Abstract
Some biostimulants, including plant origin preparations, act similarly to plant growth regulators. Moreover, the supplementation of known and unknown rooting cofactors can stimulate rhizogenesis in cuttings. The aim of this research was to assess the response of difficult-to-root and long-rooting stem cuttings of the once-blooming old variety Rosa ‘Hurdal’ to preparations of plant origin. The hypothesis was that the plant origin preparations could enhance rooting processes by inhibiting chlorophyll a/b degradation in leaves and postponing leaf senescence, simultaneously increasing the quality of cuttings. The one-bud stem cuttings were made in four phenological stages: (H1) flower buds closed, (H2) open flowers, (H3) just after petal fall, and (H4) 7–14 days after petal shedding. They were treated with either standard commercial powder preparations containing 0.4% indole-3-butyric acid (IBA) or 0.2% 1-naphthalene acetic acid (NAA) as well as with commercial plant origin preparations that this work will henceforth refer to as: Algae Extract, Organic Preparation, and Plant Extract. The cuttings were evaluated after 12 weeks of rooting them in two substrates: peat–perlite and peat–sand (v:v; 1:1). Mean root percentages for both substrates were noted after preparation from stage H1 (74.5%), H2 (59.5%), H3 (50.8%) shoots. The H4 cuttings did not root at all and were not considered further. The means for all phenology stages together were the highest by the use of 0.6% Algae Extract, 0.012% and 0.02% Organic Preparation, and 0.2% and 0.4% Plant Extract. The lowest means were reported for the control cuttings as well as NAA and IBA treatment. Plant origin preparations encouraged growth parameters but did not unequivocally inhibit the decrease of chlorophyll content in the cuttings’ leaves. The percentage of cuttings that rooted in both rooting substrates was determined by the quality of the cuttings as well as the chlorophyll a/b and soluble protein content in the leaves.
Keywords:
biostimulant; carotenoids; chlorophyll; IBA; leaf senescence; NAA; phenology; soluble proteins 1. Introduction
The propagation of woody ornamental plants, including roses, via stem cuttings harvested during flowering time is the most simple and cost-effective approach in nursery production [1]. However, roses are highly biodiverse, and considering their many species, subspecies, groups, and cultivars [2] as well as diversification connected with anatomical [3], morphological, and various phenotypical features [2], their propagation can be difficult and seems to be fallible when using the standard nursery methods [1,4].
Meanwhile, conservation and popularization, especially of old garden roses, is important on grounds of preserving a wide genetic pool and increasing plant biodiversity in gardens as an implementation of the provisions of the Convention on Biological Diversity (CBD) drawn up in Rio de Janeiro on 5th June 1992. The object of this study is the once-blooming Rosa ‘Hurdal’, one of the valuable garden old roses, probably an alba or villosa hybrid created by an unnamed breeder. Its winterhardiness as well as high tolerance to frost and fungal leaf diseases [2,5] predestines this variety to cultivation on its own roots, as is preferred in extensive cultivation. However, the rooting ability of the cuttings of this rose is low and strictly dependent on the phenological stage of shoots [6,7]. Moreover, the rooting of one-leaf stem cuttings of this rose is long, lasting 12 weeks [6,8], as is observed in the case of other wild [9] and old rose taxa [4,6,8], in contrast to modern roses such as tea hybrids, the cuttings of which root in 4–6 weeks [10].
During their lengthy rhizogenesis, the stem cuttings of Rosa ‘Hurdal’ are especially exposed to stress factors caused by a decrease in chlorophyll and reduced content of carbohydrates in its leaves [6]. The decrease of chlorophyll content and increase of carotenoids is one of the symptoms of leaf senescence processes [11,12]. Moreover, the decrease of chlorophyll content in the leaves of the ‘Hurdal’ rose, both during the process of rhizogenesis and after flowering in the stock plant, has been proven in previous research and recognized as one of the probable factors of cuttings failing to root when prepared from shoots during the fruiting phenological stage—7–14 days after petal fall [6] (BBCH 71 702) [13].
The following eco-friendly plant origin commercial preparations are used in the present study on the ‘Hurdal’ rose: Bio Rhizotonic [14], Root Juice [15], and Bio Roots [16]. These preparations can be qualified as biostimulants containing humic substances. Their use increases the content of nutrients in plant tissues, has positive effects on metabolic processes, and has mostly been considered and proved to be efficient in relation to crops and vegetable plants [17,18,19], rarely ornamentals [20], including woody plants propagation [21,22,23]. When applied exogenously, some plant preparations have similar actions to groups of known plant hormones (auxins, gibberellins, cytokinins) [24,25]. Seaweed extracts in particular can also contain growth regulators that enhance rhizogenesis [26]. A detailed characterization of these preparations is presented in Table S1 [14,15,16,27,28].
The aim of this work was to assess the response of difficult-to-root and long-rooting stem cuttings of Rosa ‘Hurdal’ to preparations of plant origin. The hypothesis was that plant origin preparations could enhance rooting processes, inhibiting chlorophyll degradation and postponing leaf senescence and at the same time increasing the quality of cuttings. In previous research, we proved the key roles of the phenological stage of shoots harvested for cuttings [6,29,30,31] and of the rooting substrate [8] in the rhizogenesis of this taxon. In this work, the results were analyzed considering four phenological stages of shoots and two rooting substrates. The possibility of using organic plant origin preparations encourages eco-friendly plant reproduction in nurseries. These ideas are aligned with the Official Journal of the European Union and EU Council Directive [32,33,34], the National Organic Program USDA [35], and the Organic Materials Review Institute [27] limiting the use of chemicals in plant production.
2. Material and Methods
2.1. Plant Material
The cuttings were harvested from shrubs of Rosa ‘Hurdal’ (Germany-Norway; origin unknown, R. × alba × R. villosa, Figure 1a) growing in the National Collection of Rose Cultivars in the Polish Academy of Sciences Botanical Garden, Centre of Biological Diversity Conservation in Powsin, Warsaw, Poland.
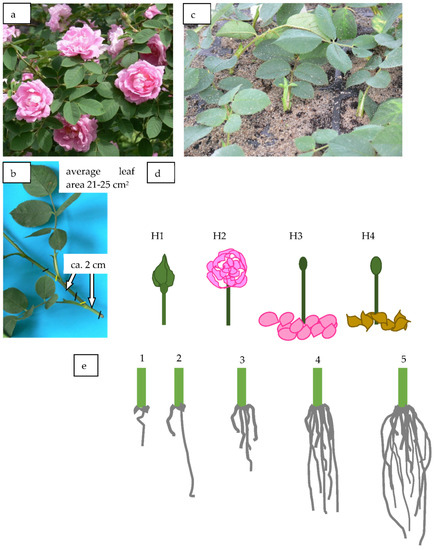
Figure 1.
The Rosa ‘Hurdal’ (a); single-node leaf cutting prepared for experiment (b); planted cuttings (c); phenological stages of shoots for cuttings’ preparation, as they figure in this work named and in BBCH scale (Meier et al. 2009) (d): H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); H4—7 × 14 days after shedding petals (70,701; fruit set: beginning of hip berry − growth on the main stem); (e) the quality of the root system (degree of rooting) on a 5-point valuation scale: 1—only callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by length; 5—strong roots, starting to form a root ball, well developed. Stages (Figure 1d) according to BBCH scale in roses [13] lasted 16 days and are denoted in the work by the letter H: H1—27 May, 54,504 (flower buds still closed), H2—1 June, 69,605 (full flowering; at least 50% of flowers open), H3—6 June, 69,629 (end of flowering of second order stem: all petals fallen), H4—11 June, 70,701 (fruit set: beginning of berry growth on the main stem) (Figure 1d).
The single-node leafy semi-hardwood shoot cuttings of Rosa ‘Hurdal’ [2] with an original leaf (leaf area 24–27 cm2) (Figure 1b,c) were taken from the middle part of generative shoots. The harvesting time of shoots was in four data and four phenological stages.
