Comparative Transcriptome Analysis Reveals the Key Genes Involved in Lipid Deposition in Pekin Ducks (Anas platyrhynchos domesticus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Data Source
2.2. Transcriptome Sequencing
2.3. Transcriptome Data Validation by qPCR
2.4. Alignment of High-Quality Reads to Reference
2.5. Analysis of Difference Expression Genes
2.6. Pathway Analysis of Kyoto Encyclopedia Genes and Genomes (KEGG)
2.7. Weight Gene Co-Expression Network Analysis
3. Results
3.1. Clustering Analysis for DEGs among Samples
3.2. Transcriptome Data Validation by qPCR
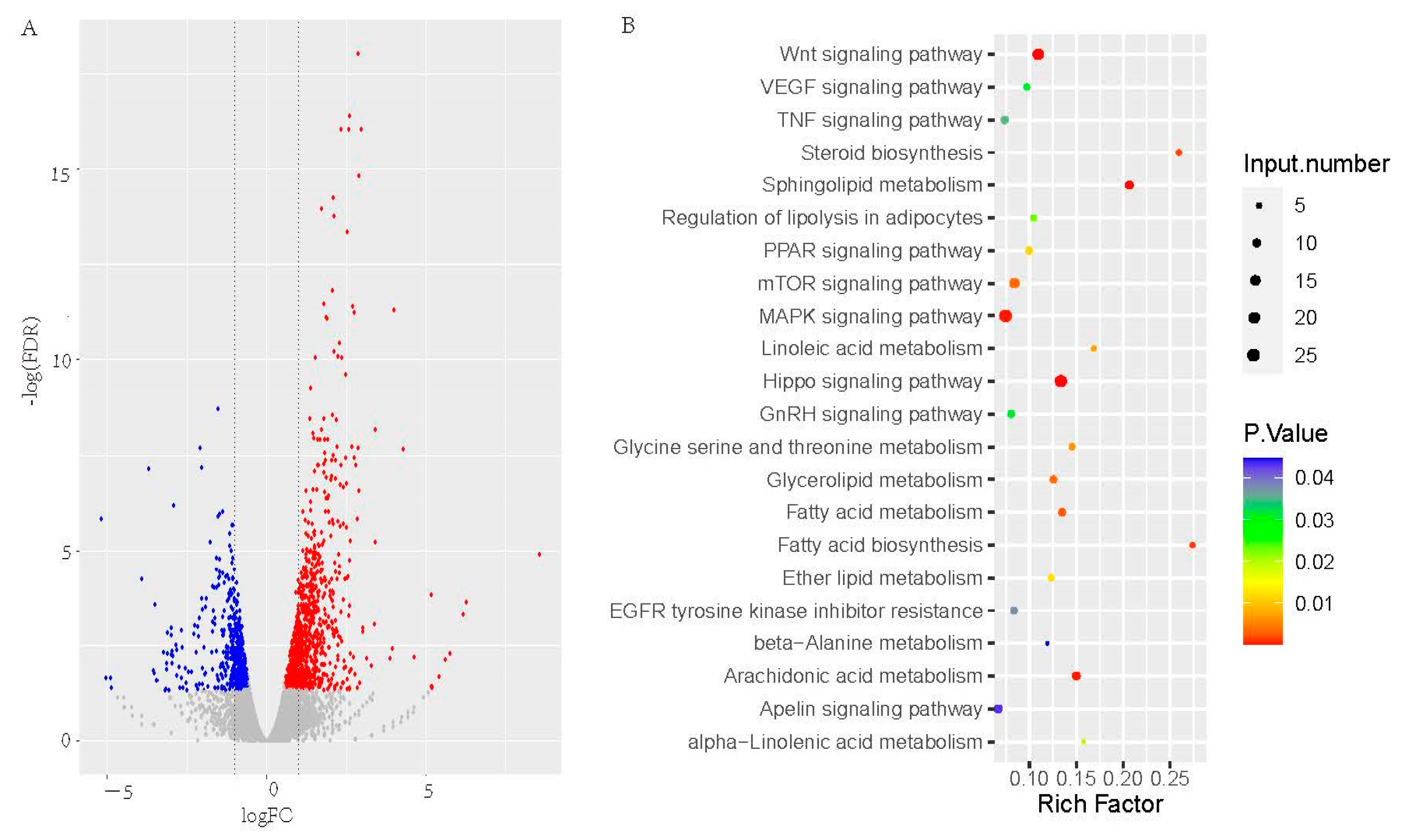
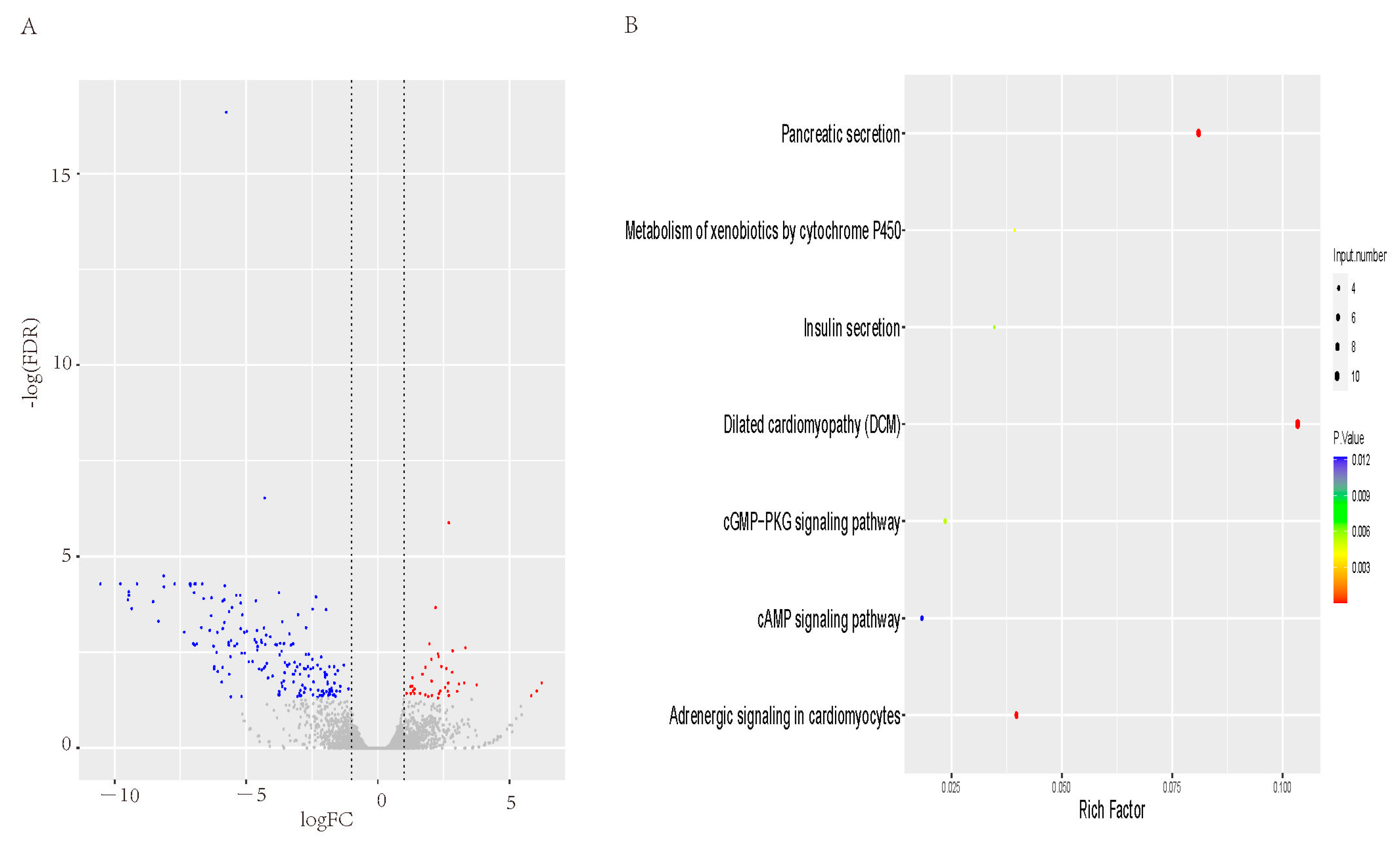
3.3. Differential Expression Genes KEGG Analysis in Liver between FT and LT Ducks
3.4. Differential Expression Genes and KEGG Analysis in Sebum between FT and LT Ducks
3.5. Differential Expression Genes and KEGG Analysis in Abdominal fat between FT and LT Ducks
3.6. Co-Expression Network Analyzed by WGCNA between Fatty and LT Ducks
3.7. Functional Annotation of Highly Correlated Module
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Xu, T.; Gu, L.; Schachtschneider, K.; Liu, X.; Huang, W.; Xie, M.; Hou, S. Identification of differentially expressed genes in breast muscle and skin fat of postnatal Pekin duck. PLoS ONE 2014, 9, e107574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, A.; Chang, W.; Hou, S.; Zhang, S.; Cai, H.; Chen, G.; Lou, R.; Liu, G. Unraveling molecular mechanistic differences in liver metabolism between lean and fat lines of Pekin duck (Anas platyrhynchos domestica): A proteomic study. J. Proteom. 2014, 98, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.R.; Li, G.S.; Chen, S.R.; Zhu, F.; Hao, J.P.; Yang, F.X.; Hou, Z.C. Comparison of carcass and meat quality traits between lean and fat Pekin ducks. Anim. Biosci. 2021, 34, 1193–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhat, A.; Chavez, E. Metabolic studies on lean and fat Pekin ducks selected for breast muscle thickness measured by ultrasound scanning. Poult. Sci. 2001, 80, 585–591. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, M.; Tang, J.; Zhou, Z.; Zhang, Y.; Chen, G.; Hou, S. Effects of genetic selection and threonine on meat quality in Pekin ducks. Poult. Sci. 2020, 99, 2508–2518. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Kuo, M.S.; Li, S.Y.; Bui, H.H.; Peake, D.A.; Sanders, P.E.; Thibodeaux, S.J.; Chu, S.; Qian, Y.-W.; Zhao, Y.; et al. AGPAT6 is a novel microsomal glycerol-3-phosphate acyltransferase. J. Biol. Chem. 2008, 283, 10048–10057. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.K.; Sukumaran, S.; Cortés, V.A.; Tunison, K.; Mizrachi, D.; Sankella, S.; Gerard, R.D.; Horton, J.D.; Garg, A. Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: Biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice. J. Biol. Chem. 2011, 286, 37676–37691. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.L.; Farese, R.V., Jr. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 2003, 278, 18532–18537. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Qian, H.; Nian, Y.; Han, Y.; Ren, Z.; Zhang, H.; Hu, L.; Prasad, B.V.V.; Laganowsky, A.; Yan, N.; et al. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature 2020, 581, 329–332. [Google Scholar] [CrossRef]
