li, M.; Gan, Q.; Yu, W.; Xiao, K.; Liu, B. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr (VI) in an aqueous solution. Sci. Total Environ. 2019, 695, 133886. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Panchal, M.; Minugu, O.P.; Gujjala, R.; Ojha, S.; Mallampati Chowdary, S.; Mohammad, A. Study of environmental behavior and its effect on solid particle erosion behavior of hierarchical porous activated carbon-epoxy composite. Polym. Compos. 2022, 43, 2276–2287. [Google Scholar] [CrossRef]
- Xiong, B.; Zydney, A.L.; Kumar, M. Fouling of microfiltration membranes by flowback and produced waters from the Marcellus shale gas play. Water Res. 2016, 99, 162–170. [Google Scholar] [CrossRef]
- Okonji, S.O.; Yu, L.; Dominic, J.A.; Pernitsky, D.; Achari, G. Adsorption by granular activated carbon and nano zerovalent iron from wastewater: A study on removal of selenomethionine and selenocysteine. Water 2020, 13, 23. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Arenas, L.R.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process. Sci. Total Environ. 2021, 791, 148175. [Google Scholar] [CrossRef]
- Altmann, J.; Rehfeld, D.; Träder, K.; Sperlich, A.; Jekel, M. Combination of granular activated carbon adsorption and deep-bed filtration as a single advanced wastewater treatment step for organic micropollutant and phosphorus removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef]
- Ambika, S.; Kumar, M.; Pisharody, L.; Malhotra, M.; Kumar, G.; Sreedharan, V.; Singh, L.; Nidheesh, P.; Bhatnagar, A. Modified biochar as a green adsorbent for removal of hexavalent chromium from various environmental matrices: Mechanisms, methods, and prospects. Chem. Eng. J. 2022, 439, 135716. [Google Scholar] [CrossRef]
- Shokry, H.; Elkady, M.; Hamad, H. Nano activated carbon from industrial mine coal as adsorbents for removal of dye from simulated textile wastewater: Operational parameters and mechanism study. J. Mater. Res. Technol. 2019, 8, 4477–4488. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Kamarehie, B.; Almasi, A.; Darvishmotevalli, M.; Salari, M.; Moradnia, M.; Azimi, F.; Ghaderpoori, M.; Neyazi, Z.; Karami, M.A. Removal of Rhodamine B from aqueous solution by stalk corn activated carbon: Adsorption and kinetic study. Biomass Convers. Biorefinery 2021, 1–10. [Google Scholar] [CrossRef]
- Feiqiang, G.; Xiaolei, L.; Xiaochen, J.; Xingmin, Z.; Chenglong, G.; Zhonghao, R. Characteristics and toxic dye adsorption of magnetic activated carbon prepared from biomass waste by modified one-step synthesis. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 43–54. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Chang, S.X. Pyrolysis temperature and steam activation effects on sorption of phosphate on pine sawdust biochars in aqueous solutions. Chem. Speciat. Bioavailab. 2016, 28, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Valentín-Reyes, J.; García-Reyes, R.; García-González, A.; Soto-Regalado, E.; Cerino-Córdova, F. Adsorption mechanisms of hexavalent chromium from aqueous solutions on modified activated carbons. J. Environ. Manag. 2019, 236, 815–822. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, P.; Li, X.; Zhang, G. Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chem. Eng. J. 2017, 322, 129–139. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Zhang, C.; Lai, C.; Zeng, G.-M.; Cheng, M.; Gong, X.-M.; Wan, J.; Luo, H. Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour. Technol. 2018, 261, 265–271. [Google Scholar] [CrossRef]
- Mubarak, M.F.; Zayed, A.M.; Ahmed, H.A. Activated Carbon/Carborundum@ Microcrystalline Cellulose core shell nano-composite: Synthesis, characterization and application for heavy metals adsorption from aqueous solutions. Ind. Crop. Prod. 2022, 182, 114896. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb (II) and Cd (II) by magnetic activated carbon and its mechanism. Sci. Total Environ. 2021, 757, 143910. [Google Scholar] [CrossRef]