2.2. Rooting Medium and Experimental Conditions
The cuttings were inserted in multipot trays (6.6 × 6.6 cm, 98 cm3) in two rooting substrates: (i) peat–perlite and (ii) peat–sand, mixture (v:v 1:1; pH 6–6.5). The peat was purchased from the Karaska company (Poland), very coarse-grained sand (1.25–2.00 mm grain size) originated from the River Vistula (Serwal Piaskarnia Warsaw Wilanów company, Poland), and perlite was imported from Italy (Tables S2 and S3).
The treatment of cuttings in the experiment is presented in detail in Table 1. According to this schema, the basal parts (1 cm) of the stems were dipped in rooting powder containing 0.4% IBA or 0.2% NAA. The remaining cuttings were watered with 10 cm3 per pot (98 cm3) with a solution of plant origin preparation or, in the control combination, water. On the 10th and 20th day of rooting, the cuttings were watered with 10 cm3 water or Algae Extract solution per pot (98 cm3) (no. 4–14 of treatment) or with the water (no. 1–3 of treatment) (Table 1).

Table 1.
The treatment of cuttings of Rosa ‘Hurdal’ at each phenological phase of the experiment.
The experiment was conducted in a foil tunnel in the commercial nursery of M&M Kryt in Wola Prażmowska (51.56° N, 20.28° E), Poland. An electronically controlled misting system maintained optimal climatic conditions (air temperature 23–25 °C, ambient relative humidity 80–90%). The cuttings were covered with a shading material that limited the photosynthetic photon flux density to 130 μmoL·m−2·s−1. The cuttings were protected against fungal diseases by applying fungicides every 7–9 days during the rooting period: Previcur Energy 840 SL (propamocarb 47.28%, fosetyl 27.65%), Amistar® 250 SC (azoxystrobin 250 g∙dm−3), Score 250 SC (difenoconazole 250 g∙dm−3).
2.3. Evaluation of the Cuttings’ Growth
The cuttings were dug up after 12 weeks, in the first days of August. The rooted cuttings were cleaned carefully from the medium in water and evaluated.
The percentage of rooted cuttings was calculated (%) in relation to the number of planted cuttings, while the percentage of cuttings with retained stock plant leaf and the percentage of cuttings that formed a new shoot were calculated in relation to the number of cuttings with roots.
The fresh weight (g) of rooted cuttings was estimated using an analytical balance (PS 6000/C/2, RADWAG, Poland). The total leaf area (cm2, original leaf with all leaves on the new shoot) was scanned with a leaf area meter (AM 300, ADC BioScientific Ltd., Hoddesdon, UK). Due to the length of young new shoots not exceeding 1 cm for most of the cuttings, this parameter was not considered or measured.
The quality of the root system was estimated as the degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated in length; 5—roots strong, starting to form a root ball, well developed (Figure 1e). Cuttings with the value ‘1′ were rejected and not evaluated further. They figure in the experiment and in the statistical evaluation of the degree of rooting as unrooted.
2.4. Protein and Pigment Analyses
For biochemical analyses, the remaining stock plant leaves of rooted cuttings were sampled after 12 weeks of the rhizogenesis process. Additionally, the leaves were taken from stock plants on the days of evaluation of the rooting cuttings in August. All leaf samples were frozen and stored at a temperature of −18 °C. Research was conducted in the laboratories of PAS Botanical Garden CBDC in Powsin.
Protein extract was used to estimate the content of soluble proteins [36]. A frozen leaf sample (about 100 mg) was grinded in liquid nitrogen with a mortar and pestle. Proteins were extracted with 1 mL of extraction buffer (0.063M TRIS, 2% SDS, 5% β-mercaptoethanol, 10% glycerol) for 30 min [36]. The extraction slurry was transferred to Eppendorf’s tube and proteins were denaturated in a water bath (95 °C, 5 min). After centrifugation at 10,000× g for 20 min. at room temperature, 300 µL of supernatant was taken to a new tube and frozen at −40 °C for future assessment.
Total soluble protein content was evaluated according to Ghosh with minor modifications [37]. Aliquot of 10 µL of proteins extract was loaded onto squared pieces (8 × 8 mm) of filter paper (Whatman 3MM) placed in cells of plastic cultivation plates. After 15 min. drying at room temperature, paper pieces were saturated with 15 µL of 0.2% CBB R-250 solution in 40% methanol and 10% acetic acid. After 20 min, the nonbonded dye was washed off three times with 0.5 mL of 20% methanol and 10% acetic acid solution and dried at 35 °C in an oven. Dried papers were transferred to a centrifuge tube (2 mL), and the protein–dye complex was eluted with 2 mL of 1% SDS solutions in an ultrasonic washer for 1 h. Protein content was assessed spectrophotometrically at 590 nm. The amount of protein was calculated using a standard curve created for BSA solutions.
For the assessment of the content of chlorophyll a/b and carotenoids, the leaf tissue sample of 0.1 g was grinded to a powder by hand in a mortar in liquid nitrogen. The extraction was conducted with 5 mL of 99.5% acetone by adding a pinch of calcium carbonate to neutralize the organic acids. The extracts were completed to a total volume of 25 mL with acetone and filtered through soft blotting paper. The assessment of the content of the pigments was made spectrophotometrically measuring the absorbance at wavelengths of 750, 662, 645, and 470 nm (GeneQuant 1300, GE). The calculations were made using the following formulas [38]:
where A is absorbance at a given wavelength, V is total volume of the extract (cm3), and W is sample weight (g).
Chlorophyll a = (12.7 × A663 − 2.7 × A645) × V × (1000 W) − 1
Chlorophyll b = (22.9 × A645 − 4.7 × A663) × V × (1000 W) − 1
Carotenoids = (1000 × M470 − 1.9 × chlorophyll a − 63.14 × chlorophyll b) × 214 − 1
2.5. Statistical Analysis
The experiment was constructed as a randomized complete block design (RCBD) [39] for each maturity phase and involved 112 combinations for three variables (treatment, phenological stage, medium). Each combination of treatment in four phenological stages of shoots (Figure 1) included four replicas of 10 cuttings. A total of 4480 cuttings was planted.
The values in percentage were transformed by using the function ARCSIN(x)1/2 according to Bliss or y = x2 + (x2 + 1)2 to compare the means and carry out analyses [39]. The data of biochemical analyses and growth parameters were analyzed by using three or two-factorial analysis of variance (ANOVA), and Duncan’s honest significant difference (HSD) test was used to determine the significance of differences between the means (p ≤ 0.05) for three or two variables—phenological stage, treatment with rooting enhancer, and medium.
Correlations between the data parameters of growth, pigments, and soluble proteins content in cuttings were carried out [39]. The results are shown together for each phenological phase and rooting substrate. The correlations between rooting percentage and evaluated parameters in relation to rooting enhancers were presented separately for each stage rooted in both substrates.
STATISTICA 13.3 software package (Statsoft Polska, Cracow, Poland) was used for statistical analysis.
3. Results
3.1. The Pivotal Role of Phenology Stage in the Rooting of Rosa ‘Hurdal’ Cuttings
The research showed a dominant role of the phenological stage of shoots in rooting the cuttings of Rosa ‘Hurdal’ due to its influence on the effectiveness of propagation and the quality of cuttings.
A higher mean rooting percentage of the stem cuttings of Rosa ‘Hurdal’ was noticed after preparing them from shoots with flower buds closed (H1; 74.5%); the means were significantly lower after attempts to root cuttings harvested from shoots with opened flowers (H2; 59.5%) or immediately after petal fall (H3; 50.8%) (Figure 2). The cuttings prepared from shoots 7–14 days after petal fall did not root at all and were not taken into further consideration. The means for all phenology stages together were the highest by the use of 0.6% Algae Extract, 0.012% and 0.02% Plant Extract, and 0.2% and 0.4% Organic Preparation. The lowest means were noticed for the control cuttings as well as NAA and IBA treatment. The rooting substrate did not affect this mean for all phenology stages of shoots (Figure 2).