- Brasaemle, D.L.; Subramanian, V.; Garcia, A.; Marcinkiewicz, A.; Rothenberg, A. Perilipin A and the control of triacylglycerol metabolism. Mol. Cell Biochem. 2009, 326, 15–21. [Google Scholar] [CrossRef]
- Zechner, R.; Kienesberger, P.C.; Haemmerle, G.; Zimmermann, R.; Lass, A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 2009, 50, 3–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Ming, X.I.E.; Zhou, Z.K.; Jing, T.A.N.G.; Chang, G.B.; Chen, G.H.; Hou, S.S. Dietary threonine deficiency affects expression of genes involved in lipid metabolism in adipose tissues of Pekin ducks in a genotype-dependent manner. J. Integr. Agric. 2022, 21, 2691–2699. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Mao, X.; Tao, C.; Luo, J.; Wei, L. KOBAS server: A web-based platform for automated annotation and pathway identification. Nucleic Acids Res. 2006, 34, W720–W724. [Google Scholar] [CrossRef] [Green Version]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadoun, A.; Leclercq, B. In vivo lipogenesis in genetically fat and lean chickens of various ages. Comp. Biochem. Physiol. B 1986, 83, 607–611. [Google Scholar] [CrossRef]
- Saadoun, A.; Leclercq, B. Comparison of in vivo fatty acid synthesis of the genetically lean and fat chickens. Comp. Biochem. Physiol. B Comp. Biochem. 1983, 75, 641–644. [Google Scholar] [CrossRef]
- Weisiger, R. Cytosolic fatty acid binding proteins catalyze two distinct steps in intracellular transport of their ligands. Mol. Cell. Biochem. 2002, 239, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Prows, D.R.; Jefferson, J.R.; Schroeder, F. Liver fatty acid-binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim. Biophys. Acta 1996, 1301, 191–198. [Google Scholar] [CrossRef]
- Luxon, B.A. Inhibition of binding to fatty acid binding protein reduces the intracellular transport of fatty acids. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 271, G113–G120. [Google Scholar] [CrossRef]
- Wu, C.; Chen, C.; Liu, Y.; Li, H.; Cheng, B. Proteomic analysis of liver tissue between fat and lean broiler lines. Br. Poult. Sci. 2021, 62, 211–218. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Zhang, X.; Gu, X.; Wang, N.; Wu, S.; Li, H. Microarray analysis of genes differentially expressed in the liver of lean and fat chickens. Anim. Int. J. Anim. Biosci. 2010, 4, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Spann, N.; Kang, S.; Li, A.; Chen, A.; Newberry, E.; Davidson, N.; Hui, S.; Davis, R. Coordinate transcriptional repression of liver fatty acid-binding protein and microsomal triglyceride transfer protein blocks hepatic very low density lipoprotein secretion without hepatosteatosis. J. Biol. Chem. 2006, 281, 33066–33077. [Google Scholar] [CrossRef] [Green Version]
- Swierczynski, J.; Zabrocka, L.; Goyke, E.; Raczynska, S.; Adamonis, W.; Sledzinski, Z. Enhanced glycerol 3-phosphate dehydrogenase activity in adipose tissue of obese humans. Mol. Cell. Biochem. 2003, 254, 55–59. [Google Scholar] [CrossRef]
- Clarke, S.D.; Nakamura, M.T. Fatty Acid Synthesis and its Regulation. Encycl. Biol. Chem. 2004, 52, 99–103. [Google Scholar]