- Zabihi, M.; Omidvar, M.; Motavalizadehkakhky, A.; Zhiani, R. Competitive adsorption of arsenic and mercury on nano-magnetic activated carbons derived from hazelnut shell. Korean J. Chem. Eng. 2022, 39, 367–376. [Google Scholar] [CrossRef]
- Lee, M.-E.; Park, J.H.; Chung, J.W. Comparison of the lead and copper adsorption capacities of plant source materials and their biochars. J. Environ. Manag. 2019, 236, 118–124. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; He, Y.; Kong, D.; Klein, B.; Yin, S.; Zhao, H. Preparation of Magnetic Activated Carbon by Activation and Modification of Char Derived from Co-Pyrolysis of Lignite and Biomass and Its Adsorption of Heavy-Metal-Containing Wastewater. Minerals 2022, 12, 665. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Kim, S.-H.; Kang, S.-W.; Jeong, C.Y.; Jeon, J.-R.; Park, K.H.; Cho, J.-S.; Delaune, R.D.; Seo, D.-C. Cadmium adsorption characteristics of biochars derived using various pine tree residues and pyrolysis temperatures. J. Colloid Interface Sci. 2019, 553, 298–307. [Google Scholar] [CrossRef]
- Laffon, B.; Pásaro, E.; Valdiglesias, V. Effects of exposure to oil spills on human health: Updated review. J. Toxicol. Environ. Health 2016, 19, 105–128. [Google Scholar] [CrossRef]
- Shokry, H.; Elkady, M.; Salama, E. Eco-friendly magnetic activated carbon nano-hybrid for facile oil spills separation. Sci. Rep. 2020, 10, 10265. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Sun, Y.; Yu, I.K.; Tsang, D.C.; Hou, D.; Gupta, J.; Bhaskar, T.; Pandey, A. Critical review on biochar-supported catalysts for pollutant degradation and sustainable biorefinery. Adv. Sustain. Syst. 2020, 4, 1900149. [Google Scholar] [CrossRef]
- Ben Hammouda, S.; Chen, Z.; An, C.; Lee, K.; Zaker, A. Buoyant oleophilic magnetic activated carbon nanoparticles for oil spill cleanup. Clean. Chem. Eng. 2022, 2, 100028. [Google Scholar] [CrossRef]
- Raj, K.G.; Joy, P.A. Coconut shell based activated carbon–iron oxide magnetic nanocomposite for fast and efficient removal of oil spills. J. Environ. Chem. Eng. 2015, 3, 2068–2075. [Google Scholar] [CrossRef]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.-H.; Kirkham, M. Multifunctional applications of biochar beyond carbon storage. Int. Mater. Rev. 2022, 67, 150–200. [Google Scholar] [CrossRef]
- Kao, T.H.; Chen, S.; Chen, C.J.; Huang, C.W.; Chen, B.H. Evaluation of analysis of polycyclic aromatic hydrocarbons by the QuEChERS method and gas chromatography–mass spectrometry and their formation in poultry meat as affected by marinating and frying. J. Agric. Food Chem. 2012, 60, 1380–1389. [Google Scholar] [CrossRef]
- Premnath, N.; Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.; Prakash, G.S.; Ananthi, V.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A crucial review on polycyclic aromatic Hydrocarbons-Environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Sridhar, K.; Chen, B.-H. Removal of polycyclic aromatic hydrocarbons from water by magnetic activated carbon nanocomposite from green tea waste. J. Hazard. Mater. 2021, 415, 125701. [Google Scholar] [CrossRef]
- Albuquerque Júnior, E.C.D.; Méndez, M.O.A.; Coutinho, A.D.R.; Franco, T.T. Removal of cyanobacteria toxins from drinking water by adsorption on activated carbon fibers. Mater. Res. 2008, 11, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Rambabu, K.; AlYammahi, J.; Bharath, G.; Thanigaivelan, A.; Sivarajasekar, N.; Banat, F. Nano-activated carbon derived from date palm coir waste for efficient sequestration of noxious 2, 4-dichlorophenoxyacetic acid herbicide. Chemosphere 2021, 282, 131103. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Omoarukhe, F.O.; Ojukwu, V.E.; Iwuozor, K.O.; Igwegbe, C.A. Cost of adsorbent preparation and usage in wastewater treatment: A review. Clean. Chem. Eng. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Luo, W.; Xiao, G.; Tian, F.; Richardson, J.J.; Wang, Y.; Zhou, J.; Guo, J.; Liao, X.; Shi, B. Engineering robust metal–phenolic network membranes for uranium extraction from seawater. Energy Environ. Sci. 2019, 12, 607–614. [Google Scholar] [CrossRef]