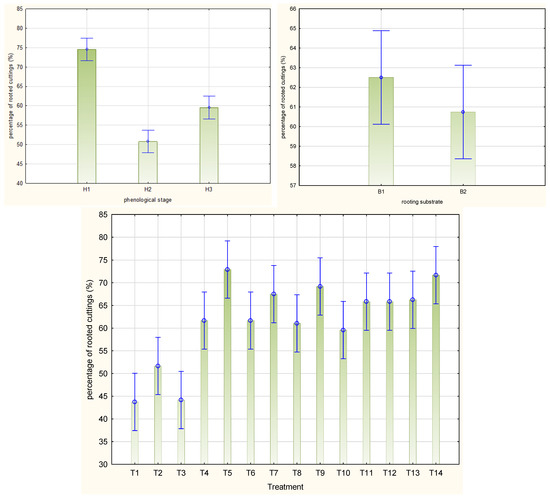
Figure 2.
Rooting percentage in Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
The highest rooting percentage of stem cuttings harvested from shoots with flower buds closed (H1) was obtained after the single treatment with 0.6% Algae Extract and rooting in peat–sand substrate (100%). A high rooting percentage was also obtained in H1 by the single use of 0.4% and three-time use of 0.6% Algae Extract in peat–sand (92.5% and 90%, respectively) as well as two-time use of 0.4% and 0.6% Algae Extract, 0.02% Plant Extract in peat–perlite (90.0%, 87.5%, and 87. 5%, respectively). In the case of H2 cuttings, the highest rooting percentage was obtained by the single use of 0.6% Algae Extract and 0.02% Plant Extract and rooting in peat–sand substrate (both 75.0%). High means for H3 cuttings were noted by the use of three-time 0.6% Algae Extract and rooting in peat–perlite (82.5%) as well as single treatment with 0.6% Algae Extract and rooting in peat–sand substrate (77.5%). The lowest rooting percentage for H1 and H2 were obtained in control cuttings or after the use of NAA and rooting in peat–sand substrate (27.5% and 37.5%, respectively, and both 32.5% for H2). The low means for H3 were obtained by the use of NAA and IBA preparations in both rooting media (Figure S1).
The highest mean rooting degree of the stem cuttings of Rosa ‘Hurdal’ was noticed after preparing them from shoots H1 and H3 as compared to H2. The means for all phenology stages together were the highest by the use of the one-time 0.6% Algae Extract and 0.012% Plant Extract, 0.2%. The rooting substrate did not affect this mean for all phenology stages of shoots (Figure 3).
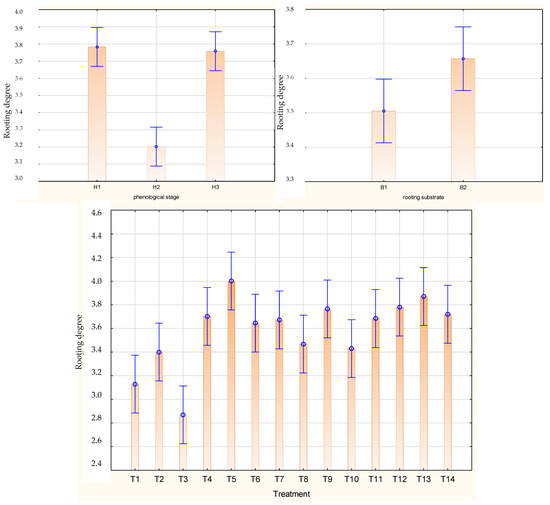
Figure 3.
Rooting degree in Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. The degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by the length; 5—strong roots starting to form a root ball, well developed. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
In the case of cuttings from H1, the highest values in rooting degree were obtained by the single use of 0.4% and 0.6% Algae Extract and 0.2% Organic Preparation rooted in peat–sand, while the lowest were reported after the use of NAA rooting in both substrates. The H2 cuttings had the highest rooting degree after the use of Plant Extract and single-use 0.6% Algae Extract rooted in peat–sand substrate, while the lowest was found in the control in both media and after the use of NAA for cuttings rooted in peat–perlite. In the case of H3 cuttings, the use of 0.6% Algae Extract provided higher means in both rooting media, while the use of NAA and IBA resulted in lower means in both rooting substrates, similarly to control cuttings rooted in peat–perlite. The rooting degree was higher for cuttings rooted in the peat–sand medium for H1 and H2 but lower for H3 (Figure S2).
The highest mean weight of rooted Rosa ‘Hurdal’ stem cuttings was observed after harvesting them from shoots with flower buds closed (H1; 1.1 g) and was significantly lower after harvesting the H3 cuttings (0.8 g) and H2 shoots (0.4 g) (Figure 4). The means for all phenology stages together were the highest for the one-time use of 0.4% and 0.6% Algae Extract, two-time use of 0.6% Algae Extract, and 0.4% Organic PreparationTM. The lowest mean was noticed for the control cuttings (Figure 4). The rooting substrate did not affect the mean cutting weight of shoots in means for all phenological stages (Figure 4).
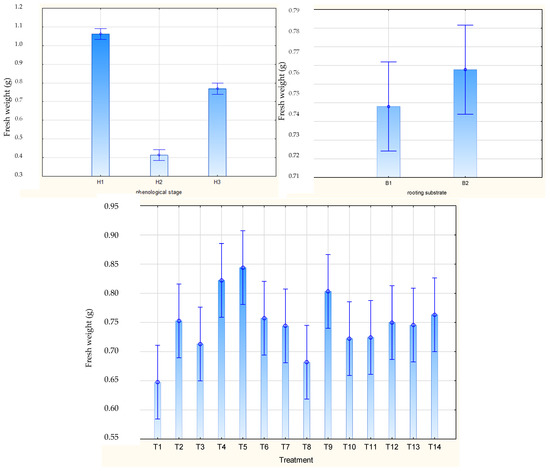
Figure 4.
Fresh weight of Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. The degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by the length; 5—strong roots starting to form a root ball, well developed. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
The weight of rooted cuttings of H1 shoots was higher after the single use of Algae Extract rooted in peat–sand and after single watering by 0.6% Algae Extract for H2 and H3 in both substrates. Moreover, a high mean was obtained for H2 cuttings using 0.02% Plant Extract. The rooting substrate did not affect this mean for H1 cuttings, while for H2 cuttings it was higher in peat–sand and for H3 in peat–perlite (Figure S3).
The highest mean percentage of Rosa ‘Hurdal’ cuttings with retained stock plant leaf was obtained after cutting from H3 shoots (H1; 95.8%) and was significantly lower after harvesting H2 cuttings (83.8%) and H3 shoots (77.1%) (Figure 5). The means for all phenology stages together were the highest by the use of one-time 0.6% Algae Extract, 0.4% Organic Preparation, and 0.012% Plant Extract. The lowest means were noticed for cuttings treated with IBA preparation and for the control cuttings (Figure 5). The rooting substrate did not affect all phenology stages of shoots in terms of the mean percentage of cuttings with a retained stock plant leaf (Figure 5).
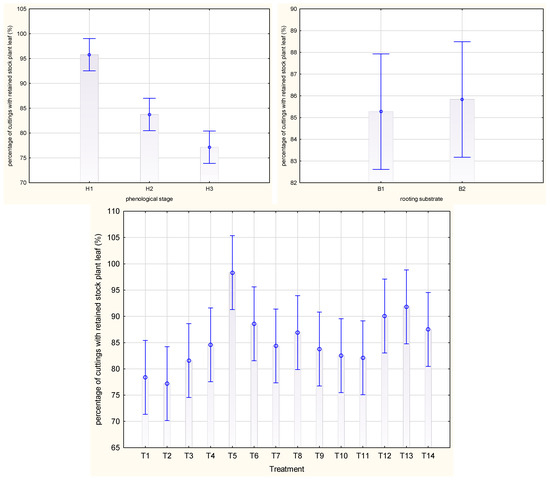
Figure 5.