- Wang, J.; Yu, L.; Schmidt, R.; Su, C.; Huang, X.; Gould, K.; Cao, G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem. Biophys. Res. Commun. 2005, 332, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Goren, M.; Fox, B. Wheat germ cell-free translation, purification, and assembly of a functional human stearoyl-CoA desaturase complex. Protein Expr. Purif 2008, 62, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daval, S.; Lagarrigue, S.; Douaire, M. Messenger RNA levels and transcription rates of hepatic lipogenesis genes in genetically lean and fat chickens. Genet. Sel. Evol. GSE 2000, 32, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourneuf, E.; Hérault, F.; Chicault, C.; Carré, W.; Assaf, S.; Monnier, A.; Mottier, S.; Lagarrigue, S.; Douaire, M.; Mosser, J.; et al. Microarray analysis of differential gene expression in the liver of lean and fat chickens. Gene 2006, 372, 162–170. [Google Scholar] [CrossRef]
- Xing, K.; Liu, H.; Zhang, F.; Liu, Y.; Wang, C. Identification of Key Genes Affecting Porcine Fat Deposition Based on Co-Expression Network Analysis of Weighted Genes. J. Anim. Sci. Biotechnol. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Jiang, X.C. Liver phospholipid transfer protein (PLTP) expression with a PLTP-null background promotes very low-density lipoprotein production in mice. Hepatology 2012, 56, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Lie, J.; Crom, R.D.; Gent, T.V.; Haperen, R.V.; Tol, A.V. Elevation of plasma phospholipid transfer protein in transgenic mice increases VLDL secretion. J. Lipid Res. 2002, 43, 1875–1880. [Google Scholar] [CrossRef] [Green Version]
- Yazdanyar, A.; Quan, W.; Jin, W.; Jiang, X. Liver-specific phospholipid transfer protein deficiency reduces high-density lipoprotein and non-high-density lipoprotein production in mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2058–2064. [Google Scholar] [CrossRef] [Green Version]
- Jauhiainen, M.; Metso, J.; Pahlman, R.; Blomqvist, S.; Ehnholm, C. Human plasma phospholipid transfer protein causes high density lipoprotein conversion. J. Biol. Chem. 1993, 268, 4032–4036. [Google Scholar] [CrossRef]
- Yu, L.; Li-Hawkins, J.; Hammer, R.E.; Berge, K.E.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Investig. 2002, 110, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Santis, M.; Hammamieh, R.; Das, R.; Jett, M. Adipocyte-fatty acid binding protein induces apoptosis in DU145 prostate cancer cells. J. Exp. Ther. Oncol. 2004, 4, 91–100. [Google Scholar] [PubMed]
- Mashek, D.; Bornfeldt, K.; Coleman, R.; Berger, J.; Bernlohr, D.; Black, P.; DiRusso, C.; Farber, S.; Guo, W.; Hashimoto, N.; et al. Revised nomenclature for the mammalian long-chain acyl-CoA synthetase gene family. J. Lipid Res. 2004, 45, 1958–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, L.; Tocher, D.; Monroig, O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Prog. Lipid Res. 2016, 62, 25–40. [Google Scholar] [CrossRef]
- Naganuma, T.; Kihara, A. Two modes of regulation of the fatty acid elongase ELOVL6 by the 3-ketoacyl-CoA reductase KAR in the fatty acid elongation cycle. PLoS ONE 2014, 9, e101823. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Chen, H.; Tang, X.; Zhao, J.; Zhang, H.; Chen, Y.; Chen, W. Δ6 fatty acid desaturases in polyunsaturated fatty acid biosynthesis: Insights into the evolution, function with substrate specificities and biotechnological use. Appl. Microbiol. Biotechnol. 2020, 104, 9947–9963. [Google Scholar] [CrossRef]