- Yadav, A.K.; Yadav, H.K.; Naz, A.; Koul, M.; Chowdhury, A.; Shekhar, S. Arsenic removal technologies for middle-and low-income countries to achieve the SDG-3 and SDG-6 targets: A review. Environ. Adv. 2022, 9, 100262. [Google Scholar] [CrossRef]
- Sawalha, H.; Bader, A.; Sarsour, J.; Al-Jabari, M.; Rene, E.R. Removal of Dye (Methylene Blue) from Wastewater Using Bio-Char Derived from Agricultural Residues in Palestine: Performance and Isotherm Analysis. Processes 2022, 10, 2039. [Google Scholar] [CrossRef]







| Type of Activation | Precursor Material | Pyrolysis Conditions | Activation Agent | Removing Material | Adsorption Capacity | Reference |
|---|---|---|---|---|---|---|
| Physical activation | Palm kernel shells | 700 W for 30 min | Steam | Herbicides | 11 mg/g | [56] |
| Biomass straw | 600 °C for 20 min | Microwave and ultrasound | Elemental mercury | 7.23 mg/g | [57] | |
| Cattle manure | 850 °C for 60 min | CO2 | Hydrogen sulfide | 868.45 mg/g | [58] | |
| Corn cob residue | 600 W for 20 min | Microwave and H3PO4 | Organic dyes | 183.3 mg/g | [59] | |
| Bamboo | 900 °C for 120 min | Steam and thermal | Xenon | 158.49 g/g | [60] | |
| Chemical activation | Prawn shells | 800 °C for 180 min | KOH and HCl | Heavy metals | 560 mg/g | [61] |
| Pistachio shells | 1000 °C for 240 min | CaHPO4 | Organic dyes | - | [48] | |
| Lignocellulosic waste | 500 °C for 120 min | H3PO4 | Pesticide | 35.7 mg/g | [62] | |
| Cashew nut shells | 500 °C for 120 min | ZnCl2 | Dyes | 476 mg/g | [63] | |
| Animal bone waste | 600 °C for 120 min | Orthophosphoric acid | Heavy metals | 27.86 mg/g | [64] | |
| Molasses | 500 °C for 120 min | H3PO4 | Organic dyes | 625 mg/g | [65] |
| Agricultural Waste | Pyrolysis Condition | Type of Metal | Surface Area (m2/g) | Maximum Adsorption (mg/g) | Reference |
|---|---|---|---|---|---|
| Coconut shell | Commercial | I− | 1220 | 41.2 | [105] |
| Bamboo waste | 700 °C for 2 h | Cd(II) | 6.79 | 73.45 | [106] |
| Pig manure | 700 °C for 2 h | Cd(II) | 11.37 | 77.34 | [106] |
| Kenaf core fiber | 400 °C for 1 h | As (III) | 1031 | 422.9 | [107] |
| Rape straw | 300 °C for 2 h | Pb(II) | 699.9 | 253.2 | [108] |
| Hazelnut shell | 700 °C for 2 h | Hg | - | 80.0 | [109] |
| Gingko leaf | 400 °C for 1.5 h | Cu(II) | 310.00 | 59.90 | [110] |
| Lignite and poplar leaves | 600 °C for 0.5 h | Pb(II) | 805.86 | 10.55 | [111] |
| Pine tree residue | 600 °C for 4 h | Cd(II) | N.A | 85.8 | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, S.; Abdul Khalil, H.P.S.; Abd Hamid, S.; Albadn, Y.M.; Suriani, A.B.; Kamaruzzaman, S.; Mohamed, A.; Allaq, A.A.; Yahya, E.B. Insights into Agricultural-Waste-Based Nano-Activated Carbon Fabrication and Modifications for Wastewater Treatment Application. Agriculture 2022, 12, 1737. https://doi.org/10.3390/agriculture12101737
Muhammad S, Abdul Khalil HPS, Abd Hamid S, Albadn YM, Suriani AB, Kamaruzzaman S, Mohamed A, Allaq AA, Yahya EB. Insights into Agricultural-Waste-Based Nano-Activated Carbon Fabrication and Modifications for Wastewater Treatment Application. Agriculture. 2022; 12(10):1737. https://doi.org/10.3390/agriculture12101737
Chicago/Turabian StyleMuhammad, Syaifullah, H. P. S. Abdul Khalil, Shazlina Abd Hamid, Yonss M. Albadn, A. B. Suriani, Suraiya Kamaruzzaman, Azmi Mohamed, Abdulmutalib A. Allaq, and Esam Bashir Yahya. 2022. "Insights into Agricultural-Waste-Based Nano-Activated Carbon Fabrication and Modifications for Wastewater Treatment Application" Agriculture 12, no. 10: 1737. https://doi.org/10.3390/agriculture12101737