Percentage of cuttings with retained stock plant leaf in Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. The degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by the length; 5—strong roots starting to form a root ball, well developed. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
In the case of H1 cuttings, the percentage of cuttings with a retained stock plant leaf was high, while the use of 0.2% Organic PreparationTM and rooted peat–perlite caused the lowest (73.4%) mean. This parameter in H2 cuttings was higher after the use of 0.2% and 0.4% Organic Preparation as well as Plant Extract. The weight of cuttings of H3 shoots was higher after the single use of 0.6% Algae Extract and rooting in peat–sand (Figure S4).
The mean percentage of Rosa ‘Hurdal’ cuttings with new shoots was similar when prepared from all three phenological stages (Figure 6). The means for all phenology stages together were the highest by the use of 0.4% Organic Preparation and 0.012% Plant Extract, while the lowest means were noticed for cuttings treated with IBA preparation and for the control cuttings (Figure 6). The percentage of cuttings with new shoots was higher for cuttings rooted in peat–sand medium (Figure 6).
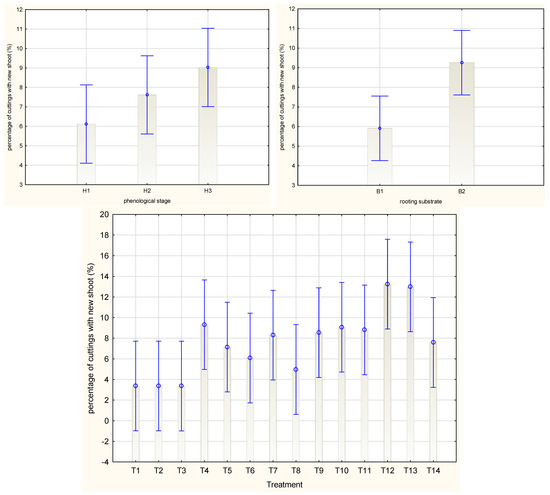
Figure 6.
Percentage of cuttings with new shoot in Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. The degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by the length; 5—strong roots starting to form a root ball, well developed. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
The percentage of cuttings with new shoots was highest by the use of 0.2% Organic Preparation in the case of H1 cuttings and after the single use of 0.6% Algae Extract and rooting in peat–sand for H2 and H3. The rooting substrate did not affect this mean for H1 and H3 cuttings, while for H2 cuttings it was higher in peat–sand (Figure S5).
The highest mean of total leaf area in Rosa ‘Hurdal’ stem cuttings was noticed after harvesting from shoots with flower buds closed (H1; 24.4 cm3) and was significantly lower after preparing the cuttings from H2 (21.4 cm3) and from H3 shoots (18.5 cm3) (Figure 7). The means for all phenology stages together were highest by the one-time use of 0.6% or two-time use of 0.4% and 0.6% Algae Extract and 0.4% Organic Preparation, compared to the control cuttings. (Figure 7) The rooting substrate did not affect the mean weight of cuttings for all phenology stages of shoots (Figure 7).
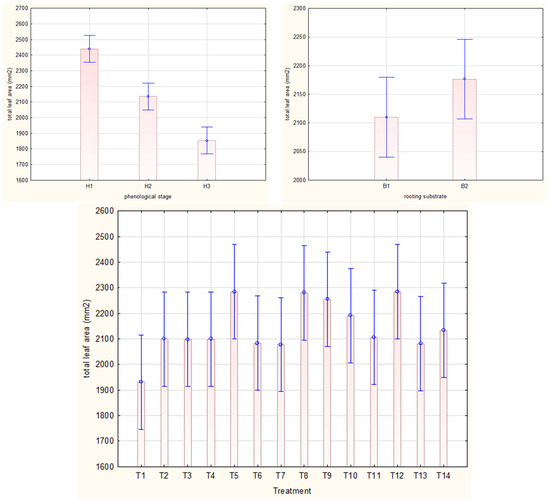
Figure 7.
Total leaf area (mm2) in Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. The degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by the length; 5—strong roots starting to form a root ball, well developed. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
The total leaf area of cuttings of H1 shoots was higher after the use of IBA, one-time watering with 0.4% or triple watering with 0.6% Algae Extract, rooted in peat–perlite, and NAA rooted in both substrates, compared to the use of 0.02% Plant Extract for cuttings rooted in peat–sand. The highest mean for H2 cuttings was obtained by the one-time use of 0.6% Algae Extract and rooting in peat–perlite as well as three-time use of 0.4% Algae Extract and rooting in peat–sand. In the case of H3 cuttings, the analysis did not show any significant interaction between treatment and substrate (Figure S6).
3.2. The Pivotal Role of the Phenological Stage of Shoots in the Progressive Aging Processes of Leaves during the Rhizogenesis of Rosa ‘Hurdal’ Cuttings
The content of chlorophyll a/b, carotenoids, and proteins in the leaves of cuttings decreased during the rhizogenesis process and were lower in rooted cuttings both before rooting and in stock plant leaves in August at the time of harvesting the cuttings. These phenomena were observed in cuttings of all the phenological phases (Figure 8, Figure 9, Figure 10 and Figure 11 and Figures S7–S10).
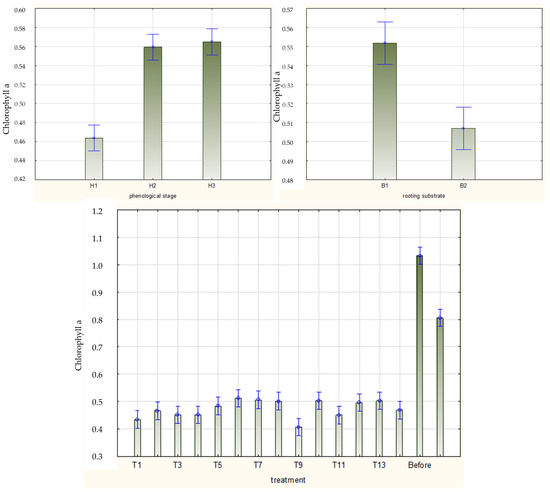
Figure 8.
Content of chlorophyll a (mg·g−1 FW) in leaves of Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. The degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by the length; 5—strong roots starting to form a root ball, well developed. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
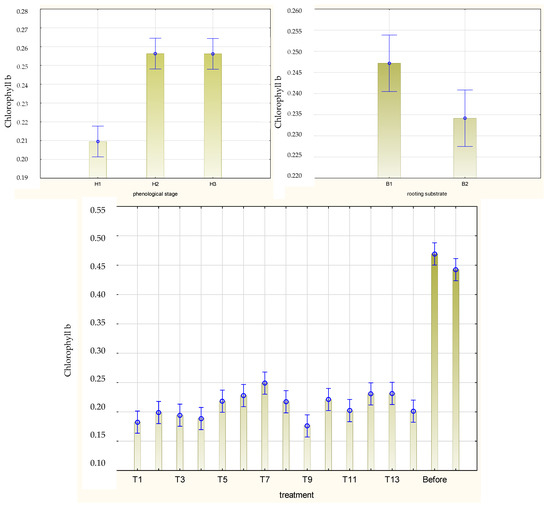
Figure 9.