- Ge, L.; Gordon, J.S.; Hsuan, C.; Stenn, K.; Prouty, S.M. Identification of the delta-6 desaturase of human sebaceous glands: Expression and enzyme activity. J. Investig. Dermatol. 2003, 120, 707–714. [Google Scholar] [CrossRef]
- Leonard, A.E.; Kelder, B.; Bobik, E.G.; Chuang, L.T.; Parker-Barnes, J.M.; Thurmond, J.M.; Kroeger, P.E.; Kopchick, J.J.; Huang, Y.S.; Mukerji, P. cDNA cloning and characterization of human Delta5-desaturase involved in the biosynthesis of arachidonic acid. Biochem. J. 2000, 347 Pt 3, 719–724. [Google Scholar] [CrossRef]
- Ellis, J.M.; Li, L.O.; Wu, P.C.; Koves, T.R.; Ilkayeva, O.; Stevens, R.D.; Watkins, S.M.; Muoio, D.M.; Coleman, R.A. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab. 2010, 12, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Li, L.O.; Mashek, D.G.; An, J.; Doughman, S.D.; Newgard, C.B.; Coleman, R.A. Overexpression of Rat Long Chain Acyl-CoA Synthetase 1 Alters Fatty Acid Metabolism in Rat Primary Hepatocytes. J. Biol. Chem. 2006, 281, 37246–37255. [Google Scholar] [CrossRef] [Green Version]
- Benítez, R.; Trakooljul, N.; Núñez, Y.; Isabel, B.; Murani, E.; De Mercado, E.; Gómez-Izquierdo, E.; García-Casco, J.; López-Bote, C.; Wimmers, K.; et al. Breed, Diet, and Interaction Effects on Adipose Tissue Transcriptome in Iberian and Duroc Pigs Fed Different Energy Sources. Genes 2019, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Resnyk, C.W.; Carré, W.; Wang, X.; Porter, T.E.; Cogburn, L.A. Transcriptional analysis of abdominal fat in genetically fat and lean chickens reveals adipokines, lipogenic genes and a link between hemostasis and leanness. BMC Genom. 2013, 14, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, B.; Leng, L.; Li, Z.; Wang, W.; Jing, Y.; Li, Y.; Wang, N.; Li, H.; Wang, S. Profiling of RNA -Methyladenosine Methylation Reveals the Critical Role of mA in Chicken Adipose Deposition. Front. Cell Dev. Biol. 2021, 9, 590468. [Google Scholar] [CrossRef] [PubMed]
- Resnyk, C.; Chen, C.; Huang, H.; Wu, C.; Simon, J.; Le Bihan-Duval, E.; Duclos, M.; Cogburn, L. RNA-Seq Analysis of Abdominal Fat in Genetically Fat and Lean Chickens Highlights a Divergence in Expression of Genes Controlling Adiposity, Hemostasis, and Lipid Metabolism. PLoS ONE 2015, 10, e0139549. [Google Scholar]
- Ghafouri, F.; Bahrami, A.; Sadeghi, M.; Miraei-Ashtiani, S.; Bakherad, M.; Barkema, H.; Larose, S. Omics Multi-Layers Networks Provide Novel Mechanistic and Functional Insights Into Fat Storage and Lipid Metabolism in Poultry. Front. Genet. 2021, 12, 646297. [Google Scholar] [CrossRef]
- Vassiliou, G.; Benoist, F.; Lau, P.; Kavaslar, G.; McPherson, R. The low density lipoprotein receptor-related protein contributes to selective uptake of high density lipoprotein cholesteryl esters by SW872 liposarcoma cells and primary human adipocytes. J. Biol. Chem. 2001, 276, 48823–48830. [Google Scholar] [CrossRef] [Green Version]