Content of chlorophyll b (mg·g−1 FW) in leaves of Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. The degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by the length; 5—strong roots starting to form a root ball, well developed. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
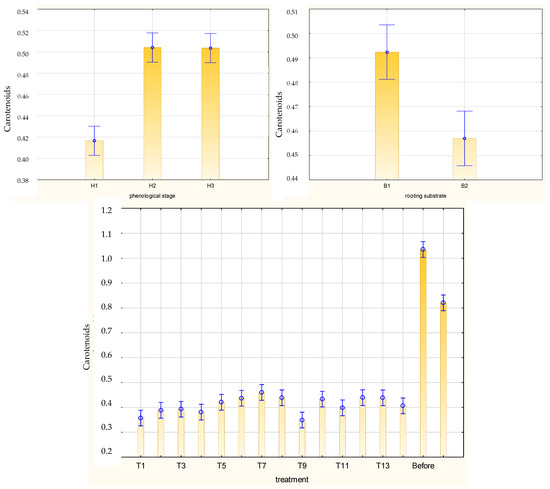
Figure 10.
Content of carotenoids (mg·g−1 FW) in leaves of Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. The degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by the length; 5—strong roots starting to form a root ball, well developed. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
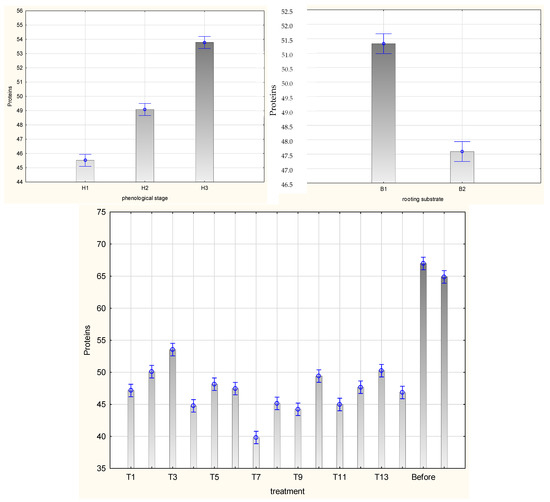
Figure 11.
Content of soluble proteins (μg·mg−1 FW) in leaves of Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. B1—peat–perlite; B2—peat–sand; H1—flower buds closed (BBCH 54,504; flower buds still closed); H2—flowers opened (69,605; full flowering, at least 50% of flowers open); H3—immediately after petal shedding (69,629; end of flowering of second order stem: all petals fallen); and treated: T1—the control cuttings; T2—0.4% IBA (Ukorzeniacz Aaqua); T3—0.2% NAA (Ukorzeniacz Baqua); T4, T5, T6, T7, T8, T9—Algae Extract: watering with 0.4% or 0.6% Bio Rhizotonic (10 mL) 1, 2, or 3 times (after planting and 10 and 20 days later); T10, T11, T12—Organic Preparation—watering with 0.1%, 0.2%, or 0.4% Root Juice™ (10 mL) after planting; T13, T14—Plant Extract—watering with 0.012% or 0.02% Bio Roots (10 mL) after planting. The degree of rooting on a 5-point scale: 1—only a callus, without formed roots; 2—at least one short root; 3—a few longer roots; 4—roots visibly developed, differentiated by the length; 5—strong roots starting to form a root ball, well developed. Vertical bars denote 95% confidence intervals for the mean of counts (one-way ANOVA).
The mean chlorophyll a/b and carotenoid contents in leaves of Rosa ‘Hurdal’ cuttings were similar when prepared from H2 and H3 shoots and higher than in phenological stage H1. The protein content was higher in H3 than in H2 and H1. The content of pigments and proteins was higher for the cuttings rooted in peat–perlite medium when considering the results for all phenological phases together (Figure 8, Figure 9 and Figure 10).
In the case of H1 cuttings, the chlorophyll a content in leaves increased after two-time treatment with Algae Extract and 0.2% IBA on cuttings rooted in peat–perlite and 0.02% Plant Extract rooted in peat–sand compared with cuttings rooted in peat–sand in the control and cuttings treated twice and three times with Algae Extract and 0.02% Plant Extract. In the case of H2 cuttings, the chlorophyll a content in leaves increased after one-time treatment with 0.4% and two-time treatment with 0.6% Algae Extract rooted in peat–perlite compared with cuttings rooted in peat–sand and treated twice or three times with 0.6% Algae Extract and rooted in peat–perlite, as well as with those treated with 0.4% Algae Extract, 0.1% Organic Preparation, and 0.02% Plant Extract. The highest means for H3 cuttings were obtained by the use of 0.1 Organic Preparation and rooting in peat–perlite as well as 0.012% Algae Extract and rooting in peat–sand (Figure S7).
The chlorophyll b content in leaves of rooted H1 cuttings was higher after two-time watering with 0.4% and 0.6% Algae Extract for cuttings rooted in peat–perlite substrate. In the case of H2 cuttings, this mean was highest after the use of 0.4 Organic Preparation and two-time use of 0.6% Algae Extract combined with rooting in peat–perlite substrate as well as after one- and two-time use of 0.6% Algae Extract and rooting in peat–sand medium. In the case of H3 cuttings, the highest means were obtained after the use of 0.4% Algae Extract, 0.1%, and 0.4% Organic Preparation twice and rooting in peat–perlite (Figure S8).
The rooting substrate did not affect the chlorophyll a/b content in leaves of rooted cuttings for H2 cuttings, while for H1 and H3 cuttings it was higher in peat–perlite (Figures S8 and S9).
The carotenoid content in leaves of rooted H1 cuttings was higher after watering twice with 0.4% and 0.6% Algae Extract and rooting in peat–perlite substrate. In the case of H2 cuttings, this mean was higher after the use of 0.4% Organic Preparation and two-time use of 0.6% Algae Extract combined with rooting in peat–sand substrate as well as one-time use of 0.6% Algae Extract and rooting in peat–perlite medium. In the case of H3 cuttings, the highest means were obtained after the use of 0.02% Plant Extract and rooting in peat–sand as well as all treatments with Organic Preparation, 0.02% Plant Extract, and 0.4% Algae Extract when rooted in peat–perlite (Figure S9).
The rooting substrate did not affect the chlorophyll a/b and carotenoid content in the leaves of rooted cuttings for H2 cuttings, while for H1 and H3 cuttings the means were higher in peat–perlite (Figures S7–S9).
The protein content in leaves of rooted H1 cuttings was higher after watering the cuttings with 0.4% Organic Preparation and 0.02% Plant Extract and rooting in peat–perlite substrate. In the case of cuttings rooted in peat–sand, the highest means were noticed for cuttings treated with 0.012% Plant Extract and 0.4% Organic Preparation. In the case of H2 cuttings rooted in peat–perlite medium, these means were higher after the use of Plant Extract, also when compared to the protein content in stock plant leaves before rooting. In the case of H3 cuttings rooted in peat–sand medium, this mean was the highest after the use of 0.4% NAA and 0.2% IBA, also when compared to the content in stock plant leaves in August. The rooting substrate did not affect the protein content in leaves of rooted H3 cuttings; for H1 cuttings, it was highest in peat–sand and for H2 in peat–perlite (Figure S10).
3.3. Interdependence of Qualitative Parameters of Cuttings and Rooting Percentage
The correlation analysis of the rooted single-node stem cuttings of Rosa ‘Hurdal’ derived from of H2–H3 shoots and rooted in peat–perlite or peat–sand showed a strict relationship between increased rooting and a high percentage of cuttings with a retained stock plant leaf. The rooting degree decreased with the increase of total leaf area in H1 cuttings rooted in peat–perlite substrate; however, the same parameter positively correlated with an increase in the percentage of cuttings with a retained stock plant leaf (H2, H3/peat–perlite, H2, H3/peat–sand), the percentage of cuttings with young shoots (H1/peat–sand), the weight of cuttings (H3/peat–perlite; H1, H2, H3/peat–sand), and chlorophyll b and carotenoid content (H3/peat–sand). The weight of cuttings was positively correlated with the total leaf area (H1, H2/peat–perlite) and the percentage of cuttings with a retained stock plant leaf (H3/peat–perlite; H3/peat–sand) (Tables S4–S15).
The chlorophyll a/b, carotenoid, and protein content in the leaves of cuttings before rooting did not correlate with any measured parameters in H1 cuttings rooted in peat–perlite or in peat–sand substrate (Tables S4 and S7), in H3 rooted in peat–perlite (Table S4), or in H2 rooted in peat–sand (Table S8). The increase of chlorophyll a/b and carotenoid content in leaves of cuttings before rooting was correlated with a decrease in the value of these parameters in H2 cuttings rooted in peat–perlite (Table S8) and of the total leaf area of H3 cuttings rooted in peat–sand (Table S9). The content of chlorophyll a/b and carotenoids measured in the leaves of rooted cuttings were strictly connected (Tables S4–S9).
Correlation analyses of the rooted cuttings treated with rooting enhancers derived from the shoots of three phenological stages (H1–H3) showed a relationship between rooting percentage following treatments in two rooting media and the pigments and soluble protein content in leaves of cuttings before rooting. The changes in growth and biochemical parameters caused an increase in rooting percentage (Tables S10–S15).
4. Discussion
Rhizogenesis in cuttings is affected by endogenous and exogenous processes [1]. Additionally, seasonal changes in plant tissues connected with the decrease of chlorophyll content and increase in carotenoids are typical for plants in the temperate zone [11,12]. The rooting ability of cuttings, and also the action of rooting enhancers, changes accordingly to the phenological stage of the plants [40]. Within a few weeks, the flowering shoots of once-blooming roses change not only in phenological stage but also in terms of biologically active components [6] and anatomical structure [7]. These phenomena are important especially in the case of once-blooming roses, because it is the phenological stage of harvesting the cuttings, rather than the period, that should be properly defined [7,41]. For this reason, the period when the shoots, having reached their highest natural rooting ability, should be harvested for cuttings is very short—about 15 days [6,7]. While once-blooming roses flower only in June, the young shoots of rose cultivars with repeating flowering can be harvested a few times throughout the summer despite undergoing similar processes. Previous research showed that among the four phenological stages of flowering Rosa ‘Hurdal’ shoots, cuttings made during the last stage—10–14 days after petal shedding—did not root [6,7,29,30]. In this experiment, none of the rooting enhancers changed this result, and neither did the use of two rooting substrates (Figure S1). In research on the ‘Maiden’s Blush’ rose, the rooting enhancers, including plant origin preparations, did not show any unambiguous impact on rooting percentage in cuttings or affect the low natural rooting ability in shoots harvested for cuttings 7–14 days after shedding petals [31].
Leaves have been proven to have a key role in the rooting of the one-leaf rose stem cuttings of ‘Madelon’ (tea hybrid) [10]. According to previous research [6], the leaf could also play a pivotal role for the ‘Hurdal’ rose due to the length of the process of rhizogenesis. The limited reserves contained in a small-sized shoot and leaf are the deciding factor of the survival and growth of softwood and semi-hardwood cuttings in woody plants [42]. In this experiment, both the increase of quality of the cuttings (the weight of cuttings and the percentage of rooted cuttings with retained stock plant leaf, new shoot, and total leaf) and the increase of photosynthetically active pigments and soluble proteins were connected with an increase of rooting percentage independently of the rooting substrate (Tables S4–S10).
The use of preparations containing indole-3-butyric acid (IBA) or 1-naphthalene acetic acid (NAA) is a standard procedure in rooting cuttings. The possibilities of applying biostimulants in rooting cuttings of various ornamental plants have been studied [30,43]. Among them, algae preparations may contain auxins or different concentrations of similar substances [17,43] that can stimulate rhizogenesis [26]. Biostimulants, and among them plant origin preparations, affected the content of chlorophyll a/b, carotenoids [31,44], and carbohydrates in roses [30], along with the rooting percentage and quality of cuttings of roses [30,43] as well as other woody ornamentals [21,22,23]. It has also been proven that plant origin preparations positively affect the quality of cuttings, indirectly increasing their rooting percentage (Tables S4–S10).
It was confirmed in this research that preparations had different effects on the ‘Hurdal’ rose, and these depended on the phenological stage. The control cuttings rooted at a higher percentage in peat–perlite than in peat–sand. However, the use of proper rooting enhancers, especially plant origin preparations, independently of rooting substrate increased the rooting ability and rooting percentage in the H1–H3 phenological stage cuttings (Figure S1) and improved their quality in terms of degree of rooting (Figure S2), weight of rooted cuttings (Figure S3), percentage of cuttings with retained stock plant leaf (Figure S4) and new shoots (Figure S5), and also the total leaf area (Figure S6). The increase of quality of cuttings after the use of plant origin preparations is in line with other research on old rose taxa stem cuttings [44]. Moreover, the rooting enhancers had an unambiguous influence on growth parameters (Figures S1–S6). NAA especially decreased the rooting percentage and rooting degree in H3 rooted in peat–perlite (Figures S1 and S2), which can be connected with the tendency to callus overgrowth showed in research on Rosa helenae ‘Semiplena’ [45] and was proved in research on Rosa species leaf cuttings [46].
The process of rooting old roses is usually long and fallible [4]. The ‘Hurdal’ cuttings rooted in 12 weeks, but the rooting degree suggested (Figure S2) that the process could take longer in nursery production. Prolonged adventitious root formation exposes cuttings to similar stresses as cut flowers [47,48]. One of the reasons for low rooting ability can be the decrease of chlorophyll a/b, carotenoids, and soluble proteins content in leaves as dynamic natural seasonal processes of leaf senescence progress [6]. However, the decrease of pigment content during the process of rhizogenesis was lower at the time of evaluation of the rooted cuttings, and the content of soluble proteins was similar to that observed in stock plant leaves in August. This confirmed that the ‘Hurdal’ rose is susceptible to senescence processes and also to a natural seasonal decrease of these components earlier than other varieties included in surveys [6]. The rooting enhancers variously affected the increase of pigments and soluble proteins content in leaves (Figures S7–S9).
The action of biostimulants, including plant origin preparations, can most likely be attributed to the supplementation of unknown rooting cofactors and IAA-like substances present in biopreparations as well as other factors such as the improved nutrition of cuttings [19]. In the case of the ‘Hurdal’ rose, the plant origin preparations slowed the decline of photosynthetic pigments in leaves (Figure 8, Figure 9 and Figure 10); however, their effect was determined by the phenological stage of shoots used for cuttings. In the case of Rosa gallica ‘Duchesse d’Angoulême’, the use of plant origin preparations in proper concentrations caused an increase of chlorophyll a/b and carotenoid content in the leaves of cuttings cut from shoots with opened flowers and just after petal shedding but not from the shoots 7–14 days after shedding petals [44]. In addition, the rooted cuttings of Physocarpus opulifolius ‘Dart’s Gold’ and ‘Red Baron’ treated with seaweed preparation Algaminoplant had higher concentrations of chlorophyll a/b in their leaves compared with the control cuttings [22].
Moreover, this experiment on the ‘Hurdal’ rose proved, in line with previous research on once-blooming roses propagated by stem cuttings, that the effectiveness of the method can often be fallible [4] and should be verified and modified for each individual cultivar before introduction to practice [6,31,44].
5. Conclusions
The experiment showed the key role of phenological stages of cuttings on both the propagation efficiency and quality of rooted cuttings. The cuttings cut from shoots 7–14 days after petal shedding did not root because of senescence processes in the leaves, connected with a decrease of chlorophyll content and related to the loss stock plant leaf.