- Argov, N.; Moallem, U.; Sklan, D. Lipid transport in the developing bovine follicle: Messenger RNA expression increases for selective uptake receptors and decreases for endocytosis receptors. Biol. Reprod. 2004, 71, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Wiese, S.; Gronemeyer, T.; Ofman, R.; Kunze, M.; Grou, C.P.; Almeida, J.A.; Eisenacher, M.; Stephan, C.; Hayen, H.; Schollenberger, L.; et al. Proteomics characterization of mouse kidney peroxisomes by tandem mass spectrometry and protein correlation profiling. Mol. Cell Proteom. 2007, 6, 2045–2057. [Google Scholar] [CrossRef] [Green Version]
- Yagita, Y.; Shinohara, K.; Abe, Y.; Nakagawa, K.; Al-Owain, M.; Alkuraya, F.S.; Fujiki, Y. Deficiency of a Retinal Dystrophy Protein, Acyl-CoA Binding Domain-containing 5 (ACBD5), Impairs Peroxisomal β-Oxidation of Very-long-chain Fatty Acids. J. Biol. Chem. 2017, 292, 691–705. [Google Scholar] [CrossRef] [Green Version]
- Ferdinandusse, S.; Falkenberg, K.D.; Koster, J.; Mooyer, P.A.; Jones, R.; van Roermund, C.W.T.; Pizzino, A.; Schrader, M.; Wanders, R.J.A.; Vanderver, A.; et al. ACBD5 deficiency causes a defect in peroxisomal very long-chain fatty acid metabolism. J. Med. Genet. 2017, 54, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.S.; Li, J.L.; Li, D.M.; Tobin, J.F.; Gimeno, R.E. Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19695–19700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukumaran, S.; Barnes, R.I.; Garg, A.; Agarwal, A.K. Functional characterization of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 10/glycerol-3-phosphate acyltransferase isoform 3. J. Mol. Endocrinol. 2009, 42, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Onorato, J.M.; Chen, L.; Nelson, D.W.; Yen, C.-L.E.; Cheng, D. Synthesis of neutral ether lipid monoalkyl-diacylglycerol by lipid acyltransferases. J. Lipid Res. 2017, 58, 1091–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V., Jr. The life of lipid droplets. Biochim. Biophys. Acta 2009, 1791, 459–466. [Google Scholar] [CrossRef]
- Ducharme, N.A.; Bickel, P.E. Lipid droplets in lipogenesis and lipolysis. Endocrinology 2008, 149, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, V.; Rothenberg, A.; Gomez, C.; Cohen, A.W.; Garcia, A.; Bhattacharyya, S.; Shapiro, L.; Dolios, G.; Wang, R.; Lisanti, M.P.; et al. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J. Biol. Chem. 2004, 279, 42062–42071. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Omatsu, N.; Morimoto, E.; Nakashima, H.; Osumi, T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J. Lipid Res. 2007, 48, 1078–1089. [Google Scholar]
- Thompson, B.; Sahai, E. MST kinases in development and disease. J. Cell Biol. 2015, 210, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Nerstedt, A.; Kurhe, Y.; Cansby, E.; Caputo, M.; Gao, L.; Vorontsov, E.; Stahlman, M.; Nunez-Duran, E.; Boren, J.; Marschall, H.-U.; et al. Lipid droplet-associated kinase STK25 regulates peroxisomal activity and metabolic stress response in steatotic liver. J. Lipid Res. 2020, 61, 178–191. [Google Scholar] [CrossRef]