Plant origin preparations have an unquestionably favorable impact on the rooting percentage of the ‘Hurdal’ rose, and they may successfully replace traditional rooting powders containing IBA or NAA in encouraging the rhizogenesis process in difficult-to-root roses. However, their effect did not involve increasing the content of photosynthetically active pigments or soluble proteins in the leaves of rooted cuttings. Moreover, none of the preparations inhibited dead processes of cuttings prepared from shoots 7–14 days after petal fall. Plant origin preparations positively influenced the quality and quantity of cuttings, e.g., the rooting degree, weight of cuttings, percentage of cuttings with retained stock plant leaf and new shoots, and the leaf area. However, the action of plant preparations is dependent on the phenological stage of shoots cut for cuttings as well as on the substrate. Plant preparations of natural origin can be recommended in ecological cultivation and plant nursery propagation of the ‘Hurdal’ rose (91/414/EEC, 2009/128/WE; OJEU), as also proved by previous research on the long and difficult-to-root gallica rose ‘Duchesse d’Angoulême’ [44] and alba ‘Maiden’s Blush’ [31].
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/agriculture12020158/s1, Table S1. Preparations used for rooting Rosa ‘Hurdal’ in the experiment. Table S2. Basic analysis of the growing media. Table S3. The physical properties of rooting media, peat–perlite and peat–sand, mixed at 1:1 ratio in the cutting propagation experiment. Table S4. Correlation matrices between growth parameters, pigments, and soluble proteins content for Rosa ‘Hurdal’ cuttings harvested from shoots with flower buds closed (H1) and rooted in peat–perlite substrate. Table S5. Correlation matrices between growth parameters, pigments, and soluble proteins content for Rosa ‘Hurdal’ cuttings harvested from shoots with flowers opened (H2) and rooted in peat–perlite substrate. Table S6. Correlation matrices between growth parameters, pigments, and soluble proteins content for Rosa ‘Hurdal’ harvested from cuttings from shoots immediately after shedding petals (H3) and rooted in peat–perlite substrate. Table S7. Correlation matrices between growth parameters, pigments, and soluble protein content for Rosa ‘Hurdal’ cuttings harvested from shoots with flower buds closed (H1) and rooted in peat–sand substrate. Table S8. Correlation matrices between growth parameters, pigments, and soluble protein content for Rosa ‘Hurdal’ cuttings harvested from shoots with flowers opened (H2) and rooted in peat–sand substrate. Table S9. Correlation matrices between growth parameters, pigments, and soluble protein content for Rosa ‘Hurdal’ cuttings harvested from shoots immediately after shedding petals (H3) and rooted in peat–sand substrate. Table S10. Correlation matrices between rooting percentage and growth parameters, pigments, and soluble protein content for Rosa ‘Hurdal’ cuttings prepared from shoots with flower buds closed (H1) rooted in peat–perlite substrate and treated with rooting enhancers. Table S11. Correlation matrices between rooting percentage and growth parameters, pigments, and soluble proteins content for Rosa ‘Hurdal’ cuttings prepared from shoots with flowers opened (H2) rooted in peat–perlite substrate and treated with rooting enhancers. Table S12. Correlation matrices between rooting percentage and growth parameters, pigments, and soluble protein content for Rosa ‘Hurdal’ cuttings prepared from shoots immediately after flower petals shedding (H3) rooted in peat–perlite substrate and treated with rooting enhancers. Table S13. Correlation matrices between rooting percentage and growth parameters, pigments, and soluble protein content for Rosa ‘Hurdal’ cuttings prepared from shoots with flower buds closed (H1) rooted in peat–sand substrate and treated with rooting enhancers. Table S14. Correlation matrices between rooting percentage and growth parameters, pigments, and soluble protein content for Rosa ‘Hurdal’ cuttings prepared from shoots with flowers opened (H2) rooted in peat–sand substrate and treated with rooting enhancers. Table S15. Correlation matrices between rooting percentage and growth parameters, pigments, and soluble protein content for cuttings prepared from shoots immediately after the shedding of flower petals (H3) of the Rosa ‘Hurdal’ rooted in peat–sand substrate and treated with rooting enhancers. Figure S1. Rooting percentage in Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. Figure S2. Rooting degree in Rosa ‘Hurdal’ cuttings prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. Figure S3. Weight of rooted cuttings in Rosa ‘Hurdal’ prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. Figure S4. Percentage of rooted cuttings with retained stock plant leaf (%) in Rosa ‘Hurdal’ prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. Figure S5. Percentage of rooted cuttings that have created a new shoot (%) in Rosa ‘Hurdal’ prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. Figure S6. The total leaf area (cm2) in rooted cuttings of Rosa ‘Hurdal’ prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. Figure S7. The chlorophyll a content (mg·g−1 FW) in leaves of rooted cuttings of Rosa ‘Hurdal’ prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. Figure S8. The chlorophyll b content (mg·g−1 FW) in leaves of rooted cuttings of Rosa ‘Hurdal’ prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. Figure S9. The carotenoid content (mg·g−1 FW) in leaves of rooted cuttings of Rosa ‘Hurdal’ prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers. Figure S10. The soluble proteins content (μg·mg−1 FW) in leaves of rooted cuttings of Rosa ‘Hurdal’ prepared from three phenological stages (H1–H3) of shoots and rooted in two rooting substrates after treatment by rooting enhancers.
Author Contributions
Conceptualization, M.J.M.; methodology, M.J.M., M.N. and K.W.; software, M.J.M.; validation, M.J.M.; formal analysis, M.J.M., M.N. and K.W.; investigation, M.J.M., K.W. and M.N.; resources, M.J.M.; data curation, M.J.M., K.W. and M.N.; writing—original draft preparation, M.J.M.; writing—review and editing, M.J.M.; visualization, M.J.M.; supervision, M.J.M.; project administration, M.J.M.; funding acquisition, M.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
The experiments were supported by the National Science Centre, research project no. NN 310008240.
Data Availability Statement
The data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
Many thanks to the commercial nursery M. M. Kryt in Wola Prażmowska for their professional staff assistance and a place for the experiment.
Conflicts of Interest
The authors declare no conflict of interest. The authors also confirm that funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant Propagation, Principles and Practices, 7th ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 2011. [Google Scholar]
- Gustavsson, L.-A. Rosen Leksikon; Rosinante Forlag A/S: Kopenhaga, Denmark, 1999. [Google Scholar]
- Zhang, S.-Y. Systematic wood anatomy of the Rosaceae. Blumea 1992, 3, 81–158. [Google Scholar]
- Ginova, A.; Tsvetkov, I.; Kondakova, V. Rosa damascena Mill.—An overview for evaluation of propagation methods. Bulg. J. Agric. Sci. 2012, 18, 514–556. Available online: https://www.agrojournal.org/18/04-11-12.pdf (accessed on 23 January 2022).
- Monder, M.J. Zasoby genowe i ocena wybranych odmian róż historycznych w kolekcji Ogrodu Botanicznego CZRB PAN. Zeszyty Problemowe Postępów Nauk Rolniczych 2007, 517, 487–494. [Google Scholar]
- Monder, M.J.; Niedzielski, M.; Woliński, K.; Pacholczak, A. The impact of seasonal changes in plant tissue on rhizogenesis of stem cuttings of the once flowering roses. Not. Bot. Horti Agrobot. 2016, 44, 92–99. [Google Scholar] [CrossRef]
- Monder, M.J.; Kozakiewicz, P.; Jankowska, A. Effect of anatomical structure of shoots in different flowering phase on rhizogenesis of once-blooming roses. Not. Bot. Horti Agrobot. 2017, 45, 408–416. [Google Scholar] [CrossRef]
- Monder, M.J. Factors affecting the rooting of cuttings of once-blooming roses. Propag. Ornam. Plants 2017, 17, 134–144. [Google Scholar]
- Hoşafçi, H.; Arslan, N.; Sarihan, E.O. Propagation of dog rose (Rosa canina L.) plants by softwood cuttings. Acta Hortic. 2005, 690, 139–142. [Google Scholar] [CrossRef]
- Costa, J.M.; Challa, H. The effect of the original leaf area on growth of softwood cuttings and planting material of rose. Sci. Hortic. 2002, 95, 111–121. [Google Scholar] [CrossRef]
- Afitlhile, M.M. Constituent Processes of Leaf Senescence in Hordeum vulgare cv. Dyan. Master’s Thesis, Rhodes University, Grahamstown, South Africa, 1993. [Google Scholar]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Brumme, H.; Bruns, E.; Mehring, B.; Proll, T.; Wiegand, J. Phenological growth stages of roses (Rosa sp.): Codification and description according to the BBCH scale. Ann. Appl. Biol. 2008, 154, 231–238. [Google Scholar] [CrossRef]
- Canna Continental, Los Angeles, USA. Bio Rhizotonic. Available online: https://www.canna.ca/biorhizotonic (accessed on 28 July 2021).
- BioBizz Worldwide, B.V. Root JuiceTM. Available online: http://www.biobizz.com/products/#root%c2%b7juice (accessed on 19 January 2017).
- Terra Aquatica (T.A.). Pro Roots. Homepage General Hydroponics Europe/T.A. Terra Aquatica. Available online: https://www.eurohydro.com/pro-roots (accessed on 28 July 2021).
- Thorsen, M.K.; Woodward, M.; McKenzie, B.M. Kelp (Laminaria digitata) increases germination and affects rooting and plant vigour in crops and native plants from an arable grassland in the Outer Hebrides, Scotland. J. Coast. Conserv. 2010, 14, 239–247. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laïné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica napus growth is promoted by Ascophyllum nodosum L. Le. Jol. Seaweed extract, microarray analysis and physiological characterization of N, C, and S metabolism. J. Plant Growth Regul. 2013, 31, 32–52. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schaivon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2015, 73, 18–23. [Google Scholar] [CrossRef]
- Khan, W.; Rayrath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Pacholczak, A.; Szydło, W.; Jacygrad, E.; Federowicz, M. Effect of auxins and the biostimulator Algaminoplant on rhizogenesis in stem cuttings of two dogwood cultivars (Cornus alba ‘Aurea’ and ‘Elegantissima’). Acta Sci. Pol. Hortorum Cultus 2012, 11, 93–103. [Google Scholar]
- Pacholczak, A.; Szydło, W.; Petelewicz, P.; Szulczyk, K. The effect if Algaminoplant on rhizogenesis in stem cuttings of Physocarpus opulifolius ‘Dart’s Gold’ and ‘Red Baron’. Acta Sci. Pol. Hortorum Cultus 2013, 12, 105–116. [Google Scholar]
- Pacholczak, A.; Nowakowska, K.; Mika, N.; Borkowska, M. The effect of the biostimulator Goteo on the rooting of ninebark stem cuttings. Folia Hortic. 2016, 28, 109–116. [Google Scholar] [CrossRef][Green Version]
- Yaronskaya, E.; Vershilovskaya, I.; Poers, Y.; Alawady, A.E.; Averina, N.; Grimm, B. Cytokinin effects on tetrapyr role biosynthesis and photosynthetic activity in barley seedlings. Planta 2006, 224, 700–709. [Google Scholar] [CrossRef]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zadope, S.T.; Patolia, J.S. Effect of seaweed extract on growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Comparison of cytokinin- and auxin-like activity in some commercially used seaweed extracts. J. Appl. Phycol. 1997, 8, 503–508. [Google Scholar] [CrossRef]
- OMRI Products List, Web Edition. Organic Materials Review Institute. Available online: https://www.omri.org/omri-lists (accessed on 28 July 2021).
- Certificaat Bio Roots. No: C8008445INP-01.2013. 2014. Available online: http://www.eurohydro.com/pdf/certif-bioboosters.pdf/ (accessed on 19 January 2017).
- Monder, M.J.; Kozakiewicz, P.; Jankowska, A. The role of plant origin preparations and phenological stage in anatomy structure changes in the rhizogenesis of Rosa ‘Hurdal’. Front. Plant Sci. 2021, 12, 696998. [Google Scholar] [CrossRef] [PubMed]
- Monder, M.J.; Pacholczak, A. Rhizogenesis and concentration of carbohydrates in cuttings harvested at different phenological stages of once-blooming rose shrubs and treated with rooting stimulants. Biol. Agric. Hortic. 2020, 3, 53–70. [Google Scholar] [CrossRef]
- Monder, M.J.; Niedzielski, M.; Woliński, K. Effect of Phenological Stage and Rooting Enhancers on Physiological Parameters in Stem Cuttings in the Process of Rhizogenesis of Rosa × alba ‘Maiden’s Blush’. Agriculture 2020, 10, 572. [Google Scholar] [CrossRef]
- EU Council. Directive no. 91/414/EEC 1991. Dziennik Urzędowy Unii Europejskiej 03/t.11/Dziennik Urzędowy Wspólnot Europejskich. Off. J. Eur. Union 1991, L230/1, 332–362. [Google Scholar]
- EU Council. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. Off. J. Eur. Union 2009, L309, 71–86. [Google Scholar]
- EU Council. Regulation (EC) No. 1107/2009 of the European Parliament and of the Council of 21 October (2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, L309, 1–50. [Google Scholar]
- United States Department of Agriculture (USDA). National Organic Program. Available online: https://www.ams.usda.gov/about-ams/programs-offices/national-organic-program (accessed on 16 March 2021).
- Moberg, U.; Winter, A.; Hyden, N.; Ek, K. Analysis of Monoclonal Antibodies during Production and Purification; A Review of Electrophoretic Methods, LKB Application Note; LKB INSTRUMENTS. Available online: https://www.lkb.com.au/ (accessed on 28 October 2020).
- Ghosh, S.; Gepstein, S.; Heikkila, J.J.; Dumbroff, E.B. Use of scaning densitometer or an ELISA Plate Reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal. Biochem. 1988, 169, 227–233. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzyme in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Wójcik, A.R.; Laudański, Z. Planowanie i Wnioskowanie Statystyczne w Doświadczalnictwie; PWN: Warsaw, Poland, 1989; p. 130. [Google Scholar]
- Torchik, V. Effect of donor plant phenological phase on root formation of stem cuttings of ornamental Juniperus L. cultivars. Propag. Ornam. Plants 2005, 5, 51–55. [Google Scholar]
- Pihlajaniemi, H.; Siurainen, M.; Rautio, P.; Laine, K.; Peteri, S.L.; Huttunen, S. Field evaluation of phenology and success of hardy, micropropagated old shrub roses in northern Finland. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2005, 55, 275–286. [Google Scholar]
- Okoro, O.O.; Grace, J. The physiology of rooting Populus cuttings. I. Carbohydrates and photosynthesis. Physiol. Plant. 1973, 36, 133–138. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul. 1993, 13, 21–29. [Google Scholar] [CrossRef]
- Monder, M.J.; Niedzielski, M.; Woliński, K. Effect of rooting preparations on protein, chlorophyll and carotenoid content in leaves of Rosa gallica ‘Duchesse d’Angoulême’ cuttings. Dendrobiology 2014, 72, 29–40. [Google Scholar] [CrossRef]
- Monder, M.J.; Kozakiewicz, P.; Jankowska, A. Anatomical structure changes in stem cuttings of rambler roses induced with plant origin preparations. Sci. Hortic. 2019, 255, 242–254. [Google Scholar] [CrossRef]
- Fouda, R.A.; Schmidt, G. Histological changes in the stems of some Rosa species propagated by leafy cuttings as affected by IBA treatments. Acta Agron. Hung. 1994, 43, 265–275. [Google Scholar]
- Ranwala, A.; Miller, W.B. Effects of gibberellin treatments on flower and leaf quality of cut hybrid lilies. Acta Agron. 2002, 570, 205–210. [Google Scholar] [CrossRef]
- Skutnik, E.; Rabiza-Świder, J. Longevity of cut shoots of Molucella laevis L. as affected by flower preservatives and growth regulators. Folia Hortic. 2004, 16, 167–173. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).