- Amrutkar, M.; Cansby, E.; Chursa, U.; Nunez-Duran, E.; Chanclon, B.; Stahlman, M.; Friden, V.; Manneras-Holm, L.; Wickman, A.; Smith, U.; et al. Genetic Disruption of Protein Kinase STK25 Ameliorates Metabolic Defects in a Diet-Induced Type 2 Diabetes Model. Diabetes 2015, 64, 2791–2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrutkar, M.; Cansby, E.; Nunez-Duran, E.; Pirazzi, C.; Stahlman, M.; Stenfeldt, E.; Smith, U.; Boren, J.; Mahlapuu, M. Protein kinase STK25 regulates hepatic lipid partitioning and progression of liver steatosis and NASH. FASEB J. 2015, 29, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Amrutkar, M.; Kern, M.; Nunez-Duran, E.; Stahlman, M.; Cansby, E.; Chursa, U.; Stenfeldt, E.; Boren, J.; Blueher, M.; Mahlapuu, M. Protein kinase STK25 controls lipid partitioning in hepatocytes and correlates with liver fat content in humans. Diabetologia 2016, 59, 341–353. [Google Scholar] [CrossRef] [PubMed]







| Gene | Genebank ID | Product (bp) | Primer Sequence |
|---|---|---|---|
| GAPDH | XM_0050167 | 104 | F: 5′-AGATGCTGGTGCTGAATACG-3′ |
| R: 5′-CGGAGATGATGACACGCTTA-3′ | |||
| ELOVL6 | XM_038178819.1 | 126 | F: 5′-CTGGCGGTGGCTGGTTTATGAC-3′ |
| R: 5′-TCTGCGACAAGGTGATGAACATGG-3′ | |||
| GPAM | XM_038181452.1 | 101 | F: 5′-AAACCTGTGCCTGCTCCTCTTTC-3′ |
| R: 5′-AACGAAGCCTCTCTACCCTCATCC-3′ | |||
| ACSL3 | XM_027464027.2 | 85 | F: 5′-TGATCCTTGGACACCTGAGACTGG-3′ |
| R: 5′-GTCCTGTTGATAGTACGCCGTAAGC-3′ | |||
| ACSL6 | XM_027468211.2 | 131 | F: 5′-GCAGGAGGTGGAGGATTGT-3′ |
| R: 5′-TCCCGTGAGTCAGCATAGC-3′ | |||
| MOGAT1 | XM_005026421.4 | 91 | F: 5′-TTCTGTACCTCGTGTGGCTCTACC-3′ |
| R: 5′-TTCCAGACAGTCCAGCTCCTGAC-3′ | |||
| STK39 | XM_038182285.1 | 80 | F: 5′-CAGGTGCGAGGTTATGACTTCAAGG-3′ |
| R: 5′-GGTGCTGCTCCTGTTGCTAACTC-3′ | |||
| FASN | XM_027471234.2 | 102 | F: 5′-TCTCTGCCATCTCCCGAACTTCC-3′ |
| R: 5′-TCTCAATTAGCCACTGTGCCAACTC-3′ | |||
| FABP7 | XM_027454608.2 | 112 | F: 5′-ACGTACAGAAGTGGGATGGCAAAG-3′ |
| R: 5′-TTCTCATAGTGGCGAACAGCAACC-3′ | |||
| SCD | XM_027460089.2 | 137 | F: 5′-CTTCCACAACTACCACCACACCTTC-3′ |
| R: 5′-CCTTGGAGACCTTCTTGCGATCAC-3′ | |||
| AGPAT9 | XM_040698969.1 | 116 | F: 5′-TGGTCTCCTGGAATCTCCTCACAAG-3′ |
| R: 5′-GAGTGGCAGAAGGAAGCAGTATCG-3′ | |||
| ATGL | NM_001310387.1 | 122 | F: 5′-CAATCACAGTGTCTCCGTTCTCAGG-3′ |
| R: 5′-GCGGTAGAGGTTGCGAAGGTTG-3′ | |||
| APOBEC2 | XM_005026827.5 | 237 | F: 5′-TCCAGGTAGCCCCGAGAAGT-3′ |
| R: 5′-CAACGCCCAGAACGGTGAAC-3′ | |||
| ABHD5 | XM_038174903.1 | 168 | F: 5′-CCACTTCGACGCTGATGCTC-3′ |
| R: 5′-ATAAGGTGTTTGACCCTCGAT-3′ | |||
| PLIN1 | NM_001310391.1 | 160 | F: 5′-CCTGGTCAGCACCGTCTCCC-3′ |
| R: 5′-CTGCCCCTCACCGTGGCTT-3′ |
| Gene | Log2FC | log2CPM | p-Value | FDR | Annotation |
|---|---|---|---|---|---|
| LIPG | 1.37 | 1.60 | 7.40 × 10−5 | 4.09 × 10−3 | lipase%2C endothelial |
| ETNK2 | 0.71 | 3.89 | 1.79 × 10−4 | 7.96 × 10−3 | ethanolamine kinase 2 |
| PLTP | 0.91 | 1.29 | 1.09 × 10−4 | 5.54 × 10−3 | phospholipid transfer protein |
| PLIN1 | 0.65 | 2.83 | 1.27 × 10−4 | 6.08 × 10−3 | perilipin 1 |
| FABP7 | 0.89 | 2.26 | 6.16 × 10−6 | 5.96 × 10−4 | fatty acid-binding protein 7%2C brain |
| PTGS1 | 0.98 | 3.31 | 1.97 × 10−6 | 2.27 × 10−4 | prostaglandin-endoperoxide synthase 1 (prostaglandin G/H synthase and cyclooxygenase) |
| GPD1 | 0.93 | 4.23 | 1.58 × 10−6 | 1.97 × 10−4 | glycerol-3-phosphate dehydrogenase [NAD(+)]%2C cytoplasmic-like |
| FASN | 0.72 | 8.10 | 2.87 × 10−5 | 2.00 × 10−4 | fatty acid synthase |
| PGM2 | 0.80 | 4.78 | 1.01 × 10−10 | 4.13 × 10−8 | phosphoglucomutase 2 |
| FTO | −0.82 | 3.89 | 1.14 × 10−4 | 5.69 × 10−3 | fat mass and obesity associated%2C transcript variant X1 |
| SLC5A1 | 1.06 | 0.25 | 1.30 × 10−3 | 3.23 × 10−2 | Sodium/glucose cotransporter 1 |
| ACSM4 | 0.63 | 4.02 | 2.00 × 10−4 | 8.60 × 10−3 | acyl-coenzyme A synthetase ACSM4%2C mitochondrial-like%2C transcript variant X1 |
| SCAP | −0.76 | 6.78 | 6.14 × 10−7 | 8.77 × 10−5 | SREBF chaperone |
| BMP7 | 1.29 | 0.50 | 1.93 × 10−4 | 8.42 × 10−3 | bone morphogenetic protein 7 |
| ANGPTL4 | −0.66 | 4.85 | 4.50 × 10−3 | 7.68 × 10−2 | angiopoietin-like 4 |
| SCD | 0.80 | 10.31 | 7.04 × 10−3 | 1.05 × 10−1 | stearoyl-CoA desaturase (delta-9-desaturase) |
| ABCG8 | 0.59 | 4.09 | 1.50 × 10−2 | 1.66 × 10−1 | ATP-binding cassette%2C sub-family G (WHITE)%2C member 8%2C transcript variant X1 |
| Gene | Log2FC | log2CPM | p-Value | FDR | Annotation |
|---|---|---|---|---|---|
| ABCA12 | 2.10 | 8.37 | 4.99 × 10−5 | 1.64 × 10−3 | ATP-binding cassette%2C sub-family A (ABC1)%2C member 12%2C transcript variant X4 |
| ABCB11 | 1.23 | 0.71 | 2.98 × 10−3 | 3.55 × 10−2 | ATP-binding cassette%2C sub-family B (MDR/TAP)%2C member 11%2C transcript variant X3 |
| ABHD12B | −0.70 | 4.13 | 1.82 × 10−3 | 2.49 × 10−2 | abhydrolase domain containing 12B |
| ABHD5 | −0.80 | 8.41 | 2.10 × 10−4 | 4.89 × 10−3 | abhydrolase domain containing 5%2C transcript variant X1 |
| ACAA2 | −0.73 | 8.15 | 4.33 × 10−4 | 8.46 × 10−3 | acetyl-CoA acyltransferase 2 |
| ACAD10 | −0.78 | 2.49 | 2.96 × 10−3 | 3.53 × 10−2 | acyl-CoA dehydrogenase family%2C member 10%2C transcript variant X2 |
| ACBD5 | −0.63 | 7.23 | 4.22 × 10−3 | 4.56 × 10−2 | acyl-CoA binding domain containing 5%2C transcript variant X3 |
| ACSL3 | 1.13 | 5.84 | 3.30 × 10−6 | 1.91 × 10−4 | acyl-CoA synthetase long-chain family member 3%2C transcript variant X1 |
| ACSL5 | 1.22 | 2.85 | 1.85 × 10−4 | 4.44 × 10−3 | acyl-CoA synthetase long-chain family member 5%2C transcript variant X3 |
| ACSL6 | 1.23 | 4.55 | 5.36 × 10−6 | 2.85 × 10−4 | acyl-CoA synthetase long-chain family member 6 |
| AGPAT1 | 1.53 | 2.93 | 4.49 × 10−6 | 2.44 × 10−4 | 1-acyl-sn-glycerol-3-phosphate acyltransferase alpha |
| AGPAT9 | 2.01 | 1.71 | 2.65 × 10−8 | 4.10 × 10−6 | 1-acylglycerol-3-phosphate O-acyltransferase 9 |
| ANGPTL4 | −1.01 | 5.91 | 1.73 × 10−4 | 4.18 × 10−3 | angiopoietin-like 4 |
| APOA1 | −1.53 | 12.45 | 3.18 × 10−12 | 2.03 × 10−9 | Apolipoprotein A-I |
| AQP5 | 1.81 | 3.67 | 5.71 × 10−4 | 1.06 × 10−2 | aquaporin 5 |
| AQP9 | −0.72 | 5.97 | 7.09 × 10−4 | 1.26 × 10−2 | aquaporin 9%2C transcript variant X1 |
| DGAT2 | −0.65 | 8.14 | 1.72 × 10−4 | 2.39 × 10−2 | diacylglycerol O-acyltransferase 2 |
| DGKB | 1.00 | 3.51 | 5.09 × 10−5 | 1.67 × 10−3 | diacylglycerol kinase beta-like |
| DGKD | 1.47 | 1.03 | 9.73 × 10−5 | 2.69 × 10−3 | diacylglycerol kinase delta |
| ELOVL6 | 1.50 | 5.89 | 2.58 × 10−8 | 4.04 × 10−6 | ELOVL fatty acid elongase 6%2C transcript variant X3 |
| FA2H | 1.46 | 5.77 | 8.18 × 10−4 | 1.39 × 10−2 | fatty acid 2-hydroxylase |
| FABP4 | −1.01 | 12.98 | 4.24 × 10−4 | 8.30 × 10−3 | Fatty acid-binding protein 4 |
| FADS1 | 1.59 | 4.48 | 2.18 × 10−7 | 2.37 × 10−3 | fatty acid desaturase 1-like |
| FADS6 | 1.06 | 6.09 | 7.02 × 10−5 | 2.11 × 10−3 | fatty acid desaturase 6 |
| FAT1 | 1.25 | 7.41 | 1.23 × 10−5 | 5.52 × 10−4 | FAT atypical cadherin 1%2C transcript variant X3 |
| FAT2 | 1.82 | 6.23 | 6.35 × 10−7 | 5.41 × 10−5 | FAT atypical cadherin 2 |
| GPAM | 1.06 | 5.73 | 2.38 × 10−6 | 1.48 × 10−4 | glycerol-3-phosphate acyltransferase%2C mitochondrial%2C transcript variant X2 |
| HK1 | 0.71 | 7.00 | 6.48 × 10−4 | 1.17 × 10−2 | hexokinase 1 |
| IGF2BP1 | −0.80 | 3.13 | 9.01 × 10−4 | 1.49 × 10−2 | insulin-like growth factor 2 mRNA binding protein 1 |
| LIPG | 1.08 | 4.73 | 2.26 × 10−6 | 1.42 × 10−4 | lipase%2C endothelial |
| LIPM | 1.94 | 3.57 | 1.31 × 10−5 | 5.86 × 10−4 | lipase member M |
| LPL | −0.67 | 9.50 | 3.43 × 10−4 | 7.02 × 10−3 | lipoprotein lipase |
| LRP8 | 0.94 | 2.14 | 2.05 × 10−3 | 2.70 × 10−2 | low-density lipoprotein receptor-related protein 8%2C apolipoprotein e receptor%2C transcript variant X2 |
| LRP2BP | 1.38 | 0.68 | 3.09 × 10−3 | 3.65 × 10−2 | LRP2 binding protein%2C transcript variant X1 |
| MOGAT1 | 2.32 | 3.09 | 7.87 × 10−10 | 1.96 × 10−7 | monoacylglycerol O-acyltransferase 1 |
| NOX1 | 1.17 | 3.61 | 1.56 × 10−3 | 2.23 × 10−2 | NADPH oxidase 1 |
| PLA2G4B | 1.28 | 7.26 | 5.78 × 10−7 | 5.10 × 10−5 | phospholipase A2%2C group IVB (cytosolic)%2C transcript variant X8 |
| PLA2G4F | 1.79 | 3.51 | 6.37 × 10−12 | 3.63 × 10−9 | phospholipase A2%2C group IVF%2C transcript variant X1 |
| PLIN1 | −1.09 | 11.38 | 1.31 × 10−8 | 2.22 × 10−6 | perilipin 1 |
| PLIN3 | −1.09 | 10.36 | 3.65 × 10−5 | 1.30 × 10−3 | perilipin-3-like |
| SCARF1 | −0.96 | 3.26 | 4.36 × 10−4 | 8.49 × 10−3 | scavenger receptor class F%2C member 1 |
| STK17A | 0.74 | 6.65 | 9.66 × 10−4 | 1.57 × 10−2 | serine/threonine kinase 17a |
| STK39 | 0.96 | 2.36 | 1.52 × 10−3 | 2.19 × 10−2 | serine threonine kinase 39%2C transcript variant X7 |
| UGT8 | 0.93 | 5.65 | 1.31 × 10−3 | 1.97 × 10−2 | UDP glycosyltransferase 8 |
| PNPLA3 | −0.63 | 7.642163 | 2.18 × 10−3 | 2.83 × 10−2 | patatin-like phospholipase domain-containing protein 3 |
| Gene | Log2FC | log2CPM | p Value | FDR | Annotation |
|---|---|---|---|---|---|
| ELOVL2 | 2.86 | 2.83 | 8.83 × 10−5 | 1.06 × 10−2 | ELOVL fatty acid elongase 2 |
| APOBEC2 | −5.18 | 2.30 | 2.32 × 10−6 | 7.71 × 10−4 | apolipoprotein B mRNA editing enzyme%2C catalytic polypeptide-like 2 |
| PLA2G1B | −5.41 | 1.47 | 1.07 × 10−5 | 2.17 × 10−3 | phospholipase A2%2C group IB (pancreas) |
| GHRHR | −3.62 | −0.29 | 1.61 × 10−5 | 2.99 × 10−3 | growth hormone-releasing hormone receptor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhuang, Z.; Jia, W.; Xie, M.; Zhou, Z.; Tang, J.; Bai, H.; Chang, G.; Chen, G.; Hou, S. Comparative Transcriptome Analysis Reveals the Key Genes Involved in Lipid Deposition in Pekin Ducks (Anas platyrhynchos domesticus). Agriculture 2022, 12, 1775. https://doi.org/10.3390/agriculture12111775
Jiang Y, Zhuang Z, Jia W, Xie M, Zhou Z, Tang J, Bai H, Chang G, Chen G, Hou S. Comparative Transcriptome Analysis Reveals the Key Genes Involved in Lipid Deposition in Pekin Ducks (Anas platyrhynchos domesticus). Agriculture. 2022; 12(11):1775. https://doi.org/10.3390/agriculture12111775
Chicago/Turabian StyleJiang, Yong, Zhong Zhuang, Wenqian Jia, Ming Xie, Zhengkui Zhou, Jing Tang, Hao Bai, Guobin Chang, Guohong Chen, and Shuisheng Hou. 2022. "Comparative Transcriptome Analysis Reveals the Key Genes Involved in Lipid Deposition in Pekin Ducks (Anas platyrhynchos domesticus)" Agriculture 12, no. 11: 1775. https://doi.org/10.3390/agriculture12111775






